Sistemas de control postural durante el sueño para los niños con parálisis cerebral
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Within subject, cross‐over study with randomised order of treatment using sealed envelopes. 2 nights attended polysomnography in paediatric research laboratory. Study ran from January to April 2007 | |
| Participants | Included
Excluded Children with poorly controlled/uncontrolled nocturnal seizures, unsettled domestic situations, and extreme behavioural problems | |
| Interventions |
| |
| Outcomes |
Polysomnography measured EEG (brain activity), EOG (eye movements), EMG (muscle tone), sleep stage, and detected arousals. Protech pressure transducer measured nasal airflow. Piezo bands measured respiratory movements. Masimo technology took oxygen measurements. Alice 5 (Respironics) software was used to integrate these physiological signals. Time locked digital video was also taken and parents completed Paediatric Sleep Questionnaire (PSQ) to look at snoring and daytime sleepiness scales | |
| Notes | Partly funded by manufacturer: Helping Hand Company (open grant with no requirement for approval of manuscript by company) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation to first night sleeping condition" Comment: Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "using a sealed envelope method" Comment: Probably done |
| Blinding of participants and personnel (performance bias) | High risk | Not reported Comment: Not possible to blind participants and personnel to the intervention; potential risk of bias, particularly for subjective outcomes |
| Blinding of outcome assessment (detection bias) | High risk | Not reported Comment: Lack of blinding; potential risks of bias, particularly subjective outcomes collected by parent‐report (PSQ). Lack of blinding less likely to influence outcomes measured by technology (e.g. polysomnography/piezo bands). It may influence video recording interpretation, but this outcome not included in results |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Subject 6 was excluded from within subject analysis because of a pyrexial illness on her second night" Comment: Not enough information to judge if missing outcome data were related to true outcome |
| Selective reporting (reporting bias) | High risk | CANTAB tests in protocol were abandoned. 2 years from study completion to publication |
| Other bias | High risk | Only 1 night in each condition. Did not invite all eligible for the study (13/22). Recruited existing users of sleep positioning systems. Did not have number of children required for intended statistical power |
| Methods | Within subject, cross‐over study with randomised order of treatment (method of randomisation not described). 4 consecutive nights sleeping in/out of sleep positioning system within their own home/residential care. Study ran from January 2009 to January 2011 | |
| Participants | Included
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funded by Nancie Finne Charitable Trust, which is now administered by the Chartered Society of Physiotherapy (CSP) Charitable Trust | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomised to either sleeping in their own sleep system or without their sleep system first" Comment: Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: Insufficient information to provide judgement |
| Blinding of participants and personnel (performance bias) | High risk | Not reported Comment: Not possible to blind participants and personnel to the intervention; potential risk of bias, particularly subjective outcomes |
| Blinding of outcome assessment (detection bias) | High risk | Not reported Comment: Lack of blinding; potential risks of bias, particularly subjective outcomes collected by parent‐report (PPP), but less likely to influence outcomes collected by Actigraph |
| Incomplete outcome data (attrition bias) | Unclear risk | Pain scores were not available for 1 child (no reasons given). Sleep data not available for 1 child as Actiwatch failed. 1 child slept without sleep positioning system for only 2/4 nights but results included in analysis without explanation as to how the data were averaged |
| Selective reporting (reporting bias) | High risk | Protocol reported measures such as motionless sleep and number of night awakenings, which were not reported in published paper. Findings were published a year after study completion |
| Other bias | High risk | Recruited existing users of sleep positioning systems and did not have number of children needed for intended statistical power |
AW: Actiwatch.
CANTAB: Cambridge Neuropsychological Testing Automated Battery.
GMFCS: Gross Motor Function Classification System.
EEG: electroencephalography.
EOG: electro‐oculography.
EMG: electromyography.
REM: rapid eye movement.
SpO₂: peripheral capillary oxygen saturation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Product evaluation of 8 different sleep positioning systems | |
| Case history of a 9‐year‐old. Outcome measures to improve symmetrical posture includes hip flexion | |
| Within subject comparison study on ventilatory function. No randomisation. Existing users of sleep positioning systems. 13/15 completed the trial. Age range 1 to 19 years | |
| Descriptive study looking at sleep position, muscle tone, pain, and quality of sleep. New users of sleep positioning systems. 28/42 still using sleep positioning systems after 1 year. Age range 9 months to 19 years | |
| Pilot prospective cohort study looking at hip migration and sleep patterns. New users of sleep positioning systems. 7/14 completed the trial. Age range 4 to 14 years | |
| Descriptive study looking at quality of sleep, role of parents and therapists, and views of children. 7/8 completed the trial. Age range 2 to 6 years | |
| Postal survey of 16 users' experiences of sleep positioning systems. Age range unclear | |
| Case history of a 6‐year‐old. Outcome measures were frequency of night awakenings and hip extension and abduction measurements | |
| Survey of professionals using sleep positioning systems within residential homes | |
| Cross‐sectional survey of 82 children. Both existing and new users of sleep positioning systems. Age range 6 to 15 years | |
| Postal survey of 448 paediatric physiotherapists | |
| Retrospective cohort study of 59 children looking at hip deformity, 24‐hour posture management systems not just sleep positioning systems. Age range 5 months to 18 years | |
| Prospective cohort study looking at hip deformity, 24‐hour posture management systems not just sleep positioning systems. 39/52 children completed the trial. Age range 18 months to 5 years | |
| Retrospective cohort study looking at hip stability and quality of life. Only conference abstract available | |
| Descriptive study looking at comfort, sleep patterns, and daytime activities. 4/9 completed the trial. Age‐range unclear but includes children under 5 years of age |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The effects of night positioning on sleep, postural deformity and pain in children and young people with cerebral palsy – an exploratory study |
| Methods | Feasibility randomised controlled trial (parallel groups design) across 4 regions of southern England. Allocation by minimisation |
| Participants | Included Aim to recruit 50 children, aged 3 to 16 years with cerebral palsy (GMFCS Levels IV to V), who are not walking independently and are not using sleep positioning systems Excluded Children with other conditions which may affect their musculoskeletal development or sleep quality |
| Interventions | Intervention group
Control group
|
| Outcomes |
Tools to be used: Chailey Sleep Questionnaire, Paediatric Pain Profile (PPP), Brief Pain Inventory (BPI), X‐rays |
| Starting date | November 2011 |
| Contact information | Dr Donna Cowan (Chailey Heritage Clinical Services), [email protected] |
| Notes | Funded by National Institute Health Research for Patient Benefit Panel |
GMFCS: Gross Motor Function Classification System.
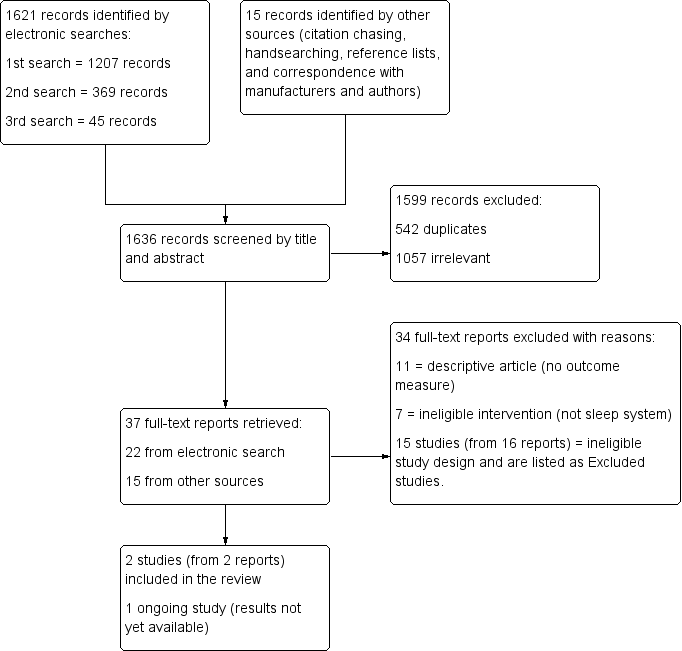
Study flow diagram

Risk of bias summary: Review authors' judgements about each risk of bias item for each included study.
| Sleeping in a sleep positioning system compared with not sleeping in a sleep positioning system for children with cerebral palsy | |||
| Population: Children with cerebral palsy Settings: United Kingdom (at home or in paediatric research laboratory) Intervention: Sleeping in sleep positioning system Comparison: Not sleeping in sleep positioning system | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Reduce hip migration/hip problems | No RCTs measured effect of sleep positioning systems on hip migration/hip problems | ‐ | ‐ |
| Effect on sleep patterns and quality | Limited data. A small number of established users of sleep positioning systems showed no significant difference in sleep quality indicators | 21 | ⊕⊝⊝⊝ |
| Effect on quality of life of child and family | No RCTs measured effect of sleep positioning systems on child and family quality of life | ‐ | ‐ |
| Effect on pain | Limited data. A small number of established users of sleep positioning systems showed no significant difference in levels of pain | 10 (1 study) | ⊕⊝⊝⊝ |
| Effect on physical functioning | No RCTs measured effect of sleep positioning systems on physical functioning | ‐ | ‐ |
| Adverse effects | No RCTs measured harms or reported adverse events | ‐ | ‐ |
| GRADE Working Group grades of evidence | |||
| GRADE: Grades of Recommendation, Assessment, Development and Evaluation. | |||
| Outcome | Variable | Definition | Study ID | Number of participants | Sleeping in sleep positioning system Mean (SD) | Sleeping out of sleep positioning system Mean (SD) | Mean difference (95% CI) | Paired t‐test | |
| t value | P value | ||||||||
| Sleep patterns/sleep quality | Sleep latency | Time (in minutes) to fall asleep | 9* | 69.1† (52.6) | 32.9 (26.0) | 36.2 (‐1.12 to 73.45) | 2.24 | 0.06 | |
| 8£ | 64.1 (54.0) | 37.0 (24.5) | 27.1 (‐8.76 to 62.89) | 1.79 | 0.12 | ||||
| 9 | 68.8 (49.8) | 80.1 (48.1) | ‐11.3 (‐30.70 to 8.03) | ‐1.35 | 0.21 | ||||
| Sleep efficiency | % of time in bed actually asleep | 9 | 80.7 (15.4) | 83.1 (12.0) | ‐2.4 (‐11.77 to 7.04) | ‐0.58 | 0.58 | ||
| 10 | 76.2 (8.3) | 73.8 (11.1) | 2.4 (‐2.98 to 7.73) | 1.00 | 0.34 | ||||
| Pain | Pain | Paediatric Pain Profile (PPP) scale (parent‐reported scores) | 10 | 11.3 (12.1) | 13.0 (14.6) | ‐1.7 (‐4.88 to 0.15) | ‐1.68 | 0.13 | |
| CI: Confidence intervals; ID: Identifier; SD: Standard Deviation | |||||||||
| * Includes one participant who fell asleep before recording started (recorded as zero), as reported by Hill 2009. | |||||||||
| Study | Variable of sleep quality | Number of participants | Sleeping in sleep positioning systems Mean (SD) | Sleeping out of sleep positioning system Mean (SD) | Sleeping in sleep positioning system Median (IQR) | Sleeping out of sleep positioning system Median (IQR) |
| Total sleep time (in minutes)¹ | 10 | 517.1 (54.4) | 509.1 (72.5) | 511.5 (52.5) | 527.5 (117.5) | |
| Total sleep time (in minutes) | 9 | 349.9 (101.1) | 427.7 (55.0) | 412.5 (143.5)* | 421.0 (89.0)* | |
| Total sleep time that was S1^ (%) | 9 | 2.4 (2.2) | 3.2 (2.4) | 1.7 (1.1)* | 3.6 (3.5)* | |
| Total sleep time that was S4^ (%) | 9 | 33.3 (10.6) | 29.0 (10.6) | 29.2 (11.3) | 28.0 (7.5) | |
| Total sleep time that was S3^ (%) | 9 | 6.4 (1.7) | 6.2 (2.4) | 6.3 (2.2) | 5.5 (3.8) | |
| Total sleep time that was S2^ (%) | 9 | 46.4 (10.0) | 50.5 (11.0) | 48.7 (11.3) | 49.7 (10.8) | |
| REM onset latency (in minutes) | 9 | 159.0 (99.4) | 204.3 (122.4) | 190.0 (18.0) | 187.0 (65.0) | |
| Number of REM cycles | 9 | 3.3 (0.9) | 2.9 (1.1) | 4.0 (1.0)* | 2.0 (2.0) | |
| Total sleep time that was REM^ (%) | 9 | 11.5 (5.1) | 11.0 (4.6) | 10.7 (1.4) | 11.1 (3.9) | |
| Total arousal index | 9 | 11.5 (6.5) | 11.4 (5.0) | 8.5 (6.0)* | 10.8 (8.2)* | |
| Central Apnoea Index (CAI) | 9 | 3.0 (8.0) | 4.0 (9.9) | 0.4 (0.4)* | 0.6 (0.9)* | |
| Respiratory Arousal Index (RAI) | 9 | 2.2 (3.7) | 1.5 (2.5) | 1.4 (1.9)* | 0.6 (1.4)* | |
| Apnoea ‐ Hypopnoea Index (AHI) | 9 | 1.9 (1.8) | 0.9 (1.2) | 2.6 (3.0) | 0.4 (1.5)* | |
| Obstructive Apnoea Index (OAI) | 9 | 0.5 (0.6) | 0.4 (0.9) | 0.3 (0.8)* | 0.1 (0.3)* | |
| % total sleep time with SpO₂ > 95% | 9 | 80.5 (29.0) | 77.2 (28.1) | 98.0 (19.9)* | 87.8 (12.6)* | |
| Average (mean) SpO₂ over total time | 9 | 95.7 (0.9) | 96.2 (1.9) | 95.0 (1.0)* | 97.0 (2.0)* | |
| Minimum SpO₂ (Nidus value) | 9 | 92.7 (1.7) | 90.6 (3.0) | 92.0 (1.0)* | 91.0 (3.0)* | |
| IQR: Interquartile range; REM: Rapid eye movement; SD: Standard deviation; SpO₂: Peripheral capillary oxygen saturation | ||||||
| ¹ Originally reported in hours and minutes, here given as minutes to be comparable. ^S1, S2, S3, S4 refer to the different stages of sleep; stages one to four. All values in this table are calculated from data supplied by study authors. For results from Hill 2009, some discrepancies were found between our calculations and the original publication. These are highlighted with an asterisk (*). | ||||||

