Monoterapia con clobazam para las crisis focales o generalizadas
Appendices
Appendix 1. Cochrane Register of Studies search strategy
1. clobazam* or Aedon or Anxirloc or Castilium or Chlorepin or Clarmyl or Clobam or Clobamax or Clobator or Clofritis or Clopax or Clorepin or Frisium or Grifoclobam or Karidium or Lucium or Mystan or Noiafren or Onfi or Sederlona or Sentil or Urbadan or Urbanil or Urbanol or Urbanyl AND INREGISTER
2. >26/01/2017:CRSCREATED AND INREGISTER
3. #1 AND #2 AND INREGISTER
4. clobazam* or Aedon or Anxirloc or Castilium or Chlorepin or Clarmyl or Clobam or Clobamax or Clobator or Clofritis or Clopax or Clorepin or Frisium or Grifoclobam or Karidium or Lucium or Mystan or Noiafren or Onfi or Sederlona or Sentil or Urbadan or Urbanil or Urbanol or Urbanyl AND CENTRAL:TARGET
5. MESH DESCRIPTOR Epilepsy EXPLODE ALL AND CENTRAL:TARGET
6. MESH DESCRIPTOR Seizures EXPLODE ALL AND CENTRAL:TARGET
7. epilep* OR seizure* OR convuls* AND CENTRAL:TARGET
8. #5 OR #6 OR #7 AND CENTRAL:TARGET
9. #4 AND #8 AND CENTRAL:TARGET
10. ((adjunct* or "add‐on" or "add on" or adjuvant* or combination* or polytherap*) not (monotherap* or alone or singl*)):TI AND CENTRAL:TARGET
11. #9 NOT #10 AND CENTRAL:TARGET
12. >26/01/2017:CRSINCENTRAL AND CENTRAL:TARGET
13. #11 AND #12 AND CENTRAL:TARGET
14. #3 OR #13
Appendix 2. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials published in Lefebvre 2011.
1. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
2. clinical trials as topic.sh.
3. trial.ti.
4. 1 or 2 or 3
5. exp animals/ not humans.sh.
6. 4 not 5
7. exp Epilepsy/
8. exp Seizures/
9. (epilep$ or seizure$ or convuls$).tw.
10. 7 or 8 or 9
11. (clobazam or frisium or urbanol or onfi).tw.
12. 6 and 10 and 11
13. ((adjunct$ or "add‐on" or "add on" or adjuvant$ or combination$ or polytherap$) not (monotherap$ or alone or singl$)).ti.
14. 12 not 13
15. remove duplicates from 14
16. limit 15 to ed=20150416‐20180319
17. 15 not (1$ or 2$).ed.
18. 17 and (2015$ or 2016$ or 2017$ or 2018$).dt.
19. 16 or 18
Appendix 3. BIOSIS Previews search strategy
Indexes=BIOSIS Previews Timespan=2014‐2018
| #7 | #6 AND #5 |
| #6 | TS=((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind*) NEAR/2 (trial OR method OR procedure OR study)) |
| #5 | #3 NOT #4 |
| #4 | TI=(adjunct* OR "add‐on" OR "add on" OR adjuvant* OR combination* OR polytherap*) NOT TI=(monotherap* OR alone OR singl*) |
| #3 | #2 AND #1 |
| #2 | TOPIC: (clobazam or frisium or urbanol or onfi) |
| #1 | TOPIC: (epilep* OR "infantile spasm" OR seizure OR convuls* OR (syndrome NEAR/2 (aicardi OR angelman OR doose OR dravet OR "landau kleffner" OR "lennox gastaut" OR ohtahara OR panayiotopoulos OR rasmussen OR rett OR "sturge weber" OR "unverricht lundborg" OR west)) OR "ring chromosome 20" OR "R20" OR "myoclonic encephalopathy" OR "pyridoxine dependency") NOTTITLE: (*eclampsia) |
Appendix 4. ClinicalTrials.gov search strategy
Epilepsy | Clobazam | First posted on or after 01/26/2017
Appendix 5. ICTRP search strategy
Condition: epilepsy AND Intervention: clobazam AND Date of registration 26/01/2017 to 19/03/2018
Appendix 6. DARE search strategy
1 (clobazam or Frisium or Urbanol or Onfi) IN DARE
2 MeSH DESCRIPTOR epilepsy EXPLODE ALL TREES IN DARE
3 MeSH DESCRIPTOR Seizures EXPLODE ALL TREES IN DARE
4 (epilep* or seizure* or convuls*) IN DARE
5 #2 OR #3 OR #4
6 #1 AND #5
7 (adjunct* OR "add‐on" OR "add on" OR adjuvant* OR combination* OR polytherap*):TI NOT (monotherap* or alone or singl*):TI IN DARE
8 #6 NOT #7
Appendix 7. Assessment of risk of bias
We recorded each of the following aspects and graded them as high risk, low risk or unclear risk.
Sequence generation
Was the allocation sequence adequately generated: e.g. coin toss, random number tables, computer generated, other?
Allocation concealment
Was allocation adequately concealed in a way that would not allow both the investigators and the participants to know or influence
the intervention group before an eligible participant is entered into the study: e.g. central randomization, or sequentially numbered,
opaque, sealed envelopes?
Incomplete outcome data
Were incomplete outcome data adequately addressed? Incomplete outcome data essentially include: attrition, exclusions and missing
data. If any withdrawals occurred, were they described and reported by treatment group with reasons given? Whether or not there
were clear explanations for withdrawals and drop‐outs in treatment groups was recorded. An example of an adequate method to address
incomplete outcome data is the use of intention‐to‐treat analysis (ITT).
Selective outcome reporting
Are reports of the study free from suggestion of selective outcome reporting? This will be interpreted as no evidence that statistically non‐significant results might have been selectively withheld from publication: e.g. selective under‐reporting of data, or selective reporting of a subset of data.
Other sources of bias
Was the study apparently free of other problems that could put it at a high risk of bias: e.g. baseline imbalance, or the use of an insensitive
instrument to measure outcomes?
Blinding
Details of blinding participants, personnel (surgeons) and outcome assessors were assessed. This will be recorded as: yes, no or not
possible, or unclear.
Quality assessment/internal validity
Quality assessment criteria will be categorized as being at low, unclear or high risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).

Study flow diagram (results from updated searches)

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
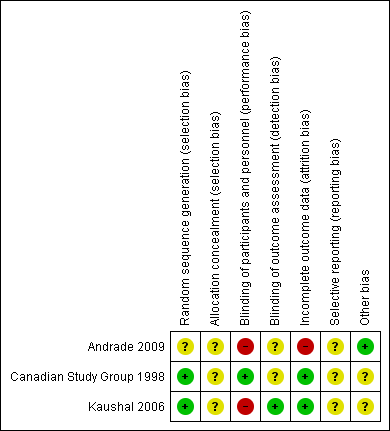
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 2 clobazam vs carbamazepine, outcome: 2.1 retention at 12 months

Forest plot of comparison: 1 clobazam versus phenytoin, outcome: 1.1 retention at 6 months
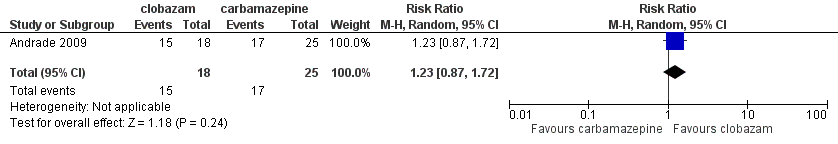
Forest plot of comparison: 1 clobazam versus carbamazepine, outcome: 1.4 terminal remission at 9 months (seizure free for last 9 months)

Comparison 1 Clobazam versus carbamazepine, Outcome 1 Retention at 12 months.
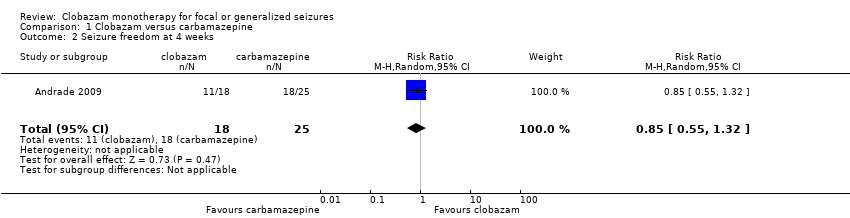
Comparison 1 Clobazam versus carbamazepine, Outcome 2 Seizure freedom at 4 weeks.
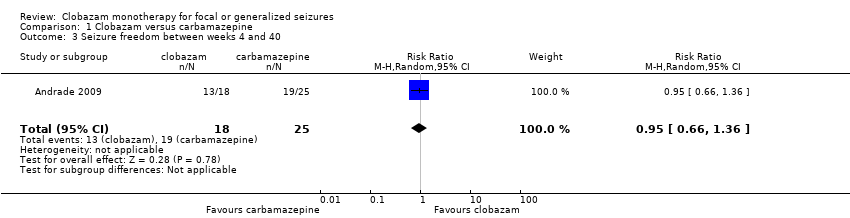
Comparison 1 Clobazam versus carbamazepine, Outcome 3 Seizure freedom between weeks 4 and 40.

Comparison 1 Clobazam versus carbamazepine, Outcome 4 Terminal remission at 9 months (seizure free for last 9 months).

Comparison 1 Clobazam versus carbamazepine, Outcome 5 50% responder rate at 4 weeks.

Comparison 1 Clobazam versus carbamazepine, Outcome 6 Reported adverse effects (number of participants).

Comparison 1 Clobazam versus carbamazepine, Outcome 7 Discontinued study medication due to adverse effects (number of participants).

Comparison 2 Clobazam versus phenytoin, Outcome 1 Retention at 6 months.

Comparison 2 Clobazam versus phenytoin, Outcome 2 Breakthrough seizure(s) during study period.

Comparison 2 Clobazam versus phenytoin, Outcome 3 Discontinued study medication due to adverse effects.

Comparison 2 Clobazam versus phenytoin, Outcome 4 Reported adverse effects.
| Clobazam versus carbamazepine for focal or generalized seizures | ||||||
| Patient or population: people with focal or generalized seizures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Carbamazepine | Clobazam | |||||
| Time on allocated treatment (retention time) | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Retention at 12 months | Study population | RR 0.83 (0.61 to 1.12) | 115 | ⊕⊕⊝⊝ | ||
| 654 per 1000 | 543 per 1000 (399 to 732) | |||||
| Seizure freedom at 4 weeks | 720 per 1000 | 611 per 1000 (396 to 950) | RR 0.85 (0.55 to 1.32) | 43 (1 study) | low2,3,4 | |
| Seizure freedom 4‐40 weeks | 760 per 1000 | 722 per 1000 (502 to 1034) | RR 0.95 (0.66 to 1.36) | 43 (1 study) | low2,3,4 | |
| 50% responder rate | 800 per 1000 | 944 per 1000 (752 to 1184) | RR 1.18 (0.94 to 1.48) | 43 (1 study) | low2,3,4 | |
| Adverse effects requiring withdrawal/discontinuation of study medication | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Blinding of participants and personnel was not done in this open‐label study. This can potentially affect assessment of this outcome. Downgraded by 1. 4Allocation concealment not stated, which could potentially introduce selection bias. Blinding of participants and personnel was not done in this open‐label study. | ||||||
| Clobazam versus phenytoin for focal or generalized seizures | ||||||
| Patient or population: drug‐naïve people with focal or generalized seizures with solitary cysticercus granuloma Settings: single Indian teaching hospital Intervention: clobazam Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Clobazam versus phenytoin | |||||
| Time on allocated treatment (retention time) | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Retention at 6 months (measured by the number of treatment failures in each group, which was defined as either breakthrough seizure or adverse effects requiring discontinuation or dose modification of study medication) | Study population | RR 1.43 | 48 | ⊕⊕⊝⊝ | ||
| 667 per 1000 | 953 per 1000 (720 to 1267) | |||||
| Seizure freedom at 4 weeks | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Seizure freedom 4‐40 weeks | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| 50% responder rate | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | Outcome not reported | |
| Adverse effects requiring withdrawal/discontinuation of study medication | Study population | RR 0.10 | 48 | ⊕⊕⊝⊝ | ||
| 222 per 1000 | 22 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Allocation concealment not stated, which could potentially introduce selection bias. Blinding of participants and personnel was not done in this open‐label study. This can potentially affect assessment of 'adverse effects requiring withdrawal' and hence 'retention time'. Downgraded by 1. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention at 12 months Show forest plot | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.61, 1.12] |
| 2 Seizure freedom at 4 weeks Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.32] |
| 3 Seizure freedom between weeks 4 and 40 Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.36] |
| 4 Terminal remission at 9 months (seizure free for last 9 months) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.87, 1.72] |
| 5 50% responder rate at 4 weeks Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| 6 Reported adverse effects (number of participants) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.16, 1.70] |
| 7 Discontinued study medication due to adverse effects (number of participants) Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 10.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Retention at 6 months Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.08, 1.90] |
| 2 Breakthrough seizure(s) during study period Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.83] |
| 3 Discontinued study medication due to adverse effects Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.65] |
| 4 Reported adverse effects Show forest plot | 1 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.32, 2.14] |
| 4.1 Sedation | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.40, 2.35] |
| 4.2 Skin problems including allergic rash | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.38] |
| 4.3 Tiredness | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.10, 1.91] |
| 4.4 Oral or gingival problems | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.65] |
| 4.5 Headache | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.73, 9.09] |
| 4.6 Weight gain | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 11.45 [0.65, 201.60] |

