Monoterapia con clobazam para las crisis focales o generalizadas
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009258.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 julio 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Epilepsia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
The review was conceived by RA and SKG. Data were extracted by VA, SKG, NG, and analyzed by SKG, RA and VA. RA and VA wrote the manuscript, which was reviewed by SKG and NG. All review authors have reviewed and approved the final version.
Sources of support
Internal sources
-
Cincinnati Children's Hospital Medical Center, USA.
Library, Biostatistics
External sources
-
National Institute for Health Research (NIHR), UK.
This review update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Ravindra Arya receives research support (total 5% effort) from Pediatric Epilepsy Research Foundation, which is unrelated to the present work
Vidhu Anand: none known
Nisha Giridharan: none known
Sushil K Garg: none known
Acknowledgements
We would like to acknowledge Cochrane Epilepsy for their support. Also, thanks to Ms. Rachael Kelly for assistance, Mr. Graham Chan and Ms. Alison Beamond for providing the search results, Dr. Yasuko Yamatogi for completing the data extraction form for their study in Japanese, and to Drs. Carol and Peter Camfield for offering their help to find the original participant data. Dr. Benedict Michael was a co‐author for the previous version of this systematic review and we acknowledge his input.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jul 11 | Clobazam monotherapy for focal or generalized seizures | Review | Ravindra Arya, Nisha Giridharan, Vidhu Anand, Sushil K Garg | |
| 2014 Oct 04 | Clobazam monotherapy for partial‐onset or generalized‐onset seizures | Review | Ravindra Arya, Vidhu Anand, Sushil K Garg, Benedict D Michael | |
| 2011 Aug 10 | Clobazam monotherapy for partial onset or generalized onset seizures | Protocol | Ravindra Arya, Sushil Kumar, Benedict Michael, Vidhu Anand | |
Differences between protocol and review
In 'Types of interventions', we decided to include studies with clobazam monotherapy given continuously for at least three months.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Adult; Child; Humans;
PICO

Study flow diagram (results from updated searches)

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
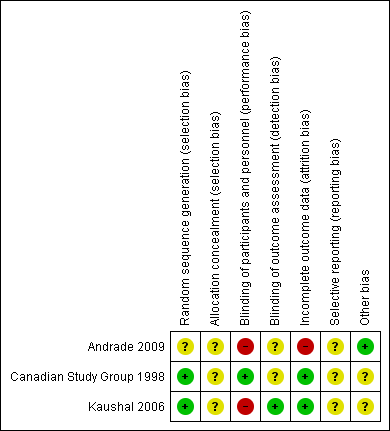
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 2 clobazam vs carbamazepine, outcome: 2.1 retention at 12 months

Forest plot of comparison: 1 clobazam versus phenytoin, outcome: 1.1 retention at 6 months
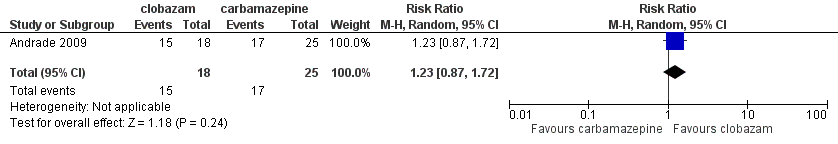
Forest plot of comparison: 1 clobazam versus carbamazepine, outcome: 1.4 terminal remission at 9 months (seizure free for last 9 months)

Comparison 1 Clobazam versus carbamazepine, Outcome 1 Retention at 12 months.
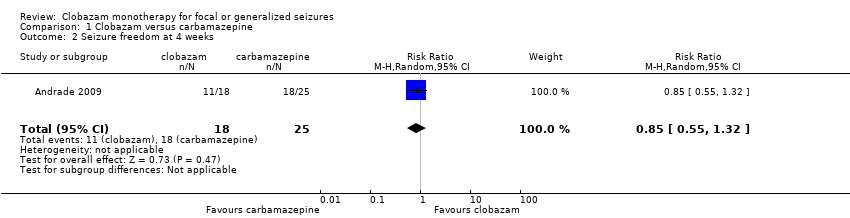
Comparison 1 Clobazam versus carbamazepine, Outcome 2 Seizure freedom at 4 weeks.
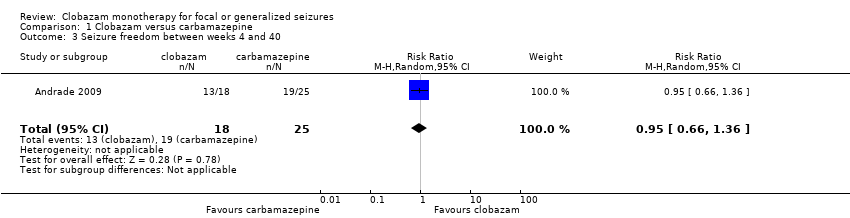
Comparison 1 Clobazam versus carbamazepine, Outcome 3 Seizure freedom between weeks 4 and 40.

Comparison 1 Clobazam versus carbamazepine, Outcome 4 Terminal remission at 9 months (seizure free for last 9 months).

Comparison 1 Clobazam versus carbamazepine, Outcome 5 50% responder rate at 4 weeks.

Comparison 1 Clobazam versus carbamazepine, Outcome 6 Reported adverse effects (number of participants).

Comparison 1 Clobazam versus carbamazepine, Outcome 7 Discontinued study medication due to adverse effects (number of participants).

Comparison 2 Clobazam versus phenytoin, Outcome 1 Retention at 6 months.

Comparison 2 Clobazam versus phenytoin, Outcome 2 Breakthrough seizure(s) during study period.

Comparison 2 Clobazam versus phenytoin, Outcome 3 Discontinued study medication due to adverse effects.

Comparison 2 Clobazam versus phenytoin, Outcome 4 Reported adverse effects.
| Clobazam versus carbamazepine for focal or generalized seizures | ||||||
| Patient or population: people with focal or generalized seizures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Carbamazepine | Clobazam | |||||
| Time on allocated treatment (retention time) | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Retention at 12 months | Study population | RR 0.83 (0.61 to 1.12) | 115 | ⊕⊕⊝⊝ | ||
| 654 per 1000 | 543 per 1000 (399 to 732) | |||||
| Seizure freedom at 4 weeks | 720 per 1000 | 611 per 1000 (396 to 950) | RR 0.85 (0.55 to 1.32) | 43 (1 study) | low2,3,4 | |
| Seizure freedom 4‐40 weeks | 760 per 1000 | 722 per 1000 (502 to 1034) | RR 0.95 (0.66 to 1.36) | 43 (1 study) | low2,3,4 | |
| 50% responder rate | 800 per 1000 | 944 per 1000 (752 to 1184) | RR 1.18 (0.94 to 1.48) | 43 (1 study) | low2,3,4 | |
| Adverse effects requiring withdrawal/discontinuation of study medication | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Blinding of participants and personnel was not done in this open‐label study. This can potentially affect assessment of this outcome. Downgraded by 1. 4Allocation concealment not stated, which could potentially introduce selection bias. Blinding of participants and personnel was not done in this open‐label study. | ||||||
| Clobazam versus phenytoin for focal or generalized seizures | ||||||
| Patient or population: drug‐naïve people with focal or generalized seizures with solitary cysticercus granuloma Settings: single Indian teaching hospital Intervention: clobazam Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Clobazam versus phenytoin | |||||
| Time on allocated treatment (retention time) | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Retention at 6 months (measured by the number of treatment failures in each group, which was defined as either breakthrough seizure or adverse effects requiring discontinuation or dose modification of study medication) | Study population | RR 1.43 | 48 | ⊕⊕⊝⊝ | ||
| 667 per 1000 | 953 per 1000 (720 to 1267) | |||||
| Seizure freedom at 4 weeks | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Seizure freedom 4‐40 weeks | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| 50% responder rate | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Adverse effects requiring withdrawal/discontinuation of study medication | Study population | RR 0.10 | 48 | ⊕⊕⊝⊝ | ||
| 222 per 1000 | 22 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Allocation concealment not stated, which could potentially introduce selection bias. Blinding of participants and personnel was not done in this open‐label study. This can potentially affect assessment of 'adverse effects requiring withdrawal' and hence 'retention time'. Downgraded by 1. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention at 12 months Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.61, 1.12] |
| 2 Seizure freedom at 4 weeks Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.32] |
| 3 Seizure freedom between weeks 4 and 40 Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.36] |
| 4 Terminal remission at 9 months (seizure free for last 9 months) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.87, 1.72] |
| 5 50% responder rate at 4 weeks Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| 6 Reported adverse effects (number of participants) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.16, 1.70] |
| 7 Discontinued study medication due to adverse effects (number of participants) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 10.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention at 6 months Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.08, 1.90] |
| 2 Breakthrough seizure(s) during study period Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.83] |
| 3 Discontinued study medication due to adverse effects Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.65] |
| 4 Reported adverse effects Show forest plot | 1 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.32, 2.14] |
| 4.1 Sedation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.40, 2.35] |
| 4.2 Skin problems including allergic rash | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.38] |
| 4.3 Tiredness | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.10, 1.91] |
| 4.4 Oral or gingival problems | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.65] |
| 4.5 Headache | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.73, 9.09] |
| 4.6 Weight gain | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 11.45 [0.65, 201.60] |

