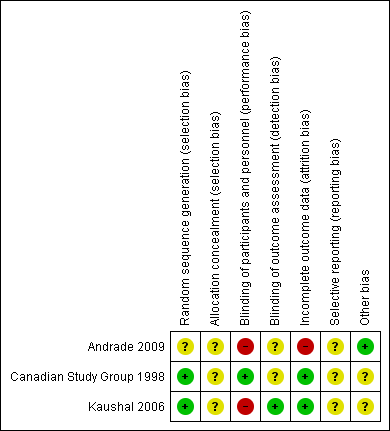
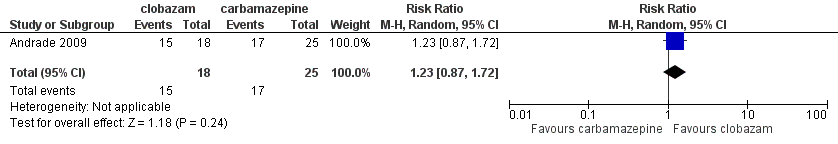
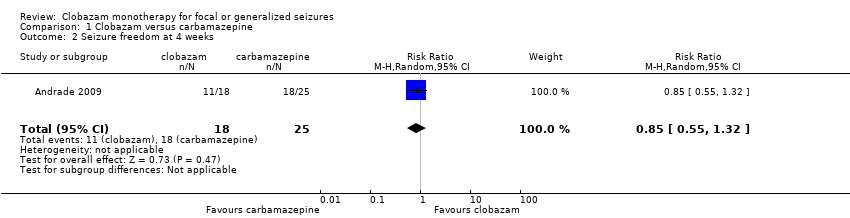
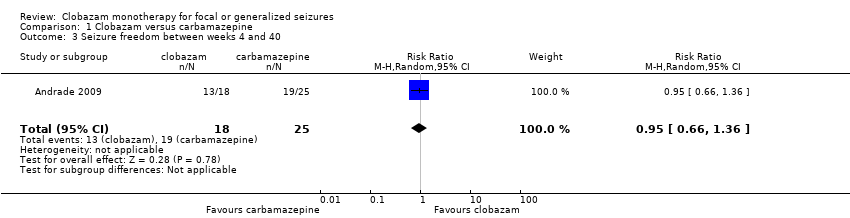
Contenido relacionado
Revisiones y protocolos relacionados
Marcus W Koch, Susanne KL Polman | 7 octubre 2009
Sarah J Nevitt, Anthony G Marson, Jennifer Weston, Catrin Tudur Smith | 9 agosto 2018
Rebecca Bresnahan, Kirsty J Martin‐McGill, John Williamson, Benedict D Michael, Anthony G Marson | 22 octubre 2019
Sarah J Nevitt, Catrin Tudur Smith, Anthony G Marson | 23 octubre 2018
Hongchang Chen, Honghu He, Yousheng Xiao, Man Luo, Hongye Luo, Jin Wang | 11 diciembre 2019
Sarah J Nevitt, Catrin Tudur Smith, Jennifer Weston, Anthony G Marson | 28 junio 2018
Sarah J Nevitt, Anthony G Marson, Catrin Tudur Smith | 18 julio 2019
Sarah J Nevitt, Catrin Tudur Smith, Anthony G Marson | 31 julio 2019
Sarah J Nevitt, Maria Sudell, Catrin Tudur Smith, Anthony G Marson | 24 junio 2019
Sarah J Nevitt, Anthony G Marson, Catrin Tudur Smith | 24 octubre 2018