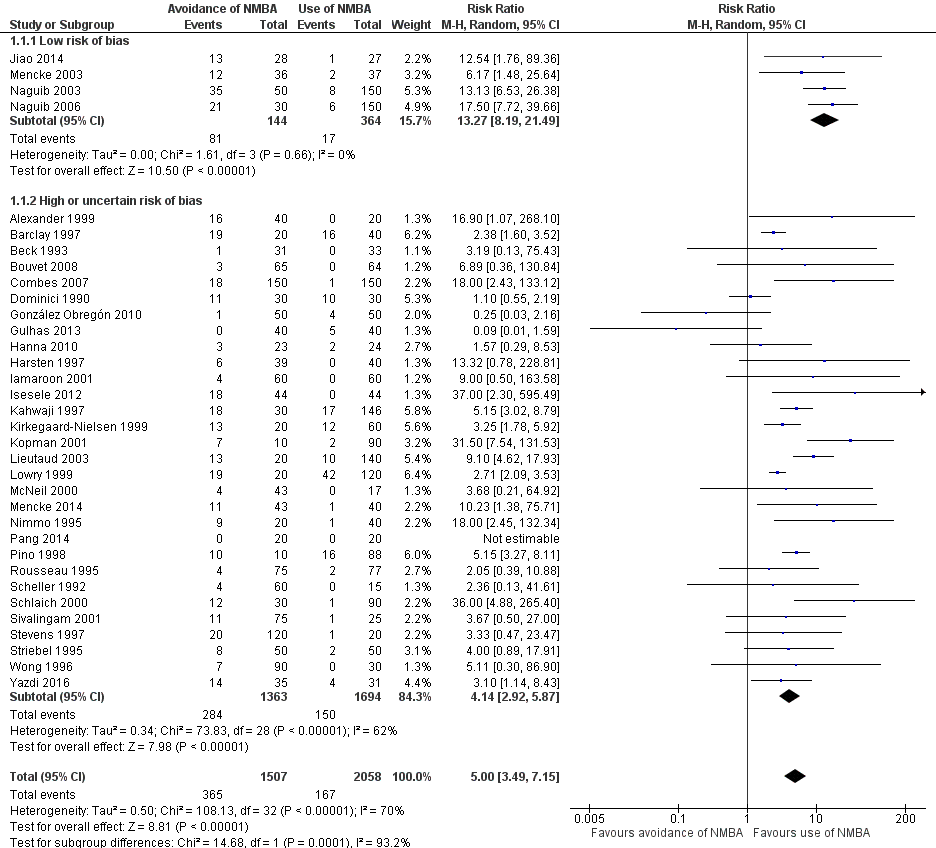
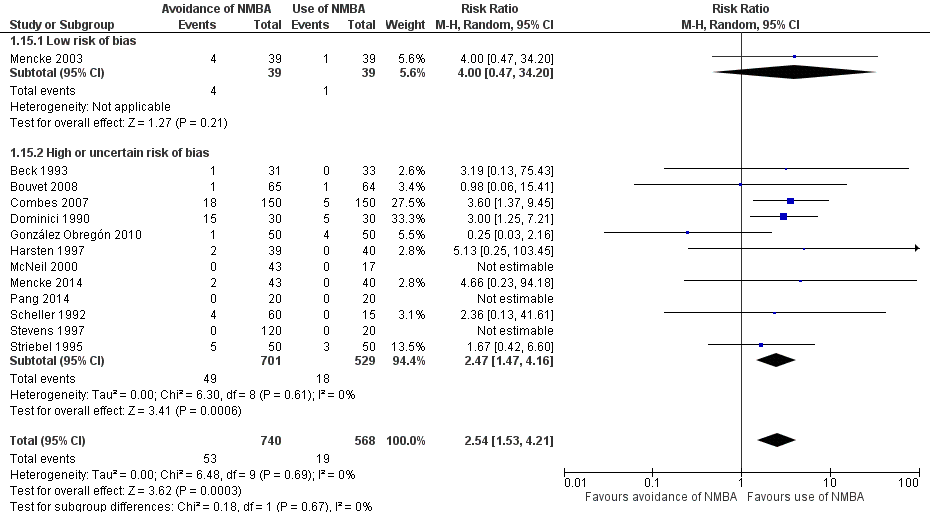
Evitación versus uso de agentes bloqueadores neuromusculares para mejorar las condiciones durante la intubación traqueal o la laringoscopia directa en adultos y adolescentes
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Parallel‐group RCT Settings: single centre Country: UK Language: English Number of control groups = 1/Number of Intervention groups = 2 Number of participants in control group = 20/Number of participants in intervention groups = 20/20 Randomized: N = 60 Analysed: N = 60 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA physical status I or II Scheduled for elective surgery Exclusion criteria Oesophageal reflux or hiatal hernia Previous difficulty with intubation or a suspected difficult airway Allergies to any of the study drugs Administration of sedative or opioid drugs in the previous 24 hours Renal or hepatic impairment | |
| Interventions | NMBA Control group S: suxamethonium 1 mg/kg Hypnotic Propofol 2 mg/kg Intervention group A: alfentanil 50 μg/kg Intervention group R: remifentanil 2 μg/kg Control group S: none None Premedicated with midazolam 0.03 mg/kg, 10 minutes before induction | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) | |
| Notes | One patient in both control group R and control group A had closed vocal cords requiring administration of suxamethonium. Tracheal intubation occurred 60 seconds after administration of the study drug Funding source: not specified Declarations of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated with a computer‐generated table to 1 of 3 groups |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | The anaesthetist who intubated the participant was unaware of the study drug and was instructed to face away from the participant; the sound of the pulse oximeter was temporarily turned off to avoid recognition of the study drug by observation of fasciculations or a decrease in heart rate |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: UK Language: English Number of control groups = 2/Number of Intervention groups = 1 Number of participants in control groups = 20/20/Number of participants in intervention group = 20 Randomized: N = 60 Analysed: N = 60 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria Aged 18 to 50 years Exclusion criteria Obese Risk of pulmonary aspiration Smoked more than 10 cigarettes per day Tracheas known to be difficult to intubate or coughing or straining after tracheal intubation | |
| Interventions | NMBA Control group 1: rocuronium 0.1 mg/kg Control group 2: rocuronium 0.3 mg/kg Hypnotic Propofol 2.5 mg/kg Alfentanil 10 μg/kg Lidocaine 10 mg IV Premedicated with temazepam 20 to 30 mg per os 1 hour before induction | |
| Outcomes | 1. Intubation conditions: modified version of "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996). The investigator assessed 3 factors: ease of jaw opening and laryngoscopy (1 easy; 2 fair; 3 difficult), position of the vocal cords and their movement (1 open; 2 moving; 3 closing), and degree of straining (“bucking”) after tracheal intubation and cuff inflation (1 none; 2 with diaphragm; 3 with abdominal muscles). The occurrence of any significant complication was recorded. Overall conditions for tracheal intubation were scored by 3 grades: optimal, suboptimal, and failure. Tracheal intubation was judged as optimal when all scores were 1 or 2, and was judged as suboptimal if any scores were 3. Failure to intubate was scored as a failure | |
| Notes | Funding source: "We also thank Organa Teknika for their generous supply of rocuronium" Declarations of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | ....an investigator, who was blinded as to the allocation..... |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: USA Language: English Number of control groups = 1/Number of Intervention groups = 1 Number of participants in control group = 33/Number of participants in intervention group = 31 Randomized: N = 64 Analysed: N = 64 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Aged 18 to 60 years Elective surgery lasting > 20 minutes Exclusion criteria Neurosurgical, obstetrical, or ophthalmological procedures | |
| Interventions | NMBA Suxamethonium 1 mg/kg Hypnotic Control group: thiopenthal 5 mg/kg Intervention group: propofol 2 mg/mL Opioid Control group: none Intervention group: alfentanil 50 μg/kg Local anaesthetic None None | |
| Outcomes | 1. Intubation condition: VAS 0 to 100, intubation condition: 0 = perfect, 100 = terrible. No defined cut‐off value indicating acceptable intubation condition 2. Laryngoscopy condition: Cormack and Lehane score (Cormack 1984) In our meta‐analyses, we categorized Cormack and Lehane scores I and II as acceptable intubation condition, and scores III and IV as unacceptable condition | |
| Notes | Funding source: not specified Declarations of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | The anaesthetist performing the intubation was independent from the study |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: France Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 64/Number of participants in intervention group = 65 Randomized: N = 130 Analysed: N = 129 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria Elective gynaecological surgery ASA class I or II Aged > 18 years Exclusion criteria History or evidence of a difficult airway (combination of Mallampati score 3 or 4, thyromental distance < 60 mm, mouth opening < 35 mm) Contraindication to use of NMBA | |
| Interventions | NMBA Cisatracurium 0.15 mg·kg–1 Hypnotic Propofol 2.5 mg·kg–1 Remifentanil 2 μg·kg–1 None Premedication when appropriate: alprazolam 0.5 mg and/or hydroxyzine 0.5 to 2 mg·kg–1 PO | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) 2. Laryngoscopy condition: Cormack and Lehane score (Cormack 1984) 3. Postoperative laryngeal symptoms and vocal cord injury: 24 hours, 48 hours, 1 month postoperative | |
| Notes | One participant in group cisatracurium was excluded because of non‐observance of the anaesthetic protocol, leaving 129 clinically evaluable participants. Time from induction to start of tracheal intubation: 270 seconds Funding source: "Financial support for this study was provided solely from institutional sources" Declarations of interest: "none declared" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by the use of coded, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | In our protocol, only the study physician (performing all tracheal intubations) was blinded to intervention allocations (along with the participant). Other personnel were aware because they had to await the disappearance of all 4 twitches in response to train‐of‐four stimulation at the adductor pollicis muscle before allowing arrival of the study physician into the operating room (contacted study author) |
| Blinding of outcome assessment (detection bias) | Low risk | "The same experienced senior physician, blinded to the anaesthetic regimen, performed all tracheal intubations..." |
| Incomplete outcome data (attrition bias) | Low risk | One participant in group cisatracurium was excluded because of non‐observance of the anaesthetic protocol |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: France Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 150/Number of participants in intervention group = 150 Randomized: N = 300 Analysed: N = 299 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA status I or II Elective surgery Exclusion criteria Predictive of difficult intubation BMI above 30 kg·m‐2 Allergy to muscle relaxants Need for a nasogastric tube Ear‐nose‐and‐throat surgery. Preoperative sore throat or hoarseness at history taking | |
| Interventions | NMBA Rocuronium 0.6 mg·kg–1 Hypnotic Propofol 2.5 mg·kg–1 Control group: alfentanil 15 µg/kg Intervention group: alfentanil 40 µg/kg None Premedication: hydroxyzine 50 to 100 mg PO | |
| Outcomes | 1. Intubation condition: Intubation Difficulty Scale (IDS) (Adnet 1997) and "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) 2. Laryngoscopy condition: Cormack and Lehane score (Cormack 1984) 3. Post‐intubation pharyngolaryngeal symptoms: 2 hours and 24 hours after extubation. Methods not specified | |
| Notes | Intubation 90 seconds after rocuronium/saline One participant left the hospital before questioning a second time on their pharyngolaryngeal symptoms. This participant (from the rocuronium arm) could not be reached Funding source: "Support was from departmental sources only" Declarations of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified |
| Allocation concealment (selection bias) | Low risk | “Patients were randomly assigned to one of the two groups by a physician not involved in the patient’s care, using numbered sealed envelopes” |
| Blinding of participants and personnel (performance bias) | Low risk | “All drugs were prepared by an independent staff anaesthetist not involved in the study. The muscle relaxant (rocuronium) and the saline solution were prepared in identical syringes and in identical volumes” |
| Blinding of outcome assessment (detection bias) | Low risk | "All drugs were prepared by an independent staff anaesthetist not involved in the study. The muscle relaxant (rocuronium) and the saline solution were prepared in identical syringes and in identical volumes” |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: France Language: French Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 30/Number of participants in intervention group = 30 Randomized: N = 60 Analysed: N = 60 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria Aged > 18 years ASA I, II, or III Endoscopies with or without laser, tonsillectomies, nose‐fracture surgeries, adenectomies, meatotomies and tympanoplastics Surgeries with duration < 90 minutes Exclusion criteria Known allergies Severe arterial hypertension, unstable heart failure Sickness related to conduction and excitability of ventricles Potassium levels above 5 mmol/L Renal failure Epilepsy | |
| Interventions | NMBA Suxamethonium 1.5 mg/kg Hypnotic Propofol 3 mg/mL Opioid Alfentanil 7 to 10µg/kg Local anaesthetic Lidocaine (2%): 1 mL injected before administration of propofol. Topical lidocaine 5% used in larynx before intubation Midazolam 0.1 mg/kg was given 30 minutes before induction | |
| Outcomes | 1. Intubation condition: quality of intubation measured by (1) position of the glottis (open or semi‐open on 1 part and closed or not visualized); (2) presence or absence of bucking; or (3) number of intubation attempts necessary for successful intubation 2. Laryngoscopy condition: position of the glottis (open or semi‐open on 1 part and closed or not visualized). Closed or not visualized defined as difficult | |
| Notes | Intubation 60 seconds after induction Funding source: not specified Declarations of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...are distributed according to a randomised list into two groups of 30" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | "...waits outside the operation room for 1 minute (to make sure fasciculations are not seen), before performing the laryngoscopy" |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Columbia Language: Spanish Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 50/Number of participants in intervention group = 50 Randomized: N = 100 Analysed: N = 100 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria Ambulatory surgery ASA physical status I or II Exclusion criteria Aged < 15 or > 60 years Expected difficult airway Full stomach Rapid sequence induction indicated Pregnant or neuromuscular disease | |
| Interventions | NMBA Rocuronium 0.6 mg/kg Hypnotic Intervention group A: oxygen 3 L/min + sevoflurane 3%; after 3 minutes, bolus of propofol 2 mg/kg Control group B: bolus of propofol of 1 to 2 mg/kg (not standardized) for a minute Opioid Intervention group A: remifentanil 0,6 microg/kg/min for 5 minutes, hereafter remifentanil dose is halved until intubation Control group B: bolus of remifentanil 1 to 2 microg/kg (not standardized) for a minute, followed by infusion of 0,15 microg/kg/min for 1 minute Local anaesthetic None None | |
| Outcomes | 1. Difficult laryngoscopy (Cormack 1984) 2. Post‐intubation pharyngolaryngeal symptoms: postoperative hoarseness, not further specified | |
| Notes | Difficult laryngoscopy was included as a surrogate for difficult intubation in our meta‐analysis Funding source: not specified Declarations of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 50 random numbers between 00 and 99 are selected by a manual/hand calculator. These numbers constitute group A, the rest group B. The group that will receive NMBA is then determined by randomization |
| Allocation concealment (selection bias) | Low risk | The result of randomization is kept in a sealed envelope |
| Blinding of participants and personnel (performance bias) | High risk | The investigator or other anaesthetist who is administering the induction according to randomization is not blinded but is not the one assessing outcomes |
| Blinding of outcome assessment (detection bias) | Low risk | The anaesthesiologist who performs tracheal intubation does not participate in randomization |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Turkey Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 40/Number of participants in intervention group = 40 Randomized: N = 80 Analysed: N = 80 Dates when the study was conducted: between November 2009 and July 2010 | |
| Participants | Inclusion criteria ASA physical status I and II Mallampati scores of I and II Aged 18 to 65 years Selective microlaryngoscopy Exclusion criteria History of head and neck Previous surgery or scheduled to undergo head and neck surgery Severe cardiovascular and pulmonary disease Neuromuscular disease Medications affecting neuromuscular junctions Strained patients | |
| Interventions | NMBA Succinylcholine 1 mg/kg Hypnotic Control group: propofol 2 mg/kg over 30 seconds Intervention group: propofol 2 mg/kg over 30 seconds Opioid Control group: remifentanil 1 μg/kg over 90 seconds Intervention group: remifentanil 4 μg/kg over 90 seconds Local anaesthetic None None | |
| Outcomes | 1. Intubation conditions: categorized by (1) jaw relaxation (complete; tone; stiff; rigid), (2) laryngoscopy (easy; fair; difficult; impossible), (3) vocal cords (open; moving; closing; closed), (4) coughing (none; slight; moderate; severe), (5) movement (none; slight; moderate; severe) (Hellbo‐Hansen 1988). In this study, no accumulated score was presented, and laryngoscopy was categorized as difficult; it was impossible to define a difficult intubation 2. Post‐intubation pharyngolaryngeal symptoms: sore throat and hoarseness evaluated. Methods and timing not described | |
| Notes | Funding source: not specified Declarations of interest: "none" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization and allocation of participants into intervention groups using computerized numbers |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Both care providers on the ward and anaesthesiologists assessing outcomes were blinded to study groups |
| Blinding of outcome assessment (detection bias) | Low risk | Both care providers on the ward and anaesthesiologists assessing outcomes were blinded to study groups |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: USA Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 24/Number of participants in intervention group = 23 Randomized: N = 50 Analysed: N = 47 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Aged 18 to 75 years Elective non‐ophthalmic surgery Exclusion criteria Anticipated difficult airway management GI reflux, hiatal hernia Ocular surgery within 6 months Long‐term opioid use Allergy to study drugs | |
| Interventions | NMBA Rocuronium 0.06 mg/kg (defasciculation) + succinylcholine 1.5 mg/kg Hypnotic Propofol 2 mg/kg Control group: none Intervention group: remifentanil 4 μg/kg Local anaesthetic Lidocaine 0.5 mg/kg IV Premedication: midazolam 2 mg and glycopyrrolate 0.2 mg IV | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) | |
| Notes | "...Three patients were excluded from the study due to IOP tonometer malfunction...." Intubation 60 seconds after induction Funding source: "supported in part by Aspect Medical Systems, Inc., Norwood, MA, USA, who provided bispectral index (BIStm) electrodes for the study. Otherwise, the study was supported by funds from the Department of Anesthesiology, Loyola University Medical Center, Maywood, IL, USA" Declarations of interest: "none" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | "...each consecutive patient contained in a sealed envelope..." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | "....two attending anesthesiologists and nurse anesthetist, blinded to patient group assignment entered the operation room." |
| Incomplete outcome data (attrition bias) | Low risk | "...Three patients were excluded from the study due to IOP tonometer malfunction...." |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Sweden Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 40/Number of participants in intervention group = 39 Randomized: N = 80 Analysed: N = 79 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Age ranging from 18 to 76 years Exclusion criteria Not specified | |
| Interventions | NMBA Suxamethonium 1 mg/kg Hypnotic Control group: thiopental 5 mg/kg Intervention group: propofol 2,5 mg/kg Alfentanil 10 μg/kg None Premedication: 0.25 mg triazolam 45 to 60 minutes before induction | |
| Outcomes | 1. Intubation condition: “Intubating conditions were assessed on the basis of jaw relaxation (0 = no relaxation, impossible to open mouth, 1 = moderate relaxation, 2 = complete relaxation), ease of the insertion of the tube (0 = vigorous movements of the vocal cords and difficult or impossible to insert tracheal tube, 1 = slight movements of the vocal cords, 2 = relaxed vocal cords without any movements) and coughing on intubation (0 = vigorous coughing, 1 = slight coughing, 2 = no coughing) (9)” In this RCT, study authors did not define any cut‐off value for acceptable intubation. For the meta‐analysis, we defined an accumulated score = 4 as clinically unacceptable. We dichotomized the score on the basis of the definition presented in "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) 2. Laryngoscopy condition: Further, patients in whom the vocal cords were not visible (equals Cormach and Lehane grade III and IV) were categorized as clinically unacceptable | |
| Notes | In the PA group, 1 patient was excluded because the induction dose of propofol was not sufficient to produce anaesthesia, and a further 2 patients because the vocal cords were not visible. In our meta‐analysis, we included the last 2 patients as "difficult to intubation" and "difficult to laryngoscope" Funding source: "The authors wish to thank Zeneca, Sweden, for the supply of propofol. This study was supported by grants from the Local Fund for Medical Research and Development of the Kristianstad County Council" Declarations of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “According to a computer generated randomisation list, patients were assigned to receive either…” |
| Allocation concealment (selection bias) | Low risk | "we used sealed non‐transparent envelopes containing a slip of paper with a description of which of the two induction methods was being used" (mail contact with study author) |
| Blinding of participants and personnel (performance bias) | High risk | "Apart from the drug administering doctor no one else knew which drugs were given" (mail contact with study author) |
| Blinding of outcome assessment (detection bias) | Low risk | “The intubating anaesthetist was not present during the induction of anaesthesia but was waiting outside the operating room. He was allowed to enter the room when the eyelash reflex or the muscle fasciculations had disappeared and was unaware of which drugs had been used” |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Thailand Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in intervention group = 60/Number of participants in control group = 60 Randomized: N = 120 Analysed: N = 120 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I and II Aged 20 to 60 years Elective non‐cardiothoracic surgery Exclusion criteria Obesity, body mass index > 30 kg/m2 Pregnancy, small bowel obstruction, history of oesophageal reflux, or hiatal hernia Difficult airway problems Hyperkalaemia Suspected malignant hyperthermia Cardiac, pulmonary, or renal disease | |
| Interventions | NMBA Suxamentonium 1.5 mg/kg Hypnotic Control group: thiopenthal 5 mg/kg + 4 L/min (N2O) and 2 L/min O2 Intervention group: sevoflurane 8% in 66% N2O and 33% O2 mixture Opioid Fentanyl 1.5 μg/kg None Diazepam 5 or 10 mg orally 1 to 2 hours before induction | |
| Outcomes | 1. Intubating conditions: Jaw relaxation was described as fully relaxed (score = 1), mildly resistant (score = 2), tight but open (score = 3), and impossible (score = 4). Vocal cord position was described as widely open (score = 1), mid position (score = 2), moving but open (score = 3), and closed (score = 4). Intubating responses were described as none (score = 1), diaphragmatic movement (score = 2), mild/moderate coughing (score = 3), and severe coughing (score = 4). Intubating conditions were graded as excellent (total score (TS) = 3), good (TS = 4 to 6), poor (TS = 7 to 9), or impossible (TS = 10 to 12). Total score of 6 or less was classified as an acceptable intubation condition, otherwise as an unacceptable condition | |
| Notes | Funding source: supported by Mahidol University research fund. Sevoflurane was supported by Abbott Laboratories Limited Declarations of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | "..... anaesthetic residents blindly participated as the intubators and the nurse anesthetists blindly participated as the observers...." |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Nigeria Language: English/French Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 48/Number of participants in intervention group = 48 Randomized: N = 96 Analysed: N = 88 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Aged 18 to 45 years Mallampati class I and II Elective surgery Exclusion criteria Inability to understand written or verbal information Presence of dental crowns Past history of difficult intubation Obvious signs of expected difficult intubation Intercurrent chronic ailments such as valvular heart disease and asthma Overt or risk of raised intracranial or intraocular pressure Risk of regurgitation and aspiration of gastric contents Antipsychotic therapy Opioid therapy | |
| Interventions | NMBA Suxamentonium 1.5 mg/kg Hypnotic Propofol 2mg/kg Opioid None Intervention group: 1.5 mg/kg intravenous lidocaine Diazepam 10 mg orally the morning of surgery | |
| Outcomes | 1. Intubation conditions: With the intubating condition scoring system, 3 parameters were assessed and scored on a scale of 0 to 2. These included jaw relaxation, ease of insertion of endotracheal tube, and response to intubation. Thus, a total intubating condition score could range from 0 (worst) to 6 (best). A score of 5 to 6 = good, 3 to 4 = moderate, and 0 to 2 = poor. Scoring system from Saarnivaara 1991 | |
| Notes | Funding source: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | All eligible participants were randomly assigned to the 2 study groups with sealed unmarked envelopes |
| Allocation concealment (selection bias) | Low risk | All eligible participants were randomly assigned to the 2 study groups with sealed unmarked envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Low risk | Eight participants, however, were excluded from further participation in the study for various reasons: 5 of these for improper documentation of the data collection form, 2 for electrocardiographic abnormalities, and 1 for abnormal haemodynamic values before induction of anaesthesia |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: China Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 27/Number of participants in intervention group = 28 Randomized: N = 55 Analysed: N = 55 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Aged 18 to 60 years Elective gynaecological laparoscopic Exclusion criteria BMI mass index > 30 Mallampati airway grade 2 to 4 Known allergy to propofol, egg, or opioids Alcohol or drug abuse History of gastro‐oesophageal reflux disease Neuromuscular disease History of upper respiratory tract infection or other airway hyperactivity disease in the recent 2 weeks | |
| Interventions | NMBA Suxamentonium 0.6 mg/kg Hypnotic Propofol 2 mg/kg Opioid Control group: remifentanil 1.0 μg/kg Intervention group: remifentanil 1.5 μg/kg None None | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) | |
| Notes | 1 participant in group C was graded as 4 by Cormach‐Lehane grading and was not improved in laryngeal exposure after addition of succinylcholine, then was transferred for video laryngoscopic intubation. We included this participant as difficult to intubate in our meta‐analyses. Additional succinylcholine was administered for 1 participant in the control group before intubation. This participant was included int the meta‐analyses as difficult to intubate Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ...with random number table |
| Allocation concealment (selection bias) | Low risk | ...given random numbers by a study co‐ordinator, who also encodes the drugs with matching random numbers. The study co‐ordinator also prepared all drugs....... (contact with study author) |
| Blinding of participants and personnel (performance bias) | Low risk | Remifentanil in each group was diluted with normal saline to 10 mL, and injectors were labelled with “2”. The injectors were infused with succinylcholine (diluted with normal saline to 10 mL) in group S and with normal saline 10 mL in group C (labelled with “3”) |
| Blinding of outcome assessment (detection bias) | Low risk | Intubation was attempted by an experienced anaesthesiologist blinded to grouping of the study |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: multi‐centre Country: USA Language: English Number of control groups = 5/Number of intervention groups = 1 Number of participants in control group = 30/27/32/28/29/Number of participants in intervention group = 30 Randomized: N = 181 Analysed: N = 178 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria Aged 19 to 85 years ASA physical status I to III Elective surgical procedures with anticipated duration ≥ 1 hour Exclusion criteria Significant neurological, renal, or hepatic disease Receiving drugs that could interfere with normal neuromuscular function Preoperative evaluation indicated that difficult tracheal intubation was anticipated | |
| Interventions | NMBA Type: ORG 9487 (rapacuronium) Control group 1: 0.5 mg/kg Control group 2: 1.0 mg/kg Control group 3: 1.5 mg/kg Control group 4: 2.0 mg/kg Control group 5: 2.5 mg/kg Hypnotic Thiopental (3 to 6 mg/kg) Fentanyl 0.5 to 3 μg/kg None None | |
| Outcomes | 1. Intubation conditions: Intubating conditions were assessed by a blind observer, using a 4‐point scale (excellent, good, poor, impossible) ‐ modification of "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) 2. Serious adverse experience: defined as an adverse effect that was fatal, life‐threatening, or permanently disabling, and required prolonged hospitalization, or was an overdose. Investigators stated whether they considered the adverse effect to be related to administration of ORG 9487 | |
| Notes | Three elderly participants were excluded before administration of the study drug owing to equipment failure, contraindicated drug administration, or clinical decision Time from induction to start of tracheal intubation: 90 seconds Adverse event: Two of these events (tachycardia, with heart rate from 85 to 150 bpm, and bronchospasm) occurred in one 29‐year‐old, 100‐kg, ASA physical status I male participant within 30 seconds of administration of 2.0 mg/kg ORG 9487 and were followed by erythema of the arms, shoulders, and face. The bronchospasm was treated with salbutamol, and all symptoms gradually subsided. These events were considered to meet the criteria for serious adverse experiences Funding source: supported by Organon Inc., Akzo Nobel Inc., West Orange, NJ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | "...before injection of one of five doses of ORG 9487 (0.5, 1.0, 1.5, 2.0, 2.5 mg/ kg) or placebo given in a randomized, blind sequence..." |
| Incomplete outcome data (attrition bias) | Low risk | ".......Violations included administration of isoflurane for induction before administration of ORG 9487, administration of enflurane before TOF had returned to 0.7, administration of an incorrect dose of ORG 9487, or allocation to the incorrect age group....." |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: USA Language: English Number of control groups = 3/Number of intervention groups = 1 Number of participants in control group = 20/20/20/Number of participants in intervention group = 20 Randomized: N = 80 Analysed: N = 80 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria Adults ASA class I and II Elective surgical procedures Exclusion criteria Aged > 60 yr or < 18 years Gastro‐oesophageal reflux Weighing > 30% more than ideal body weight Neuromuscular disease, or undergoing treatment with drugs known to interfere with neuromuscular transmission | |
| Interventions | NMBA Control group 1: rocuronium 0.4 mg/kg Control group 2: rocuronium 0.8 mg/kg Control group 3: rocuronium 1.2 mg/kg Hypnotic Propofol 2 mg/kg Fentanyl 2 μg/kg None Premedication: midazolam 2 mg IV | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996). Further, if the endotracheal tube was not passed successfully within 30 seconds, i.e. 70 seconds after rocuronium or saline solution administration, this was recorded as a failed intubation | |
| Notes | Intubation 40 seconds after induction Funding source: Support was provided solely by institutional and/or departmental sources | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | The dose each participant received was decided on a random basis by selection of an unmarked envelope containing details of the dose |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | The investigator performing the intubation and assessing conditions was blinded to the dose of rocuronium administered |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: USA Language: English Number of control groups = 3/Number of intervention groups = 1 Number of participants in control group = 30/30/30/Number of participants in intervention group = 10 Randomized: N = 100 Analysed: N = 100 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA physical status I or II Aged 18 to 65 years Elective surgical procedures Body mass index ≥ 17.5 and ≤ 27.5 Exclusion criteria Neuromuscular disease | |
| Interventions | NMBA Intervention group 1: rapacuronium 1.0 mg/kg Intervention group 2: rapacuronium 1.2 mg/kg Intervention group 3: rocuronium 0.50 mg/kg Hypnotic Propofol 2.0 mg/kg IV Alfentanil 12.5 μg/kg None Midazolam (maximum 2 mg) for induction | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) | |
| Notes | Tracheal Intubation 75 seconds after induction After 10 participants had been recruited into the saline group, it became obvious that the induction sequence in this group produced conditions for intubation that were clinically unacceptable in most patients. As a consequence, no further participants were added to this group. Each of the other 3 groups consisted of 30 participants Funding source: supported, in part, by an unrestricted grant from Organon, Inc., of West Orange, NJ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...I generated the random number sequence using a Microsoft Excel spreadsheet. " (contacted study author by mail) |
| Allocation concealment (selection bias) | High risk | After 10 participants had been recruited into the saline group, it became obvious that the induction sequence in this group produced conditions for intubation that were clinically unacceptable in most patients. As a consequence, no further participants were added to this group. Each of the other 3 groups consisted of 30 participant |
| Blinding of participants and personnel (performance bias) | High risk | After 10 participants had been recruited into the saline group, it became obvious that the induction sequence in this group produced conditions for intubation that were clinically unacceptable in most patients. As a consequence, no further participants were added to this group. Each of the other 3 groups consisted of 30 participants |
| Blinding of outcome assessment (detection bias) | Low risk | "All attempts at intubation were performed by AFK, who was not informed which test drug was administered" |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: France Language: English Number of control groups = 3/Number of intervention groups = 1 Number of participants in control group = 45/48/47/Number of participants in intervention group = 20 Randomized: N = 160 Analysed: N = 160 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA physical status I or II Aged 18 to 65 years Scheduled for abdominal or breast surgery Exclusion criteria Abnormal airway Significant cardiovascular, respiratory, hepatic, neuromuscular, or renal disease Administration of any drug known or suspected to interact with neuromuscular transmission | |
| Interventions | NMBA Control groups 'L', 'M', 'H' (atracurium 0.5 mg/kg) Hypnotic Control group 'L' (propofol 1.5 mg/kg) Control group 'M' (propofol 2.0 mg/kg) Control group 'H' (propofol 2.5 mg/kg) Intervention group 'WA' (propofol 2.5 mg/kg) Fentanyl 3 μm/kg None None | |
| Outcomes | 1. Intubation conditions: The scale distributes intubating conditions into 4 classes (excellent, good, poor, impossible). Intubating conditions were pooled as “clinically acceptable” (excellent or good) or “not clinically acceptable” (poor or impossible) (Krieg 1980). The scoring scale is a composite score based on scores of laryngoscopy, cough, and position of vocal cords | |
| Notes | Intubation 240 after induction in control group and in intervention groups based upon TOF response Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of randomization (4 groups) was designed to assign 2 participants in each group every 8 inclusions |
| Allocation concealment (selection bias) | Low risk | Allocation of the participant was sealed in an opaque envelope, which was opened upon arrival in the operating room |
| Blinding of participants and personnel (performance bias) | High risk | "...Laryngoscopy and intubation were performed when all train‐of‐four responses were abolished at the orbicularis oculi in groups high (H), medium (M) and low (L)......" Hereby, personnel performing the treatment were not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Same physician, blinded to the anaesthetic procedure, performed all intubations |
| Incomplete outcome data (attrition bias) | Low risk | Study was discontinued in group WA after the first intermediate analysis because the incidence of “clinically not acceptable” (poor and impossible) intubating conditions was unacceptable (13 of 20 patients, 65%) |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: UK Language: English Number of control groups = 6/Number of intervention groups = 2 Number of participants in control groups = 20/20/20/20/20/10/Number of participants in intervention groups = 10/10 Randomized: N = 140 Analysed: N = 140 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria Aged 18 to 65 years ASA classes I and II Udergoing elective surgery Exclusion criteria Concurrent medication known to interfere with neuromuscular transmission Weighing > 30% outside the ideal for height Patients with anticipated difficult intubation | |
| Interventions | NMBA Control group 1: rocuronium 0.3 mg/kg Control group 2: rocuronium 0.45 mg/kg Control group 3: rocuronium 0.6 mg/kg Control group 4: rocuronium 0.3 mg/kg Control group 5: rocuronium 0.45 mg/kg Control group 6: rocuronium 0.6 mg/kg Hypnotic Control groups 1 to 3 and intervention group 1: propofol 2 to 3 mg/kg Control groups 4 to 6 and Intervention group 2: sevoflurane 8% in oxygen, via a vital capacity technique Fentanyl 1 μm/kg None None | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) | |
| Notes | Intubation 60 seconds after induction Funding source: This study was supported by a grant from Abbott (UK) Ltd. Dr Lowry was in receipt of a DHSS (Northern Ireland) Clinical Research Fellowship | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "....according to prior computer‐generated random allocation...." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | "...Propofol was administered at a rate of 10–15 mg/sec until the loss of eyelash reflex. Patients in the sevoflurane group were asked to take vital capacity breaths of sevoflurane 8%...." |
| Blinding of outcome assessment (detection bias) | Low risk | ".....by an experienced anaesthetist blinded to both the method of induction used and the dose of rocuronium administered. In order to achieve this the intubator did not enter the operating room until 45 sec after the administration of the muscle relaxant...." |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: UK Language: English Number of control groups = 1/Number of intervention groups = 2 Number of participants in control groups = 17/Number of participants in intervention groups = 20/23 Randomized: N = 60 Analysed: N = 60 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA 1 or 2 Non‐obese Elective surgery Aged 18 to 65 years Exclusion criteria Obstetrical, neurosurgical, ophthalmic patients Mallampati score > 2 Gastro‐oesophageal reflux | |
| Interventions | NMBA Succinylcholine 1 mg/kg Propofol 2 mg/kg Control group: none Intervention group 1: remifentanil 2 μg/kg Intervention group 2: remifentanil 4 μg/kg None After induction, participants were mask ventilated with Sevo 2% + 50% N2O until end of fasciculation and before laryngoscopy | |
| Outcomes | 1. Intubation conditions: Scoring system included jaw mobility, mask ventilation, vocal cord visibility, vocal cord position, and participant movement during intubation (all assessed by a 3‐grade grading system) In this RCT, study authors did not define any cut‐off value for acceptable intubation. In our meta‐analyses, intubation condition was categorized as acceptable if the vocal cords were open or in mid‐position. If the vocal cords were closed, it was categorized as unacceptable 2. Difficult laryngoscopy: Vocal cord visibility was categorized as (1) vocal cords and arytenoids completely visible, (2) vocal cords and arytenoids partly visible, or (3) vocal cords and arytenoids not seen. Score = 3 was defined as a difficult laryngoscopy | |
| Notes | In all patients, vocal cord was visible (Coemack and Lehane score = 1 and 2) Intubation 30 seconds after induction of remifentanil and after fasciculation in intervention group Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Participants were randomized into 3 groups by opening unmarked envelopes |
| Blinding of participants and personnel (performance bias) | High risk | "....was initiated with 2% sevoflurane in 50% nitrous oxide in oxygen at a total flow 8 litres min‐1 and continued in group PS until fasciculation had ceased....." |
| Blinding of outcome assessment (detection bias) | Low risk | An experienced blinded anaesthetist took over airway control and attempted tracheal intubation |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Germany Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 39(37)/Number of participants in intervention group = 39(36) Randomized: N = 80 Analysed: N = 78 for laryngoscpy conditions and N = 73 for intubation condition and post‐intubation pharyngolaryngeal symptoms Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA status I or II Aged 18 to 76 years Elective surgery of the ear Exclusion criteria Obesity (defined as weight exceeding 20% of normal weight) Pregnancy Suspected to have a difficult airway (i.e. abnormal airway anatomy (Mallampati score 3 or 4); and mouth opening < 3.5 cm or cervical spine disease, difficult intubation (i.e. Cormack and Lehane score ≥ 3)) Pathological findings of the larynx revealed by initial stroboscopic examination the day before surgery | |
| Interventions | NMBA Attracurium 0.5 mg/kg Hypnotic Propofol 2.5 to 3 mg/kg Fentanyl 2 to 3 μg/kg None Premedication: ‐midazolam 7.5 mg 1 hour before, as requested | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) 2. Post‐intubation pharyngolaryngeal symptoms: Postoperative hoarseness was assessed at 24, 48, and 72 hours by a standardized interview. Vocal cords were examined by stroboscopy before and 24 and 72 hours after surgery 3. Laryngoscopy condition: It was possible to retrieve information on the laryngoscopy categorized by Cormack and Lehane (Cormack 1984) | |
| Notes | Five participants were excluded from analyses of intubation conditions and post‐intubation upper airway symptoms because of a Cormack grade of 3 or greater (1 in the atracurium group and 4 in the saline group). Moreover, 1 participant in each group had unexpected surgery of the pharynx and therefore had to be excluded Time from induction to start of tracheal intubation: 180 seconds Funding source: Support was provided solely by institutional and departmental sources | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized to 2 groups of 40 participants each, via random number draws |
| Allocation concealment (selection bias) | Low risk | Concealment was ensured. The investigator in the operating theatre was blinded; the investigator performing the interview concerning hoarseness was blinded, too. The ENT physician, who performed the stroboscopy, did not know the participant's group. Participants were blinded throughout the study (contacted study author) |
| Blinding of participants and personnel (performance bias) | Low risk | "The study drugs were administered in a double‐blind fashion, and syringes were prepared (adjusted to a 5‐ml volume) by an investigator who did not participate in the evaluation of intubating conditions, intubating score, and assessment.." |
| Blinding of outcome assessment (detection bias) | Low risk | "The study drugs were administered in a double‐blind fashion, and syringes were prepared (adjusted to a 5‐ml volume) by an investigator who did not participate in the evaluation of intubating conditions, intubating score, and assessment .." |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Germany Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 40(40)/Number of participants in intervention group = 43(39) Randomized: N = 83 Analysed: N = 83 for laryngoscopy conditions and N = 79 for intubation condition and post‐intubation pharyngolaryngeal symptoms Dates when the study was conducted: Between April 2012 and January 2013 | |
| Participants | Inclusion criteria ASA status I or II Aged 18 to 80 years Surgery of the ear Exclusion criteria Known or suspected difficult airway, such as mouth opening < 3.5 cm or Mallampati score 4 or Cormack grade 3 or 4 Obesity Disease of the larynx or vocal cords Hoarseness before surgery Preexisting severe vocal cord pathology | |
| Interventions | NMBA Rocuronium 0.45 mg·kg‐1 Hypnotic Control group: propofol 1.5 mg·kg‐1 was given (if necessary, 30 mg was supplemented) Intervention group: propofol 1.5 mg·kg‐1 was given (if necessary, 30 mg was supplemented). After propofol, the SEVO group received sevoflurane at an inspired concentration of 3.0 to 3.5 Vol% (fresh gas flow 8 L·min‐1). After 2 to 3 minutes, when the end‐tidal sevoflurane concentration reached 1.0 MAC (stable for 20 seconds), intubation was performed. Opioid Remifentanil 0.30 μg·kg·min‐1 for 3 minutes None Midazolam 7.5 mg orally before arrival in the anaesthetic room | |
| Outcomes | 1. Intubation conditions: "Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision" (Fuchs‐Buder 2007) 2. Post‐intubation vocal cord injury: All participants underwent laryngoscopy by an ENT physician who was blinded to the participant's group. Slight changes, such as erythema, and vocal cord injuries, such as oedema, haematoma, and granuloma, were noted by videolaryngoscopy 3. Laryngoscopy condition: It was possible to retrieve information on the laryngoscopy categorized by Cormack and Lehane (Cormack 1984) | |
| Notes | Two participants from the intervention (SEVO) group could be intubated only after administration of rocuronium. The vocal cords were closed and did not open after propofol 30 mg IV; to avoid vocal cord injury, rocuronium 0.45 mg·kg‐1 was given. These 2 participants were included in our meta‐analyses as difficult to intubate. Other participants from the intervention (SEVO) group had a Cormack and Lehane score of 3. These participants were included in our meta‐analyses as difficult to intubate All participants received dexamethasone 4.0 mg IV. This may have reduced the prevalence of postoperative upper airway discomfort/injury Funding source: "There was no funding for any of the authors and there was no funding for the manuscript preparation" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "....a randomization program was used..." |
| Allocation concealment (selection bias) | Low risk | Concealment was ensured. The investigator in the operating theatre was blinded when assessing intubating conditions. The investigator who performed the interview regarding hoarseness was blinded. The ENT physician, who performed the endoscopy, did not know the participant's group. Participants were blinded throughout the study (contacted study author) |
| Blinding of participants and personnel (performance bias) | High risk | Anaesthetists and nurses responsible for treatment were aware of the intervention to ensure a safe general anaesthesia if problems had occurred (contacted study author) |
| Blinding of outcome assessment (detection bias) | Low risk | All intubating variables and scores were blinded for randomization (contacted study author) Assessment of upper airway injury was blinded to participants' group assignment (direct from paper) |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Saudi Arabia Language: English Number of control groups = 3/Number of intervention groups = 1 Number of participants in control group = 50/50/50/Number of participants in intervention group = 50 Randomized: N = 200 Analysed: N = 200 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I status Elective procedures Exclusion criteria Neuromuscular, renal, hepatic, or cardiovascular disease Taking any drug known to interfere with neuromuscular function Drug or alcohol abuse Gastro‐oesophageal reflux or hiatus hernia Reactive airway disease Allergies to any of the study drugs Administration of sedative or narcotic drugs in the previous 24 hours Renal or hepatic impairment Anticipated difficult intubation | |
| Interventions | NMBA Control group I: succinylcholine 0.3 mg/kg Control group II: succinylcholine 0.5 mg/kg Control group III: succinylcholine 1.0 mg/kg Hypnotic Propofol 2 mg/kg Opioid Fentanyl 2 μg/kg Local anaesthetic None Premedication: 2 mg oral lorazepam 90 minutes before operation | |
| Outcomes | 1. Intubation conditions: acceptable vs unacceptable "GCRP in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) | |
| Notes | Intubation 60 seconds after induction Funding source: Support was provided solely by institutional and/or departmental sources | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation (contacted study author by mail) |
| Allocation concealment (selection bias) | Low risk | Yes (contacted study author by mail) |
| Blinding of participants and personnel (performance bias) | Low risk | Yes (contacted study author by mail) |
| Blinding of outcome assessment (detection bias) | Low risk | Because observation of fasciculations would identify the drug administered as succinylcholine, the anaesthesiologist performing and grading intubation was positioned with his back to the participant until just before beginning the intubation sequence |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Saudi Arabia Language: English Number of control groups = 5/Number of intervention groups = 1 Number of participants in control group = 30/30/30/30/30/Number of participants in intervention group = 30 Randomized: N = 180 Analysed: N = 180 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA physical status I Participants underwent elective procedures Exclusion criteria Neuromuscular, renal, cardiovascular, or hepatic disease Patient was taking any drug known to interfere with neuromuscular function History of drug or alcohol abuse Gastro‐oesophageal reflux or hiatal hernia Reactive airway disease Allergies to any of the study drugs Administration of sedative or narcotic drugs in the previous 24 hours Anticipated difficult intubation | |
| Interventions | NMBA Control group 1: succinylcholine 0.3 mg/kg Control group 2: succinylcholine 0.5 mg/kg Control group 3: succinylcholine 1.0 mg/kg Control group 4: succinylcholine 1.5 mg/kg Control group 5: succinylcholine 2.0 mg/kg Hypnotic Propofol 2 mg/kg Opioid Fentanyl 2 μm/kg None None | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) | |
| Notes | Intubation was subsequently performed 60 seconds after succinylcholine administration Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization schedule provided in sealed envelopes according to a computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Randomization schedule provided in sealed envelopes according to a computer‐generated list |
| Blinding of participants and personnel (performance bias) | Low risk | Yes (contacted study author by mail) |
| Blinding of outcome assessment (detection bias) | Low risk | The anaesthesiologist performing and grading the intubation was positioned with his back to the participant until just before beginning the intubation sequence |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: USA Language: English Number of control groups = 2/Number of intervention groups = 1 Number of participants in control group = 20/20/Number of participants in intervention group = 20 Randomized: N = 60 Analysed: N = 60 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Elective oral surgery under general anaesthesia necessitating nasal intubation Exclusion criteria Aged < 16 years or > 50 years Appeared clinically to present difficulty in intubation Anaesthetic technique was unsuitable | |
| Interventions | NMBA Control group 1: suxamethonium 0.25 mg/kg Control group 2: suxamethonium 0.5 mg/kg Hypnotic Propofol 2.5 mg/kg Opioid Alfentanil 15 μg/kg None | |
| Outcomes | 1. Intubation conditions: jaw and cord relaxation (Young, Clarke & Dundee). Overall intubation conditions (three grade assessment.Incidence of coughing on intubation (four grade assessment), duration of apnoea (Lund 1969) 2. Postoperative myalgia and sore throat: incidence of postoperative myalgia (four grade assessment). Later on the day of operation (day 1), participants were interviewed by one of the investigators (not participating in the anaesthetic) regarding muscle pain and sore throat and were given a questionnaire to return 5 days after the operation (day 5) with the same questions. Muscle pain and sore throat were scored as none, mild, moderate, or severe | |
| Notes | In the Intervention group, intubation was unsuccessful in 2 participants because of a combination of poor vision and poor cord relaxation. In both participants, the trachea was successfully intubated after a dose of suxamethonium 50 mg. These two participants were included in the meta‐analysis as patients difficult to intubate Postoperative sore throat was present in more than 50% of participants in all 3 groups at both 1 and 5 days after operation. Investigators noted no significant difference in the incidence of sore throat between the 3 groups. However, they did not report the specific number of participants in the 2 groups. Therefore, we did not include this study in the meta‐analysis Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Syringes with medicine were prepared by one of the investigators not participating in the anaesthetic, and 20 participants were allocated randomly to each of the 3 groups |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Unclear risk | The exact incidence of sore throat was not reported |
| Methods | Parallel‐group RCT Settings: single centre Country: China Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 20/Number of participants in intervention group = 20 Randomized: N = 40 Analysed: N = 40 Dates when the study was conducted: between July 2010 and January 2011 | |
| Participants | Inclusion criteria Aged 18 to 65 years Body mass index 18.5 to 25 kg/m2 ASA class I or II Mallampati class I or II Elective suspension laryngoscopic excision under intubation without muscular relaxation Exclusion criteria Medical history of myopathy Known allergy to study drugs Drug abuse History of upper respiratory tract infection within 3 weeks of enrolment Gastrointestinal reflux Intracranial pathology Suspected difficult airway Serious cardiopulmonary or hepatorenal insufficiency | |
| Interventions | NMBA Control group: cisatracurium 0.1 mg/kg Hypnotic Propofol target control Opioid Remifentanil target control Superficial anaesthesia with 10 mg/mL tetracaine | |
| Outcomes | 1. Intubation condition:Hellbo‐Hansen 1988 2. Laryngoscopy condition: Cormack‐Lehane classification (Cormack 1984) | |
| Notes | Funding source: This work was supported by Jilin Provincial Department of Science and Technology under the International Collaborative Initiative (#20110759) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‑generated random number table was used to randomly and equally assign participants |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of personnel performing drug administration. Participants were blinded to treatment assignment throughout the study |
| Blinding of outcome assessment (detection bias) | Low risk | An independent anaesthetist performed tracheal intubation |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: USA Language: English Number of control groups = 5/Number of intervention groups = 1 Number of participants in control group = 30/15/14/14/15/Number of participants in intervention group = 10 Randomized: N = 100 Analysed: N = 98 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Normal upper airway anatomy Aged 18 to 65 years Within 30% of ideal body weight Exclusion criteria History of malignant hyperthermia Abnormal plasma cholinesterase levels Neuromuscular, neurological, hepatic, and renal conditions that might influence neuromuscular function Use of drugs that might alter the response to neuromuscular blockade or might affect histamine release | |
| Interventions | NMBA Control group 1: mivacurium 0.25 mg/kg Control group 2: rocuronium 0.45 mg/kg Control group 3: rocuronium 0.6 mg/kg Control group 4: rocuronium 0.9 mg/kg Control group 5: rocuronium 1.2 mg/kg Hypnotic Propofol 2 mg/kg Opioid Fentanyl 2 μm/kg None Midazolam 1 to 2 mg for induction | |
| Outcomes | 1. Intubation conditions ‐ Excellent: easy passage of endotracheal tube without coughing; vocal cords relaxed and abducted ‐ Good: passage of endotracheal tube with slight coughing or bucking; vocal cords relaxed and abducted ‐ Poor: passage of endotracheal tube with moderate coughing or bucking; vocal cords moderately abducted ‐ Not possible: unable to intubate | |
| Notes | Two participants were excluded from analysis because they were mistakenly entered into the study twice Intubation 90 seconds after administration of midazolam Funding source: supported by a grant from GlaxoWellcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of assessor: intubator not involved in protocol; waited outside OP room until last minute before intubation attempt |
| Incomplete outcome data (attrition bias) | Low risk | Two participants were excluded from analysis because they were mistakenly entered into the study twice |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: France Language: French Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group (77)/Number of participants in intervention group = 75 Randomized: N = 152 Analysed: N = 152 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria > 18 years of age ASA I Mallampati I Elective surgery not needing relaxant Exclusion criteria Suspect difficult intubation Former allergies Abnormal ECG Bradycardia < 50 Urgent surgery | |
| Interventions | NMBA Vecuronium 0.08 mg/kg Hypnotic Propofol 2.5 mg/kg Alfentanil 0.03 mg/kg Intervention group: lidocaine 1.5 mg/kg Premedication: prazepam 40 mg and hydroxyzine 100 mg (1 hour before induction) | |
| Outcomes | 1. Intubation conditions: Physician performing the intubation evaluated mouth opening, opening of the glottis, and the occurrence of coughing (Saarnivaara 1991) Mouth opening: impossible 3, medium 2, complete 0; opening of the glottis: moving or closed 3, half closed 2, open 0; cough at intubation: significant (“Importante”) 3, a little 1, absent 0. Total score < 3 considered good intubating conditions. A score is calculated for each intubation attempt | |
| Notes | 107 oral intubations, 45 nasal intubations, all intubations by direct laryngoscopy Intubation after induction: 60 seconds in control group and 180 seconds in intervention group Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Different times from infusion to intubation in the 2 groups (1 minute for Vecu0 and 3 minutes for Vecu+) make it easy to discern the 2 groups from each other;no mention of measures of concealment of allocation towards assessor, who might have been present from infusion to finish (not mentioned) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Different times from infusion to intubation in the 2 groups (1 minute for Vecu0 and 3 minutes for Vecu+) make it easy to discern the 2 groups from each other; no mention of measures of concealment of allocation towards assessor, who might have been present from infusion to finish (not mentioned) |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: USA Language: English Number of control groups = 1/Number of intervention groups = 4 Number of participants in control group (15)/Number of participants in intervention group = 15/15/15/15 Randomized: N = 75 Analysed: N = 75 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria Same‐day surgery for a variety of surgical procedures ASA physical status I or II Mallampati class I airway anatomy Exclusion criteria History of intravenous drug use, alcohol addiction Full stomach Coronary artery disease Reactive airway disease | |
| Interventions | NMBA d‐Tubocurarine 3 mg and succinylcholine 1 mg/kg Hypnotic Control group 1: thiamylal 4 mg/kg Intervention groups 1 to 4: propofol 2 mg/kg Control group: no opioids Intervention group 1: alfentanil 30 µg/kg Intervention group 2: alfentanil 40 µg/kg Intervention group 3: alfentanil 50 µg/kg Intervention group 4: alfentanil 60 µg/kg None All participants received midazolam 1 mg IV before induction | |
| Outcomes | 1. Intubation conditions: Vocal cords, position of vocal cards, jaw mobility, and participant movement during and within 1 minute of attempted intubation of the trachea. Participants whose tracheas could not be intubated after receiving assigned induction drugs were so noted, and succinylcholine (1 mg/kg IV) was administered. Tracheal intubation was then re‐attempted, and exposure of the larynx and outcome of intubation attempt were recorded In this RCT, study authors did not define any cut‐off value for acceptable intubation and provided no composite measure of the 4 variables. For the meta‐analysis, we defined complete or partial exposure of the vocal cords as clinically acceptable. Vocal cord exposure was categorized as: (1) vocal cords and arytenoids completely visible; (2) vocal cords or arytenoids partially visible; or (3) vocal cords or arytenoids not seen 2. Laryngoscopy condition: vocal cords or arytenoids not seen defined as difficult laryngoscopy | |
| Notes | Induction after 90 seconds Funding source: Dr Scheller is a recipient of a B.B. Sankey Foundation Award. This work was supported in part by a grant from Janssen Pharmaceutica, Piscataway, NJ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "......randomized to one of five groups by having an assistant pick one of five cards describing the induction sequence....." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | "...Note that we were not able to be completely blinded as to group because we could generally distinguish the thiamylal/succinylcholine group from the others..." |
| Blinding of outcome assessment (detection bias) | High risk | "...Note that we were not able to be completely blinded as to group because we could generally distinguish the thiamylal/succinylcholine group from the others..." |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Germany Language: English Number of control groups = 3/Number of intervention groups = 1 Number of participants in control group = 30/30/30/Number of participants in intervention group = 30 Randomized: N = 120 Analysed: N = 120 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA status I or II Adult Scheduled for elective ambulatory surgery up to 90 minutes Exclusion criteria Pregnancy Neuromuscular disorder or receiving medications known to interact with neuromuscular function Suspected to have a difficult airway | |
| Interventions | NMBA Control group 1 (0.6 mg/kg rocuronium) Control group 2 (0.45 mg/kg rocuronium) Control group 3 (0.3 mg/kg rocuronium) Hypnotic Propofol 2 to 2.5 mg/kg Remifentanil 0.5 µg/kg/min None Premedication: midazolam 7.5 mg orally 1 hour before | |
| Outcomes | 1. Intubation condition: "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996) | |
| Notes | Time from induction to start of tracheal intubation: 180 seconds Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | "....All patients were intubated by the same experienced anaesthetist blinded to the treatment...." |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: New Zealand Language: English Number of control groups = 1/Number of intervention groups = 3 Number of participants in control group = 25/Number of participants in intervention group = 25/25/25/25 Randomized: N = 100 Analysed: N = 100 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Aged 18 to 65 years Mixed surgery Exclusion criteria Smokers Known or anticipated difficult tracheaI intubation Risk of aspiration Adverse effects of coughing or straining Taking beta‐blocker with absolute contraindication to use of sevoflurane, suxamethonium, and alfentanil | |
| Interventions | NMBA Suxamethonium 1 mg/kg Hypnotic Sevoflurane 7% and nitrous oxide 60% Control group 1: alfentanil 10 μg/kg Intervention group 1: alfentanil 20 μg/kg Intervention group 2: alfentanil 25 μg/kg Intervention group 3: alfentanil 30 μg/kg Local anaesthetic None Atropine 0.3 mg | |
| Outcomes | 1) Intubation conditions: modified version of Saarnivaara L, Klemola UM (Saarnivaara 1991). The scoring scale is a composite score based on scores of jaw relaxation, movement of limbs, movement of vocal cords, and coughing. Based on a score ranging from 3 to 12 points 2) Sore throat: postoperative, self‐reporting, time and method of reporting not defined | |
| Notes | One participant from intervention group 1 was intubated by rescue suxamethonium. We included this participant in our meta‐analyses as difficult to intubate Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The patients were randomly allocated to receive…”; method not specified |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes, opened immediately before induction |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Low risk | "...one of the investigators who was blinded to the group allocation, entered the operation room to perform direct laryngoscopy...." |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: USA Language: English Number of control groups = 1/Number of intervention groups = 6 Number of participants in control group = 20/Number of participants in intervention group = 20/20/20/20/20/20 Randomized: N = 140 Analysed: N = 140 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Outpatients Aged 18 to 60 years Scheduled for elective surgery All enrolled participants had Mallampati class I or II airway anatomy Exclusion criteria Coronary artery disease, hypertension, reactive airway disease Obesity > 30% above ideal body weight History of drug or alcohol abuse, or gastro‐oesophageal reflux Taking narcotics or drugs known to interfere with neuromuscular transmission | |
| Interventions | NMBA Control group: d‐tubocurarine 3 mg and succinylcholine 1 mg/kg Hypnotic Control group: thiopental 4 mg/kg Intervention groups 1 and 2: etomidate 0.3 mg/kg Intervention groups 3 and 4: propofol 2 mg/kg Intervention groups 5 and 6: thiopental 4 mg/kg Opioid Control group: none Intervention groups 1 to 6: alfentanil 40 μg/kg Local anaesthetic Lidocaine 1 mg/kg (intervention groups 2, 4, 6) Premedication: midazolam 0.03 mg/kg IV 5 minutes before induction | |
| Outcomes | 1. Intubation conditions: "The intubating anaesthesiologist, assessed each patient on four variables: jaw relaxation, exposure of the vocal cords, vocal cord position, and patient response to intubation and slow (5‐s) inflation of the endotracheal tube cuff" Participants who could not be intubated on the first attempt were given succinylcholine 1 mg/kg, and intubation was completed In this RCT, study authors did not define any cut‐off value for acceptable intubation and provided no composite measure of the 4 variables. For the meta‐analysis, we defined closed vocal cords as difficult intubation, and open or midline as acceptable intubation conditions 2. Laryngoscopy conditions: Exposure of the vocal cords was defined as 'complete', 'partial', or 'not seen'. Difficult laryngoscopy was defined as 'not seen' | |
| Notes | For all participants, it was possible to expose the vocal cords. In the various alfentanil groups, 17 required succinylcholine to complete intubation. Thirty‐five percent of participants (7 of 20) in intervention group 5 (alfentanil/ thiopental) required succinylcholine to complete intubation compared with 3 of 20 (15%), 2 of 20 (10%), 2 of 20 (10%), and 0% and 3 of 20 (15%) in Intervention groups 1 to 4 and 6, respectively. It was not possible to identify specific participants who had closed vocal cords and therefore required succinyl chloride Intubation was performed 90 seconds after induction Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Participants were randomly allocated to 1 of 7 groups (n = 20/group) by means of previously prepared envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | "The induction sequence was conducted using four prepared syringes in all patients"...."Opaque tape was applied to Syringe 3 to disguise the color of the hypnotic drug".... "Injection of all syringes was performed by an assistant behind a drape so that the intubating anesthesiologist (one of three of the authors) was blinded to the color and volume of the IV drugs" |
| Blinding of outcome assessment (detection bias) | Low risk | "The induction sequence was conducted using four prepared syringes in all patients"...."Opaque tape was applied to Syringe 3 to disguise the color of the hypnotic drug".... "Injection of all syringes was performed by an assistant behind a drape so that the intubating anesthesiologist (one of three of the authors) was blinded to the color and volume of the IV drugs" |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Germany Language: German Number of control groups = 2/Number of intervention groups = 2 Number of participants in control group = 25/25/Number of participants in intervention group = 25/25 Randomized: N = 100 Analysed: N = 100 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Gynaecological surgery Exclusion criteria Not specified | |
| Interventions | NMBA Control groups 1 and 2: vecuronium 1 mg + succinylcholine 1 mg/kg Hypnotic Control group 1: demand‐adapted thiopental (5.5 ± 3.14 mg/kg) Control group 2: demand‐adapted propofol (2.2 ± 0.48 mg/kg) Intervention group 1: demand‐adapted propofol (2.4 ± 0.63 mg/kg) Intervention group 2: demand‐adapted propofol (2.2 ± 0.48 mg/kg) Opioid Control group 1: fentanyl 0.1 mg Control group 2: fentanyl 0.1 mg Intervention group 1: fentanyl 0.1 mg Intervention group 2: fentanyl 0.2 mg Local anaesthetic None Premedication: midazolam 7.5 mg | |
| Outcomes | 1) Intubation conditions: (1) very good, (2) good, (3) satisfactory, (4) sufficient, (5) inadequate, (6) insufficient. Acceptable conditions 1 to 4, unacceptable conditions 5 and 6 2) Laryngoscopy conditions: Comack & Lehane (Cormack 1984). Unproblematic laryngoscopy: grades I and II. Difficult laryngoscopy: grades III and IV | |
| Notes | Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | All medicaments were blinded to personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Anaesthetist performing the intubation was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Malaysia Language: English Number of control groups = 1/Number of intervention groups = 3 Number of participants in control group = 30/Number of participants in intervention group = 30/30/30 Randomized: N = 120 Analysed: N = 120 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Elective surgery Aged 18 to 60 years Exclusion criteria Expected difficult airway Aspiration risk Head and neck surgery Suxamethonium contraindicated | |
| Interventions | NMBA Control group: succinylcholine 1 mg/kg Hypnotic Propofol 200 mg/min until loss of verbal response Control group: none Intervention group 1: alfentanil 15 μg/kg Intervention group 2: alfentanil 30 μg/kg Intervention group 3: none Local anaesthetic None Premedication: midazolam 7 mg 1 hour before induction | |
| Outcomes | 1. Intubation conditions: The scoring scale is a composite score based on scores of jaw relaxation, movement of the vocal cords, and coughing. Based on a score ranging from 0 to 6 points. Intubation conditions were categorized as good = 5 to 6 points, moderate = 3 to 4 points, poor = 1 to 2 points or failed (Saarnivaara 1991) | |
| Notes | One participant in intervention group 3 was excluded because of an unanticipated difficult intubation. We included this participant in our meta‐analysis as a difficult tracheal intubation patient The mean dose requirement for induction by propofol was higher in groups without alfentanil (3 mg/kg vs 2.5 mg/kg) Funding source: "....Janssen Pharmaceutica for supplying the alfentanil" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | "....The drug was given by an anaesthetic trainee who was unaware of the drugs before its administration....." |
| Blinding of outcome assessment (detection bias) | Low risk | ".....who was unaware of the randomisation process entered the operating room 30 seconds after completion of drug administration....." |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
| Methods | Parallel‐group RCT Settings: single centre Country: Iran Language: English Number of control groups = 1/Number of intervention groups = 1 Number of participants in control group = 31/Number of participants in intervention group = 35 Randomized: N = 66 Analysed: N = 66 Dates when the study was conducted: not specified | |
| Participants | Inclusion criteria ASA I or II Elective surgery Aged 15 to 65 years Exclusion criteria Expected difficult airway (Mallampati score III or IV) Chronic alcohol or opioid use Allergy to study medications | |
| Interventions | NMBA Control group: atracurium 0.5 mg/kg Hypnotic Propofol 2.5 mg/kg Control group: none Intervention group: remifentanil 2 μg/kg IV over 30 seconds Local anaesthetic None Atropine 0.5 mg | |
| Outcomes | 1. Intubation conditions: "...anaesthesiologist checked the intubation condition using criteria based on jaw relaxation, vocal cord movement and bucking on tracheal tube as primary measurements of comparison (Table 1). Intubation condition defined as optimal (score 1 or 2 in all categories), suboptimal (score 3 in at least 1 of the 3 categories), or fail (fail to intubation) | |
| Notes | "Unsuccessful intubation due to closed vocal cords were seen in 2 patients in remifentanil group, the patients ventilated and received atracurium and intubation was applied again" Funding source: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study was categorized as "double‐blinded". However, no detailed description was provided in the manuscript |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study was categorized as "double‐blinded". However, no detailed description was provided in the manuscript |
| Incomplete outcome data (attrition bias) | Low risk | Numbers and reasons for (possible) dropouts and withdrawals are described |
| Selective reporting (reporting bias) | Low risk | Relevant and reasonably expected outcomes are reported |
ASA = American Society of Anesthesiologists ASA physical status classification system; BMI = body mass index; ECG = electrocardiography; ENT= ear, nose, and throat; GCRP = "Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents" (Viby‐Mogensen 1996); GI = gastrointestinal; IV = intravenous; kg = kilogram; mg = milligram; mmol/L = millimoles per litre; N = number of cases; NMBA = neuromuscular blocking agent; PO = per os; RCT = randomized controlled trial; sec = second; TOF = 'train of four'; TS = total score; µg = microgram; VAS = visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| An unspecified number of participants were intubated blind nasal | |
| Study was terminated for the control group because of unacceptable intubation conditions. Thus, randomization and blinding were violated | |
| Participants were intubated blind nasal. No evaluation of direct laryngoscopy was performed |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difficult tracheal intubation: low risk of bias vs high or uncertain risk of bias Show forest plot | 34 | 3565 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [3.49, 7.15] |
| Analysis 1.1 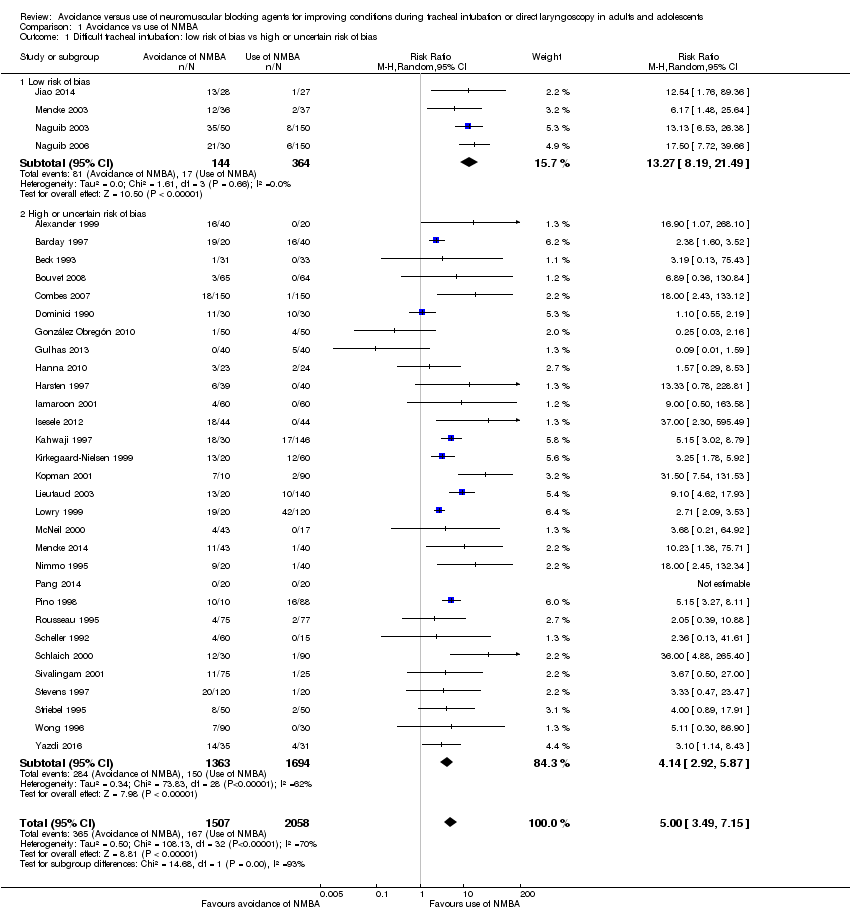 Comparison 1 Avoidance vs use of NMBA, Outcome 1 Difficult tracheal intubation: low risk of bias vs high or uncertain risk of bias. | ||||
| 1.1 Low risk of bias | 4 | 508 | Risk Ratio (M‐H, Random, 95% CI) | 13.27 [8.19, 21.49] |
| 1.2 High or uncertain risk of bias | 30 | 3057 | Risk Ratio (M‐H, Random, 95% CI) | 4.14 [2.92, 5.87] |
| 2 Difficult tracheal intubation: depolarizing vs non‐depolarizing NMBA Show forest plot | 32 | 3413 | Risk Ratio (M‐H, Random, 95% CI) | 5.25 [3.61, 7.63] |
| Analysis 1.2 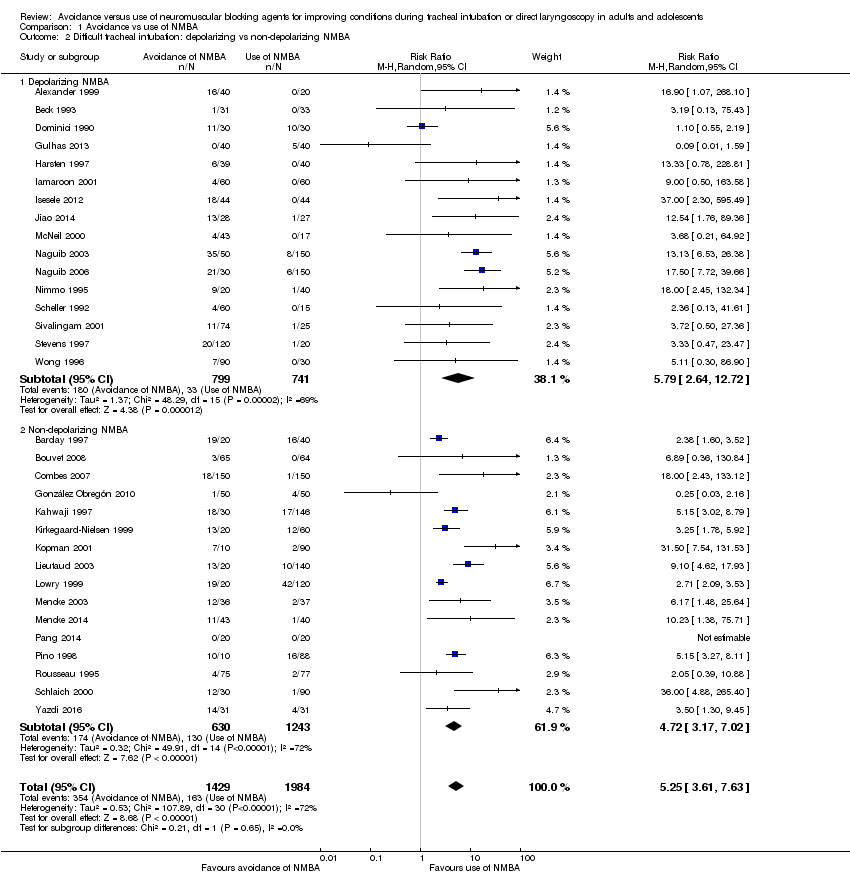 Comparison 1 Avoidance vs use of NMBA, Outcome 2 Difficult tracheal intubation: depolarizing vs non‐depolarizing NMBA. | ||||
| 2.1 Depolarizing NMBA | 16 | 1540 | Risk Ratio (M‐H, Random, 95% CI) | 5.79 [2.64, 12.72] |
| 2.2 Non‐depolarizing NMBA | 16 | 1873 | Risk Ratio (M‐H, Random, 95% CI) | 4.72 [3.17, 7.02] |
| 3 Difficult tracheal intubation: remifentanil vs no remifentanil Show forest plot | 26 | 3008 | Risk Ratio (M‐H, Random, 95% CI) | 5.64 [3.82, 8.31] |
| Analysis 1.3  Comparison 1 Avoidance vs use of NMBA, Outcome 3 Difficult tracheal intubation: remifentanil vs no remifentanil. | ||||
| 3.1 Remifentanil | 4 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 15.86 [4.43, 56.71] |
| 3.2 No remifentanil | 22 | 2636 | Risk Ratio (M‐H, Random, 95% CI) | 5.23 [3.54, 7.74] |
| 4 Difficult tracheal intubation: alfentanil vs no alfentanil Show forest plot | 26 | 2618 | Risk Ratio (M‐H, Random, 95% CI) | 4.77 [3.25, 7.01] |
| Analysis 1.4  Comparison 1 Avoidance vs use of NMBA, Outcome 4 Difficult tracheal intubation: alfentanil vs no alfentanil. | ||||
| 4.1 Alfentanil | 6 | 511 | Risk Ratio (M‐H, Random, 95% CI) | 4.46 [1.66, 11.98] |
| 4.2 No alfentanil | 20 | 2107 | Risk Ratio (M‐H, Random, 95% CI) | 5.10 [3.34, 7.79] |
| 5 Difficult tracheal intubation: local anaesthesia vs no local anaesthesia Show forest plot | 31 | 3184 | Risk Ratio (M‐H, Random, 95% CI) | 5.04 [3.48, 7.29] |
| Analysis 1.5  Comparison 1 Avoidance vs use of NMBA, Outcome 5 Difficult tracheal intubation: local anaesthesia vs no local anaesthesia. | ||||
| 5.1 Local anaesthesia | 5 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.14, 3.18] |
| 5.2 No local anaesthesia | 26 | 2877 | Risk Ratio (M‐H, Random, 95% CI) | 6.26 [4.15, 9.44] |
| 6 Difficult tracheal intubation: excluded anticipated DTI vs included anticipated DTI Show forest plot | 34 | 3564 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [3.50, 7.16] |
| Analysis 1.6  Comparison 1 Avoidance vs use of NMBA, Outcome 6 Difficult tracheal intubation: excluded anticipated DTI vs included anticipated DTI. | ||||
| 6.1 Exclusion of patients with anticipated difficult intubation | 25 | 2886 | Risk Ratio (M‐H, Random, 95% CI) | 5.32 [3.54, 8.00] |
| 6.2 No exclusion of patients with anticipated difficult intubation | 9 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 4.40 [1.71, 11.29] |
| 7 Difficult tracheal intubation: "best‐case scenario" Show forest plot | 34 | 2410 | Risk Ratio (M‐H, Random, 95% CI) | 5.99 [3.46, 10.38] |
| Analysis 1.7 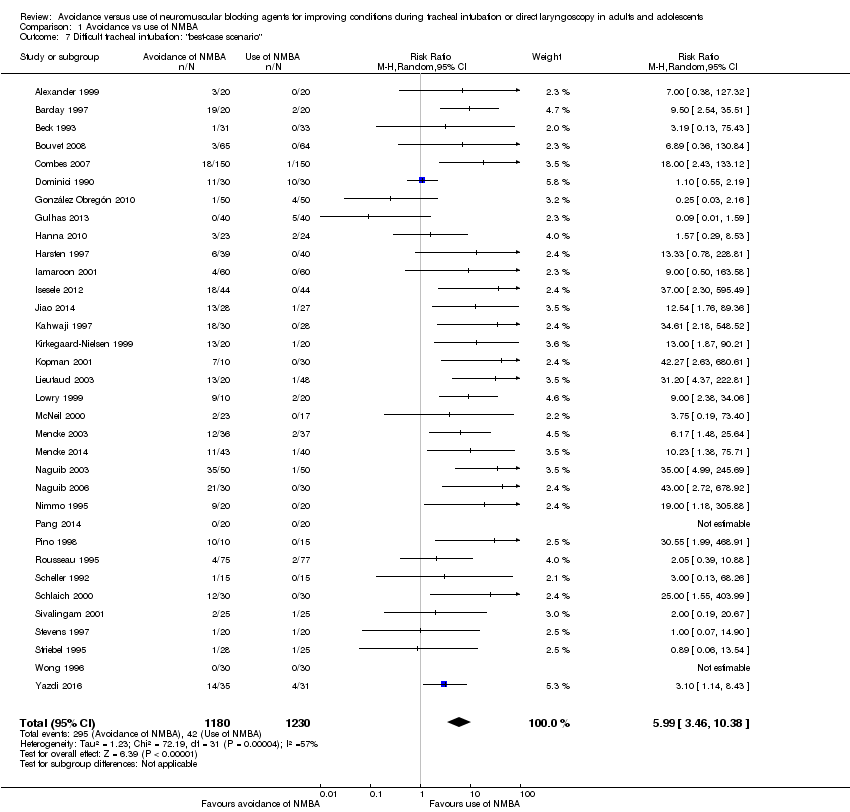 Comparison 1 Avoidance vs use of NMBA, Outcome 7 Difficult tracheal intubation: "best‐case scenario". | ||||
| 8 Difficult tracheal intubation excluding dose‐finding studies Show forest plot | 16 | 1536 | Risk Ratio (M‐H, Random, 95% CI) | 3.40 [1.63, 7.10] |
| Analysis 1.8  Comparison 1 Avoidance vs use of NMBA, Outcome 8 Difficult tracheal intubation excluding dose‐finding studies. | ||||
| 9 Difficult tracheal intubation: funding from pharmaceutical industry Show forest plot | 34 | 3565 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [3.49, 7.15] |
| Analysis 1.9 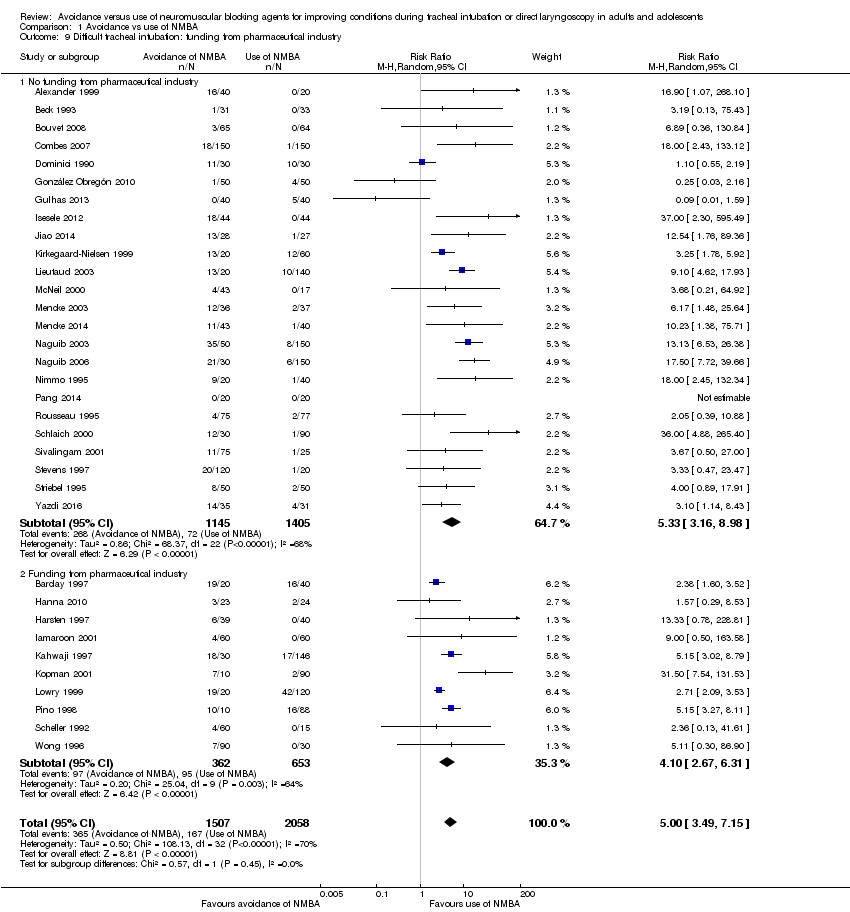 Comparison 1 Avoidance vs use of NMBA, Outcome 9 Difficult tracheal intubation: funding from pharmaceutical industry. | ||||
| 9.1 No funding from pharmaceutical industry | 24 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 5.33 [3.16, 8.98] |
| 9.2 Funding from pharmaceutical industry | 10 | 1015 | Risk Ratio (M‐H, Random, 95% CI) | 4.10 [2.67, 6.31] |
| 10 One or more events of upper airway discomfort or injury: low risk of bias vs high or uncertain risk of bias Show forest plot | 7 | 844 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.08, 1.71] |
| Analysis 1.10 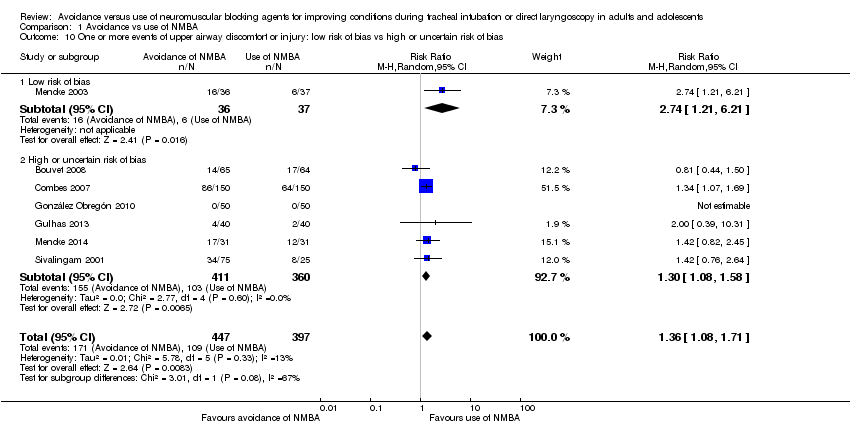 Comparison 1 Avoidance vs use of NMBA, Outcome 10 One or more events of upper airway discomfort or injury: low risk of bias vs high or uncertain risk of bias. | ||||
| 10.1 Low risk of bias | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 2.74 [1.21, 6.21] |
| 10.2 High or uncertain risk of bias | 6 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.08, 1.58] |
| 11 One or more events of upper airway discomfort or injury: depolarizing vs non‐depolarizing NMBA Show forest plot | 7 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.09, 1.74] |
| Analysis 1.11  Comparison 1 Avoidance vs use of NMBA, Outcome 11 One or more events of upper airway discomfort or injury: depolarizing vs non‐depolarizing NMBA. | ||||
| 11.1 Depolarizing NMBA | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.83, 2.65] |
| 11.2 Non‐depolarizing NMBA | 5 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.97, 1.94] |
| 12 One or more events of upper airway discomfort or injury: remifentanil vs no remifentanil Show forest plot | 7 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.09, 1.74] |
| Analysis 1.12  Comparison 1 Avoidance vs use of NMBA, Outcome 12 One or more events of upper airway discomfort or injury: remifentanil vs no remifentanil. | ||||
| 12.1 Remifentanil | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.61, 2.08] |
| 12.2 No remifentanil | 5 | 653 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.16, 1.75] |
| 13 One or more events of upper airway discomfort or injury: alfentanil vs no alfentanil Show forest plot | 5 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.85, 2.53] |
| Analysis 1.13  Comparison 1 Avoidance vs use of NMBA, Outcome 13 One or more events of upper airway discomfort or injury: alfentanil vs no alfentanil. | ||||
| 13.1 No alfentanil | 5 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.85, 2.53] |
| 14 One or more events of upper airway discomfort or injury: excluded anticipated DTI vs included anticipated DTI Show forest plot | 7 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.09, 1.74] |
| Analysis 1.14 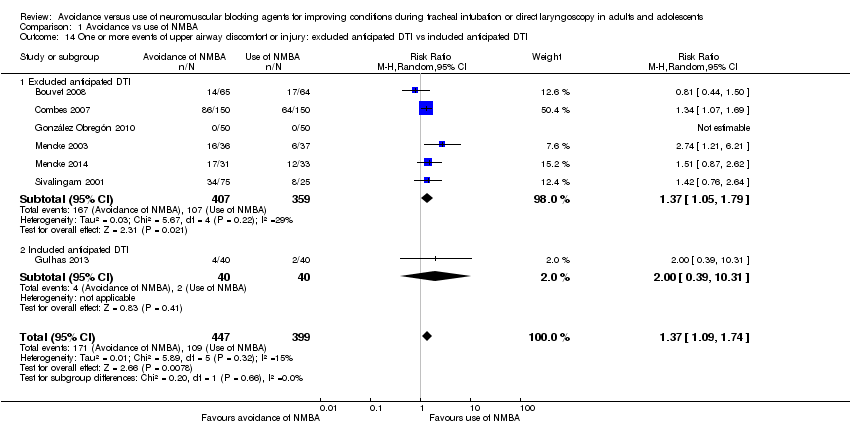 Comparison 1 Avoidance vs use of NMBA, Outcome 14 One or more events of upper airway discomfort or injury: excluded anticipated DTI vs included anticipated DTI. | ||||
| 14.1 Excluded anticipated DTI | 6 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.05, 1.79] |
| 14.2 Included anticipated DTI | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.39, 10.31] |
| 15 Difficult laryngoscopy: low risk of bias vs high or uncertain risk of bias Show forest plot | 13 | 1308 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.53, 4.21] |
| Analysis 1.15  Comparison 1 Avoidance vs use of NMBA, Outcome 15 Difficult laryngoscopy: low risk of bias vs high or uncertain risk of bias. | ||||
| 15.1 Low risk of bias | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.47, 34.20] |
| 15.2 High or uncertain risk of bias | 12 | 1230 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.47, 4.16] |
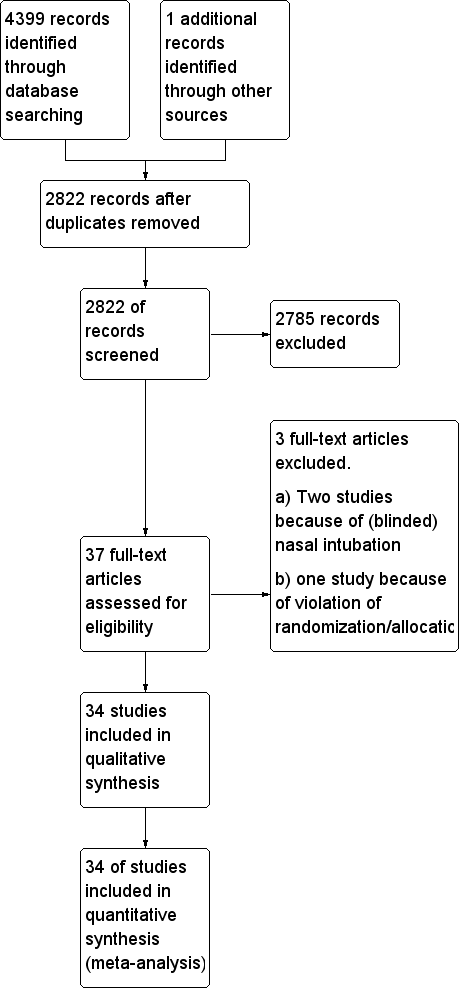
Study flow diagram.
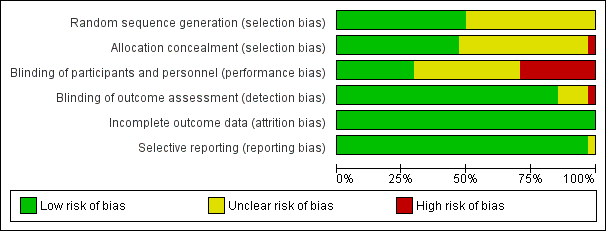
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 Avoidance vs use of NMBA, outcome: 1.1 Difficult tracheal intubation: low risk of bias vs high or uncertain risk of bias.
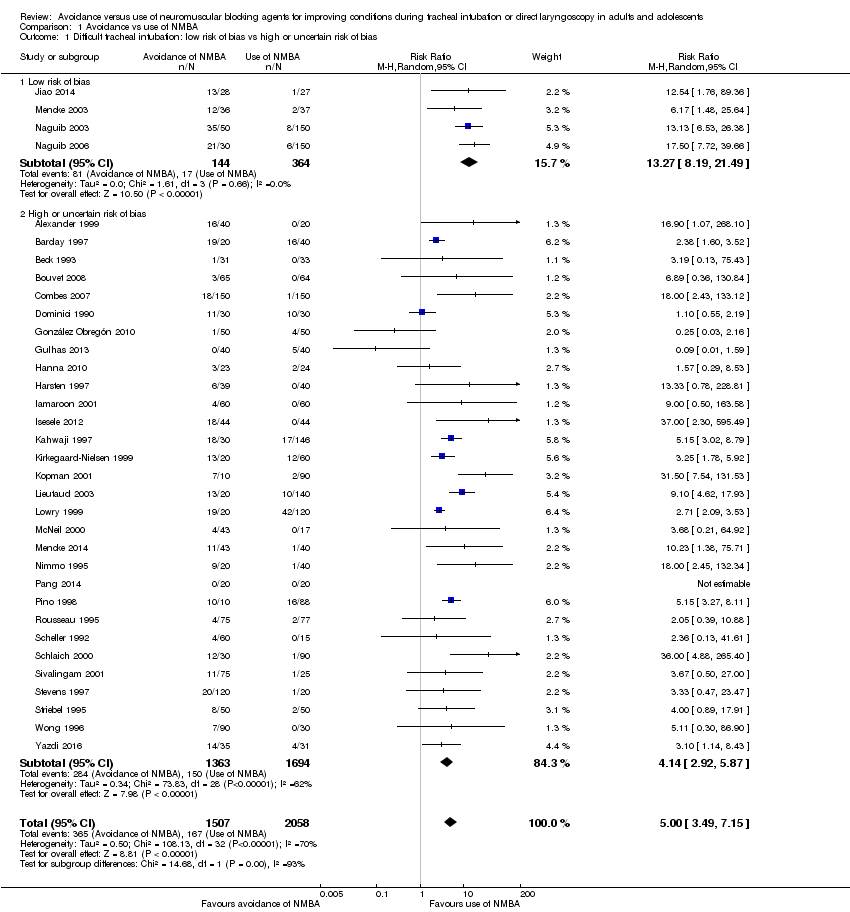
Comparison 1 Avoidance vs use of NMBA, Outcome 1 Difficult tracheal intubation: low risk of bias vs high or uncertain risk of bias.

Comparison 1 Avoidance vs use of NMBA, Outcome 2 Difficult tracheal intubation: depolarizing vs non‐depolarizing NMBA.

Comparison 1 Avoidance vs use of NMBA, Outcome 3 Difficult tracheal intubation: remifentanil vs no remifentanil.
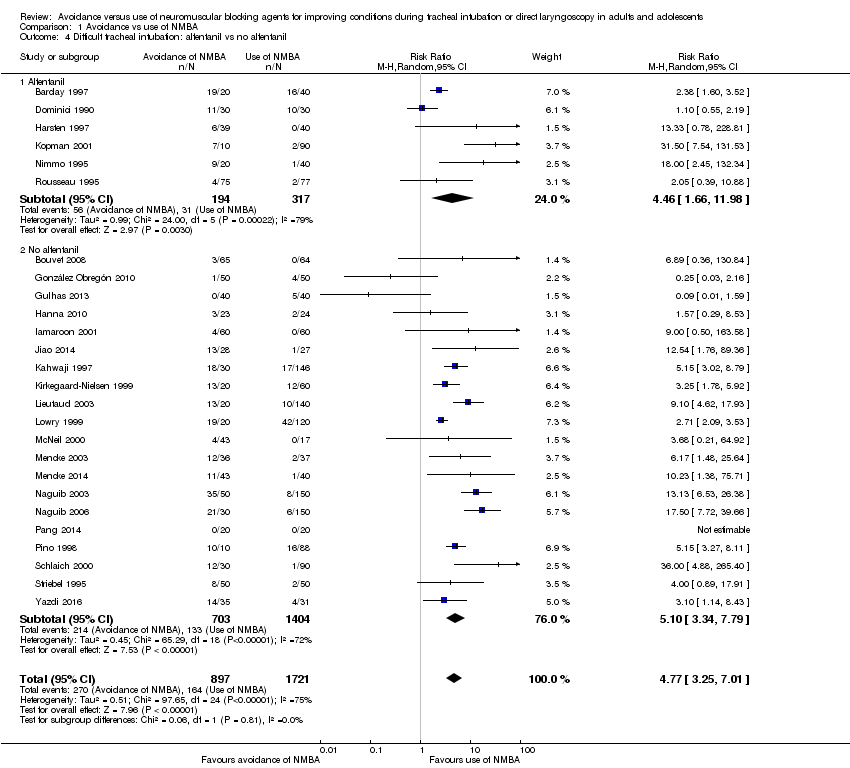
Comparison 1 Avoidance vs use of NMBA, Outcome 4 Difficult tracheal intubation: alfentanil vs no alfentanil.

Comparison 1 Avoidance vs use of NMBA, Outcome 5 Difficult tracheal intubation: local anaesthesia vs no local anaesthesia.

Comparison 1 Avoidance vs use of NMBA, Outcome 6 Difficult tracheal intubation: excluded anticipated DTI vs included anticipated DTI.

Comparison 1 Avoidance vs use of NMBA, Outcome 7 Difficult tracheal intubation: "best‐case scenario".

Comparison 1 Avoidance vs use of NMBA, Outcome 8 Difficult tracheal intubation excluding dose‐finding studies.

Comparison 1 Avoidance vs use of NMBA, Outcome 9 Difficult tracheal intubation: funding from pharmaceutical industry.
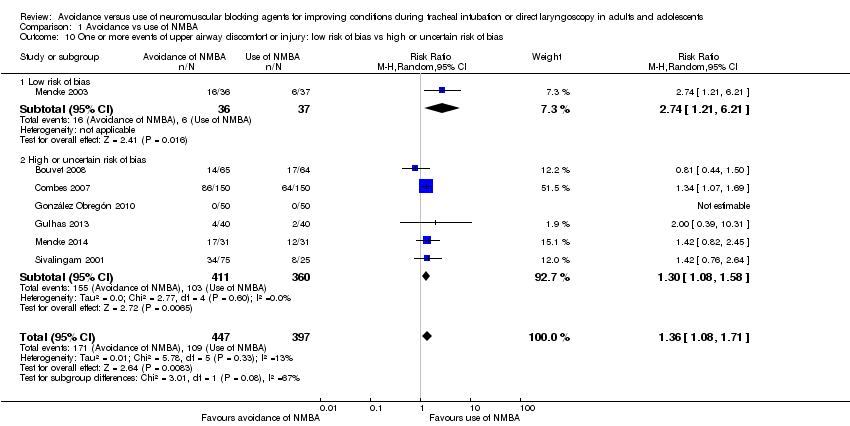
Comparison 1 Avoidance vs use of NMBA, Outcome 10 One or more events of upper airway discomfort or injury: low risk of bias vs high or uncertain risk of bias.
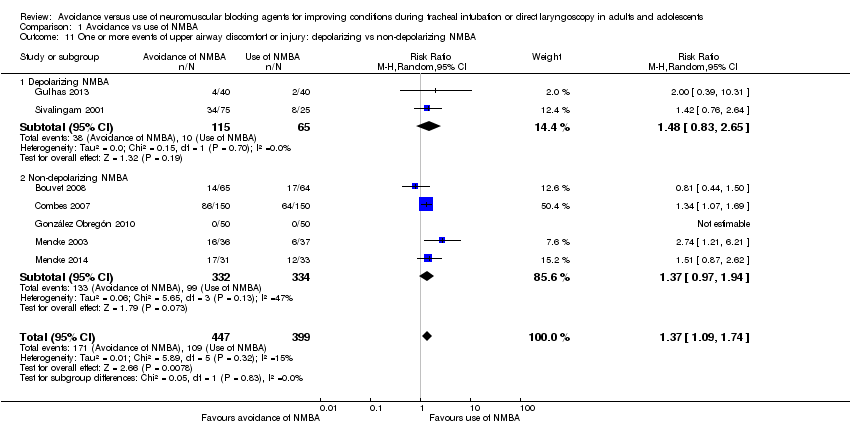
Comparison 1 Avoidance vs use of NMBA, Outcome 11 One or more events of upper airway discomfort or injury: depolarizing vs non‐depolarizing NMBA.
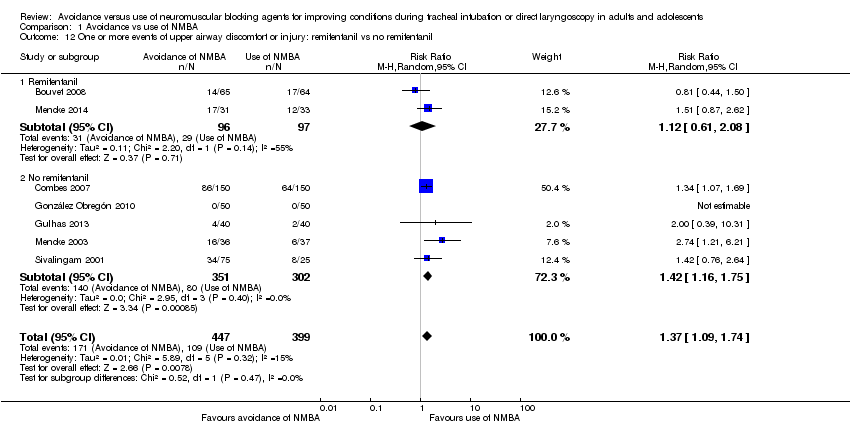
Comparison 1 Avoidance vs use of NMBA, Outcome 12 One or more events of upper airway discomfort or injury: remifentanil vs no remifentanil.
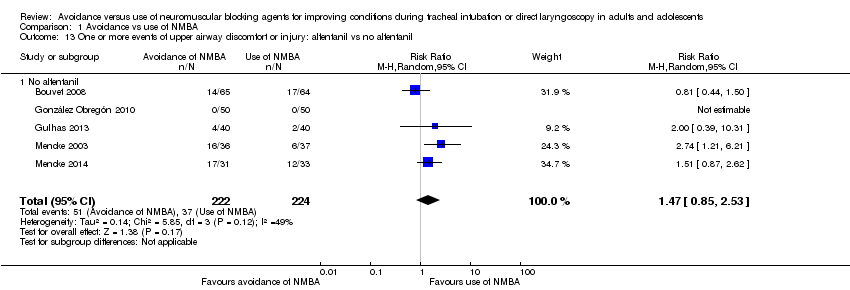
Comparison 1 Avoidance vs use of NMBA, Outcome 13 One or more events of upper airway discomfort or injury: alfentanil vs no alfentanil.
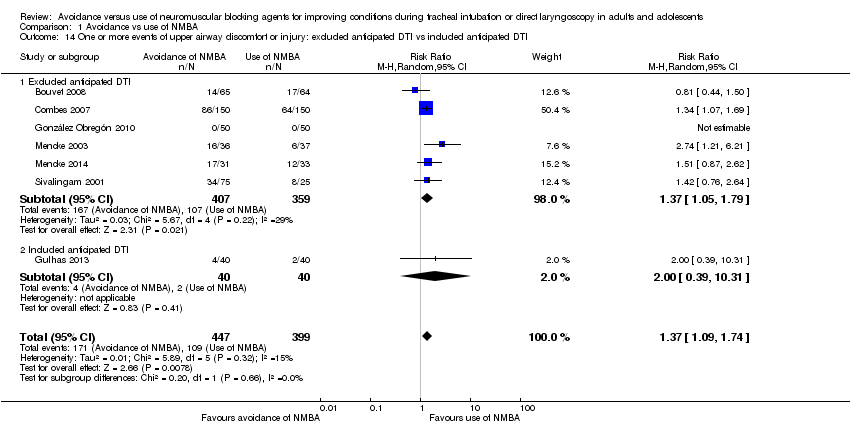
Comparison 1 Avoidance vs use of NMBA, Outcome 14 One or more events of upper airway discomfort or injury: excluded anticipated DTI vs included anticipated DTI.
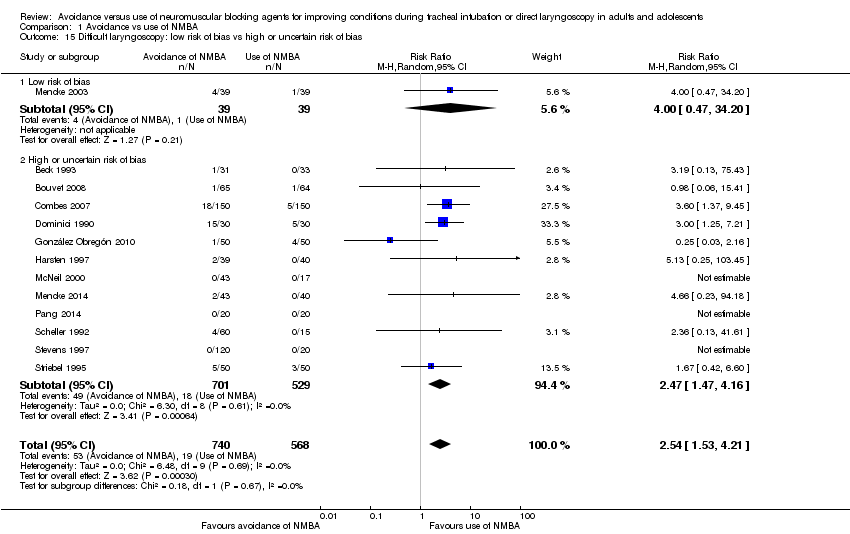
Comparison 1 Avoidance vs use of NMBA, Outcome 15 Difficult laryngoscopy: low risk of bias vs high or uncertain risk of bias.
| Avoidance vs use of neuromuscular blocking agent for improving conditions during tracheal intubation in adults and adolescents | ||||||
| Patient or population: improving conditions during tracheal intubation or direct laryngoscopy in adults and adolescents | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect of avoidance vs use of NMBA | Number of participants | Quality of the evidence | Comments | |
| Assumed risk Risk with use of NMBA | Corresponding risk Risk with avoidance of NMBA | |||||
| Primary outcomes | ||||||
| Difficult tracheal intubation: low risk of bias trials | Study population | RR 13.27 | 508 | ⊕⊕⊕⊝ Moderatea | TSA shows that the required information size of 8195 for a 20% RRR has not been achieved, but the trial sequential monitoring boundary has been crossed and the TSA‐adjusted CI for the RR is 1.85 to 95.04 | |
| 47 per 1000 | 620 per 1000 | |||||
| Difficult tracheal intubation: all trials | Study population | RR 5.00 | 3565 | ⊕⊕⊝⊝ Lowb | TSA shows that the required information size of 44,661 for a 20% RRR has not been achieved, but the trial sequential monitoring boundary has been crossed and the TSA‐adjusted CI for the RR is 1.20 to 20.77. | |
| 81 per 1000 | 406 per 1000 | |||||
| One or more events of upper airway discomfort or injury: low risk of bias trials | Study population | RR 2.74 | 73 | See comments | Because only 1 low risk of bias trial was identified, no quality of evidence assessment was performed | |
| 162 per 1000 | 444 per 1000 | |||||
| One or more events of upper airway discomfort or injury: all trials | Study population | RR 1.37 | 846 | ⊕⊕⊕⊝ Moderatec | TSA shows that the required information size of 1981 for a 20% RRR has not been achieved, but the trial sequential monitoring boundary has been crossed and the TSA‐adjusted CI for the RR is 1.00 to 1.86. | |
| 273 per 1000 | 374 per 1000 | |||||
| Mortality | Not estimated | Not estimated | Not estimated | 0 (34 studies) | Not estimated | |
| Secondary outcomes | ||||||
| Difficult laryngoscopy: low risk of bias trials | Study population | RR 4.00 | 78 | See comments | Because only 1 low risk of bias trial was identified, no quality of evidence assessment was performed | |
| 26 per 1000 | 103 per 1000 | |||||
| Difficult laryngoscopy: all trials | Study population | RR 2.54 | 1308 | ⊕⊕⊝⊝ Lowd | TSA shows that the required information size of 22,911 for a 20% RRR was not achieved, and in no trials were sequential monitoring boundaries crossed. The TSA‐adjusted CI for the RR is 0.27 to 21.75. | |
| 33 per 1000 | 85 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because of indirectness bDowngraded two levels because of indirectness, heterogeneity, and high or uncertain risk of bias cDowngraded one level because of high or uncertain risk of bias dDowngraded two levels because of imprecision and high or uncertain risk of bias | ||||||
| Study ID | NMBA | Country | Language | Randomized | Sex | Age, years | Weight, kg | BMI | ASA class included | Expected difficult airway excluded | Overweight excluded |
| C1: suxamethonium 1 mg/kg | UK | English | 60 | C: 12F/8M I1: 11F/9M I2: 11F/9M | C: 41.7 (17.4) I1: 40.3 (10.6) I2: 44.2 (15.0) | C: 76.3 (15.0) I1: 75.5 (15.1) I2: 76.6 (16.8) | ns | I‐II | yes | ||
| C1: rocuronium 0.1 mg/kg C2: rocuronium 0.13 mg/kg | UK | English | 60 | ns | C1: 30 C2: 29 I1: 27 | C1: 74 C2: 72 I1: 64 | ns | ns | yes | yes | |
| C1: suxamethonium 1 mg/kg | USA | English | 64 | C1: 22F/11M I1: 21F/10M | C1: 34 (11) I1: 35 (11) | C1: 69 (14) I1: 65 (13) | I‐II | ||||
| C1: cisatracurium 0.15 mg/kg | France | English | 130 | C1: 65F I1: 65F | C1: 41.5 (12.9) I1: 40.7 (15.2) | ns | C1: 24.6 (5.4) I1: 23.0 (3.5) | I‐II | yes | ||
| C1: rocuronium 0.6 mg/kg | France | English | 300 | C1: 73F/77M I1: 69F/81M | C1: 41 (18‐70) I1: 43 (18‐66) | C1: 73 (13) I1: 70 (13) | ns | I‐II | yes | yes | |
| C1: suxamethonium 1.5 mg/kg | France | French | 60 | C1: 9F/21M I1: 9F/21M | C1: 48.4 (3.4) I1: 50.1 (2.9) | C1: 62.8 (2.5) I1: 61.6 (2.1) | ns | I‐III | |||
| C1: rocuronium 0.6 mg/kg | Columbia | Spanish | 100 | C1: 33F/17M I1: 33F/17M | C1: 34.7 (11.0) I1: 32.8 (12.3) | ns | ns | I‐II | yes | ||
| C1: succinylcholine 1 mg/kg | Turkey | English | 80 | C1: 19F/21M I1: 22F/18M | C1: 49.6 (8.4) I1: 47.9 (8.7) | C1: 77.3 (13.1) I1: 73.2 (14.4) | ns | I‐II | |||
| C1: rocuronium 0.06 mg/kg + succinylcholine 1.5 mg/kg | USA | English | 50 | C1: 15F/9M I1: 5F/18M | C1: 39.0 (13.3) I1: 43.0 (14.5) | C1: 75.0 (15.0) I1: 81.0 (13.0) | C1: 25.9 (4.6) I1: 26.5 (2.9) | I‐II | yes | ||
| C1: suxamethonium 1 mg/kg | Sweden | English | 80 | C1: 26F/13M I1: 23F/14M | C1: 41.8 (13) I1: 39.5 (14) | ns | ns | I‐II | |||
| C1: suxamethonium 1.5 mg/kg | Thailand | English | 120 | C1: 54F/6M I1: 54F/6M | C1: 40.6 (9.1) I1: 39.7 (9.2) | C1: 55.8 (10.8) I1: 55.1 (9.1) | ns | I‐II | yes | yes | |
| C1: suxamethonium 1.5 mg/kg | Nigeria | English | 96 | C1: 12F/32M I1: 21F/23M | C1: 30.8 (9.0) I1: 32.6 (8.0) | C1. 69.0 (7.4) I1: 68.3 (6.6) | I‐II | yes | |||
| C1: suxamethonium 0.6 mg/kg | China | English | 55 | C1: 27F/0M I1: 28F/0M | C1: 38.4 (10.9) I2: 36.3 (9.9) | C1: 58.1 (7.0) I2: 58.2 (7.9) | ns | I‐II | yes | ||
| C1: ORG 9487 (rapacuronium) 0.5 mg/kg C2: ORG 9487 (rapacuronium) 1.0 mg/kg C3: ORG 9487 (rapacuronium) 1.5 mg/kg C4: ORG 9487 (rapacuronium) 2.0 mg/kg C5: ORG 9487 (rapacuronium) 2.5 mg/kg | USA | English | 181 | C1: 20F/10M C2: 18F/9M C3: 17F/15M C4: 15F/13M C5: 22F/9M I1: 19F/11M | C1: 51.3 C2: 49.6 C3: 52.0 C4: 50.6 C5: 50.2 I1: 52.6 | C1: 67.3 C2: 70.5 C3: 68.6 C4: 71.3 C5: 75.9 I1: 69.7 | ns | I‐III | yes | ||
| C1: rocuronium 0.4 mg/kg C2: rocuronium 0.8 mg/kg C3: rocuronium 1.2 mg/kg | USA | English | 80 | C1: 2F/18M C2: 6F/14M C3: 10F/10M I1: 5F/15M | C1: 39.7 (7.5) C2: 39.5 (14.3) C3: 39.2 (10.5) I1: 39.3 (11.8) | C1: 75.0 (16.9) C2: 78.6 (15.8) C3: 67.4 (14.8) I1: 73.4 (16.6) | ns | I‐II | yes | ||
| C1: rapacuronium 1.0 mg/kg C2: rapacuronium 1.2 mg/kg C3: rocuronium 0.50 mg/kg | USA | English | 100 | ns | range: 18‐65 | ns | range: 17.5‐27.5 | I‐II | yes | ||
| C1: atracurium 0.5 mg/kg C2: atracurium 0.5 mg/kg C3: atracurium 0.5 mg/kg | France | English | 170 | C1: 3F/42M C2: 7F/41M C3: 8F/39M I1: 2F/18M | C1: 52.9 (11.8) C2: 51.3 (12.6) C3: 56.3 (11.9) I1: 50.4 (10.7) | ns | C1: 23.7 (3.2) C2: 23.1 (3.2) C3: 23.6 (3.4) I1: 23.3 (3.9) | I‐II | yes | ||
| C1: rocuronium 0.3 mg/kg C2: rocuronium 0.45 mg/kg C3: rocuronium 0.6 mg/kg C4: rocuronium 0.3 mg/kg C5: rocuronium 0.45 mg/kg C6: rocuronium 0.6 mg/kg | UK | English | 140 | C1: 4F/16M I1: 2F/8M I2: 4F/6M | C1: 29 (11) I1: 29 (11) I2: 30 (9) | C1: 77(16) I1: 72 (12) I2: 73 (15) | ns | I‐II | yes | yes | |
| C1: succinylcholine 1 mg/kg | UK | English | 60 | ns | C1: 44 (15) I1: 39 (11) I2: 40 (13) | C1: 75 (10) I1: 76 (15) I2: 71 (12) | ns | I‐II | yes | yes | |
| C1: atracurium 0.5 mg/kg | Germany | English | 80 | C1: 19F/18M I1: 18F/18M | C1: 47.2 (13.2) I1: 47.7 (14.3) | C1: 77.7 (16) I1: 74.2 (15) | I‐II | yes | yes | ||
| I1: rocuronium 0.45 mg·kg‐1 | Germany | English | 83 | C1: 16F/24M I1: 16F/23M | C1: 50 (16) I1: 48 (17) | C1: 83.8 (16) I1: 79.6 (15) | C1: 28.2 (4.3) I1: 26.5 (3.7) | I‐III | yes | yes | |
| C1: succinylcholine 0.3 mg/kg C2: succinylcholine 0.5 mg/kg C3: succinylcholine 1.0 mg/kg | Saudi Arabia | English | 200 | C1: 25F/25M C2: 23F/27M C3: 28F/22M I1: 23F/27M | C1: 30.9 (28‐34) | C1: 66.6 (64‐70) | ns | I | yes | ||
| C1: succinylcholine 0.3 mg/kg C2: succinylcholine 0.5 mg/kg C3: succinylcholine 1.0 mg/kg C4: succinylcholine 1.5 mg/kg C5: succinylcholine 2.0 mg/kg | Saudi Arabia | English | 180 | C1: 17F13M I1: 19F/11M | C1: 33.5 (8.7) | C1: 67.8 (10.3) | C1: 25.6 (2.8) | I | yes | ||
| C1: suxamethonium 0.25 mg/kg C2: suxamethonium 0.5 mg/kg | USA | English | 60 | C1: 12F/8M C2: 12F/8M I1: 14F/6M | C1: 28.6 (17‐55) C2: 29.0 (16‐53) I1: 27.0 (18‐53) | C1: 66.2 (13.6) C2: 64.4 (11.2) I1: 68.1 (13.6) | ns | I‐II | |||
| C1: cisatracurium 0.1 mg/kg | China | English | 40 | C1: 14F/6M I1: 9F/11M | C1: 45.2 (7.4) I1: 43.3 (6.7) | C1: 63.8 (9.5) I1: 64.6 (7.9) | C1: 23.7 (2.8) I1: 23.3 (3.1) | I‐II | yes | yes | |
| C1: mivacurium 0.25 mg/kg C2: rocuronium 0.45 mg/kg C3: rocuronium 0.6 mg/kg C4: rocuronium 0.9 mg/kg C5: rocuronium 1.2 mg/kg | USA | English | 100 | ns | ns | ns | ns | I‐II | yes | yes | |
| C1: vecuronium 0.08 mg/kg | France | French | 152 | ns | C1: 23 (5) I1: 25 (8) | C1: 71 (10) I1: 71 (11) | ns | I | yes | ||
| C1: d‐tubocurarine 3 mg and succinylcholine 1 mg/kg | USA | English | 75 | C1: 8F/7M I1: 10F/5M I2: 11F/4M I3: 13F/2M I4: 10F/5M | C1: 37 (10) I1: 33 (9) I2: 30 (10) I3: 35 (11) I4: 36 (16) | C1: 77 (20) I1: 65 (11) I2: 66 (15) I3: 66 (12) I4: 68 (16) | ns | I | yes | ||
| C1: rocuronium 0.6 mg/kg C2: rocuronium 0.45 mg/kg C3: rocuronium 0.3 mg/kg | Germany | English | 120 | C1: 13F/17M C2: 13F/17M C3: 14F/16M I1: 14F/16M | C1: 37 (11) C2: 35 (11) C3: 36 (12) I1: 37 (11) | C1: 72 (14) C2: 75 (13) C3: 75 (12) I1: 70 (14) | ns | I‐II | yes | ||
| C1: suxamethonium 1 mg/kg | New Zealand | English | 100 | C1: 7F/18M I1: 9F/16M I2: 8F/17M I3: 10F/15M | C1: 34.3 (14.0) I1: 36.8 (12.6) I2: 29.6 (9.7) I3: 37.7 (12) | C1: 66 (10) I1: 62 (11) I2: 63 (15) I3: 61 (11) | ns | I‐II | yes | ||
| C1: d‐tubocurarine 3 mg and succinylcholine 1 mg/kg | USA | English | 140 | C1: 12F/8M I1: 12F/8M I2: 15F/5M I3: 17F/3M I4: 17F/3M I5: 15F/5M I6: 14F/6M | C1: 35 (9) I1: 38 (12) I2: 34 (11) I3: 37 (10) I4: 34 (9) I5: 33 (11) I6: 37 (14) | C1: 70 (8) I1: 72 (17) I2: 70 (14) I3: 72 (10) I4: 72 (13) I5: 72 (18) I6: 70 (13) | ns | I‐II | yes | yes | |
| C1: vecuronium 1 mg + succinylcholine 1 mg/kg C2: vecuronium 1 mg + succinylcholine 1 mg/kg | Germany | German | 100 | C1: 25F C2: 25F I1: 25F I2: 25F | C1: 47.8 (11.7) C2: 43.8 (9.5) I1: 46.5 (12.7) I2: 46.0 (12.4) | C1: 62.6 (9.4) C2: 68.2 (14) I1: 64.9 (10.1) I2: 70.8 (14.6) | ns | I‐II | |||
| C1: succinylcholine 1 mg/kg | Malaysia | English | 120 | C1: 16F/14M I1: 13F/17M I2: 18F/12M I3: 12F/17M | C1: 35.7 (16) I1: 35.5 (12) I2: 35.4 (13) I3: 35.7 (11) | C1: 60.2 (8.9) I1: 66.0 (13.1) I2: 63.4 (12.9) I3: 60.1 (10.8) | ns | I‐II | yes | ||
| C1: atracurium 0.5 mg/kg | Iran | English | 66 | 69.7% M | 31.6 (13) | ns | ns | I‐II | yes | ||
| ns = not specified; The values in parentheses are standard deviation or range | |||||||||||
| Study ID | NMBA | Randomized/ Analysed | Hypnotic | Opioid | Local anaesthetic | Difficult intubation events/ | Difficult laryngos‐ copy events/ | Upper airway discomfort or injury events/total |
| C1: suxamethonium 1 mg/kg | 60/60 | C1: propofol 2 mg/kg I1: propofol 2 mg/kg I2: propofol 2 mg/kg | C1: none I1: alfentanil 50 μg/kg I2: remifentanil 2 μg/kg | None | C1: 0/20 I1: 3/20 I2: 13/20 | ns | ns | |
| C1: rocuronium 0.1 mg/kg C2: rocuronium 0.3 mg/kg | 60/60 | C1: propofol 2.5 mg/kg C2: propofol 2.5 mg/kg I1: propofol 2.5 mg/kg | C1: alfentanil 10 μg/kg C2: alfentanil 10 μg/kg I1: alfentanil 10 μg/kg | Lidocaine 10 mg IV | C1: 14/20 C2: 2/20 I1: 19/20 | ns | ns | |
| C1: suxamethonium 1 mg/kg | 64/64 | C1: thiopenthal 5 mg/kg I1: propofol 2 mg/mL | C1: none I1: alfentanil 50 μg/kg | None | C1: 0/33 I1: 1/31 | C1: 0/33 I1: 1/31 | ns | |
| C1: cisatracurium 0.15 mg/kg | 130/129 | C1: propofol 2.5 mg/kg I1: propofol 2.5 mg/kg | C1: remifentanil 2 μg/kg I1: remifentanil 2 μg/kg | None | C1: 0/64 I1: 3/65 | C1: 1/64 I1: 1/65 | C1: 17/64 I1: 14/65 | |
| C1: rocuronium 0.6 mg/kg | 300/300 | C1: propofol 2.5 mg/kg I1: propofol 2.5 mg/kg | C1: alfentanil 15 µg/kg I1: alfentanil 40 µg/kg | None | C1: 1/150 I1: 18/150 | C1: 5/150 I1: 18/150 | C1: 64/150 I1: 86/150 | |
| C1: suxamethonium 1.5 mg/kg | 60 | C1: propofol 3 mg/mL I1: propofol 3 mg/mL | C1: alfentanil 7‐10 µg/kg I1: alfentanil 7‐10 µg/kg | Lidocaine (2%): IV + topical Lidocaine 5% | C1: 10/30 I1: 11/30 | C1: 5/30 I1: 15/30 | ns | |
| C1: rocuronium 0.6 mg/kg | 100/100 | C1: propofol 1‐2 mg/kg I1: Sevoflurane 3% + propofol 2 mg/kg | C1: remifentanil 1‐2 μg/kg in 1 min + 0.15 µ/kg/min in1 min I1: remifentanil 0.6 µ/kg/min for 5 min | None | C1: 4/50 I1: 1/50 | C1: 4/50 I1: 1/50 | C1: 0/50 I1: 0/50 | |
| C1: succinylcholine 1 mg/kg | 80/80 | C1: propofol 2 mg/kg I1: propofol 2 mg/kg | C1: remifentanil 1 μg/kg I1: remifentanil 4 μg/kg | None | C1: 5/40 I1: 0/40 | ns | C1: 2/40 I1: 4/40 | |
| C1: rocuronium 0.06 mg/kg + succinylcholine 1.5 mg/kg | 50/47 | C1: propofol 2 mg/kg I1: propofol 2 mg/kg | C1: none I1: remifentanil 4 μg/kg | Lidocaine 0.5 mg/kg IV | C1: 2/24 I1: 3/23 | ns | ns | |
| C1: suxamethonium 1 mg/kg | 80/79 | C1: thiopental 5 mg/kg I1: propofol 2.5 mg/kg | C1: alfentanil 10 μg/kg I1: alfentanil 10 μg/kg | None | C1: 0/40 I1: 6/39 | C1: 0/40 I1: 2/39 | ns | |
| C1: suxamentonium 1.5 mg/kg | 120/120 | C1: thiopenthal 5 mg/kg + (N2O) I1: sevoflurane 8% | C1: fentanyl 1.5 μg/kg I1: fentanyl 1.5 μg/kg | None | C1: 0/60 I1: 4/60 | ns | ns | |
| C1: suxamethonium 1.5 mg/kg | 96/88 | C1: propofol 2.0 mg/kg I1: propofol 2.0 mg/kg | None | C1: none I1: lidocaine IV 1.5 mg/kg | C1: 0/44 I1: 18/44 | ns | ns | |
| C1: suxamethonium 0.6 mg/kg | 55/55 | C1: propofol 2 mg/kg I1: propofol 2 mg/kg | C1: remifentanil 1 μg/kg I1: remifentanil 1.5 μg/kg | None | C1: 1/27 I2: 13/28 | ns | ns | |
| C1: ORG 9487 (rapacuronium) 0.5 mg/kg C2: ORG 9487 (rapacuronium) 1.0 mg/kg C3: ORG 9487 (rapacuronium) 1.5 mg/kg C4: ORG 9487 (rapacuronium) 2.0 mg/kg C5: ORG 9487 (rapacuronium) 2.5 mg/kg | 181/176 | C1: thiopental 3‐6 mg/kg I1: thiopental 3‐6 mg/kg | C1: fentanyl 0.5‐3 μm/kg I1: fentanyl 0.5‐3 μm/kg | None | C1: 9/30 I1: 18/30 | ns | ns | |
| C1: rocuronium 0.4 mg/kg C2: rocuronium 0.8 mg/kg C3: rocuronium 1.2 mg/kg | 80/80 | C1: propofol 2 mg/kg C2: propofol 2 mg/kg C3: propofol 2 mg/kg I1: propofol 2 mg/kg | C1: fentanyl 2 μm/kg C2: fentanyl 2 μm/kg C3: fentanyl 2 μm/kg I1: fentanyl 2 μm/kg | None | C1: 9/20 C2: 2/20 C3: 1/20 I1: 13/20 | ns | ns | |
| C1: rapacuronium 1.0 mg/kg C2: rapacuronium 1.2 mg/kg C3: rocuronium 0.50 mg/kg | 100/100 | C1: propofol 2.0 mg/kg IV C2: propofol 2.0 mg/kg IV C3: propofol 2.0 mg/kg IV I1: propofol 2.0 mg/kg IV | C1: alfentanil 12.5 μg/kg C2: alfentanil 12.5 μg/kg C3: alfentanil 12.5 μg/kg I1: alfentanil 12.5 μg/kg | None | C1: 2/30 C2: 0/30 C3: 0/30 I1: 7/10 | |||
| C1: atracurium 0.5 mg/kg C2: atracurium 0.5 mg/kg C3: atracurium 0.5 mg/kg | 170/160 | C1: propofol 1.5 mg/kg C2: propofol 2.0 mg/kg C3: propofol 2.5 mg/kg I1: propofol 2.5 mg/kg | C1: fentanyl 3 μm/kg C2: fentanyl 3 μm/kg C3: fentanyl 3 μm/kg I1: fentanyl 3 μm/kg | None | C1: 7/47 C2: 1/48 C3: 2/45 I1: 13/20 | ns | ns | |
| C1: rocuronium 0.3 mg/kg C2: rocuronium 0.45 mg/kg C3: rocuronium 0.6 mg/kg C4: rocuronium 0.3 mg/kg C5: rocuronium 0.45 mg/kg C6: rocuronium 0.6 mg/kg | 140/140 | C1: propofol 2‐3 mg/kg C2: propofol 2‐3 mg/kg C3: propofol 2‐3 mg/kg C4: sevoflurane 8% C5: sevoflurane 8% C6: sevoflurane 8% I1: propofol 2‐3 mg/kg I2: sevoflurane 8% | C1: fentanyl 1 μm/kg C2: fentanyl 1 μm/kg C3: fentanyl 1 μm/kg C4: fentanyl 1 μm/kg C5: fentanyl 1 μm/kg C6: fentanyl 1 μm/kg I1: fentanyl 1 μm/kg I2: fentanyl 1 μm/kg | None | C1: 11/20 C2: 4/20 C3: 2/20 C4:14/20 C5: 9/20 C6: 2/20 I1:10/10 I2: 9/10 | ns | ns | |
| C1: succinylcholine 1 mg/kg | 60/60 | C1: propofol 2 mg/kg I1: propofol 2 mg/kg I2: propofol 2 mg/kg | C1: none I1: remifentanil 2 μg/kg I2: remifentanil 4 μg/kg | None | C1: 0/17 I1: 2/23 I2: 2/20 | C1: 0/17 I1: 0/23 I2: 0/20 | ns | |
| C1: atracurium 0.5 mg/kg | 80/73 | C1: propofol 2.5‐3 mg/kg I1: propofol 2.5‐3 mg/kg | C1: fentanyl 2‐3 μg/kg I1: fentanyl 2‐3 μg/kg | None | C1: 2/37 I1: 12/36 | C1: 1/39 I1: 4/39 | C1: 6/37 I1: 16/36 | |
| C1: rocuronium 0.45 mg·kg/kg | 83/83 | C1: propofol 1.5 mg·kg‐1 + sevoflurane 3.0‐3.5 Vol%,8 l·min‐1 in 2‐3 minutes I1: propofol 1.5 mg/kg | C1: remifentanil 0.30 μg/kg/min for 3 minutes I1: remifentanil 0.30 μg/kg/min for 3 minutes | None | C1: 1/40 I1: 11/43 | C1: 0/40 I1: 2/43 | C1: 12/33 I1: 17/31 | |
| C1: succinylcholine 0.3 mg/kg C2: succinylcholine 0.5 mg/kg C3: succinylcholine 1.0 mg/kg | 200/200 | C1: propofol 2 mg/kg C2: propofol 2 mg/kg C3: propofol 2 mg/kg I1: propofol 2 mg/kg | C1: fentanyl 2 μg/kg C2: fentanyl 2 μg/kg C3: fentanyl 2 μg/kg I1: fentanyl 2 μg/kg | None | C1: 4/50 C2: 3/50 C3: 1/50 I1: 15/50 | ns | ns | |
| C1: succinylcholine 0.3 mg/kg C2: succinylcholine 0.5 mg/kg C3: succinylcholine 1.0 mg/kg C4: succinylcholine 1.5 mg/kg C5: succinylcholine 2.0 mg/kg | 180/180 | C1: propofol 2 mg/kg C2: propofol 2 mg/kg C3: propofol 2 mg/kg C4: propofol 2 mg/kg C5: propofol 2 mg/kg I1: propofol 2 mg/kg | C1: fentanyl 2 μm/kg C2: fentanyl 2 μm/kg C3: fentanyl 2 μm/kg C4: fentanyl 2 μm/kg C5: fentanyl 2 μm/kg I1: fentanyl 2 μm/kg | None | C1: 2/30 C2: 2/30 C3: 1/30 C4: 1/30 C5: 0/30 I1: 21/30 | ns | ns | |
| C1: suxamethonium 0.25 mg/kg C2: suxamethonium 0.5 mg/kg | 60/60 | C1: propofol 2.5 mg/kg C2: propofol 2.5 mg/kg I1: Propofol 2.5 mg/kg | C1: alfentanil 15 μg/kg C2: alfentanil 15 μg/kg I1: alfentanil 15 μg/kg | None | C1: 0/20 C2: 1/20 I1: 9/20 | ns | ns | |
| C1: cisatracurium 0.1 mg/kg | 20/20 | C1: propofol target control I1: propofol target control | C1: remifentanil target control I1: remifentanil target control | C1: tetracaine 10 mg/mL I1: tetracaine 10 mg/mL | C1: 0/20 I1: 0/20 | C1: 0/20 I1: 0/20 | ns | |
| C1: mivacurium 0.25 mg/kg C2: rocuronium 0.45 mg/kg C3: rocuronium 0.6 mg/kg C4: rocuronium 0.9 mg/kg C5: rocuronium 1.2 mg/kg | 100/98 | C1: propofol 2 mg/kg C2: propofol 2 mg/kg C3: propofol 2 mg/kg C4: propofol 2 mg/kg C5: propofol 2 mg/kg I1: propofol 2 mg/kg | C1: fentanyl 2 μm/kg C2: fentanyl 2 μm/kg C3: fentanyl 2 μm/kg C4: fentanyl 2 μm/kg C5: fentanyl 2 μm/kg I1: fentanyl 2 μm/kg | None | C1: 2/30 IC2: 9/15 C3: 4/14 C4: 1/14 C5: 0/15 I1: 10/10 | ns | ns | |
| C1: vecuronium 0.08 mg/kg | 152/152 | C1: propofol 2.5 mg/kg I1: propofol 2.5 mg/kg | C1: alfentanil 0.03 mg/kg I1: alfentanil 0.03 mg/kg | C1: none I1: lidocaine 1.5 mg/kg | C1: 2/77 I1: 4/75 | ns | ns | |
| C1: d‐tubocurarine 3 mg and succinylcholine 1 mg/kg | 75/75 | C1: thiamylal 4 mg/kg I1: propofol 2 mg/kg I2: propofol 2 mg/kg I3: propofol 2 mg/kg I4: propofol 2 mg/kg | C1: none I1: alfentanil 30 µg/kg I2: alfentanil 40 µg/kg I3: alfentanil 50 µg/kg I4: alfentanil 60 µg/kg | None | C1: 0/15 I1: 1/15 I2: 1/15 I3: 1/15 I4: 1/15 | C1: 0/15 I1: 1/15 I2: 1/15 I3: 1/15 I4: 1/15 | ns | |
| C1: rocuronium 0.6 mg/kg C2: rocuronium 0.45 mg/kg C3: rocuronium 0.3 mg/kg | 120/120 | C1: propofol 2‐2.5 mg/kg C2: propofol 2‐2.5 mg/kg C3: propofol 2‐2.5 mg/kg I1: propofol 2‐2.5 mg/kg | C1: remifentanil 0.5 µg/kg/min C2: remifentanil 0.5 µg/kg/min IC3: remifentanil 0.5 µg/kg/min I1: remifentanil 0.5 µg/kg/min | None | C1: 0/30 C2: 1/30 C3: 0/30 I1: 12/30 | ns | ns | |
| C1: suxamethonium 1 mg/kg | 100/100 | C1: Sevoflu 7% + N2O60% I1: Sevoflu 7% + N2O60% I2: Sevoflu 7% + N2O60% I3: Sevoflu 7% + N2O60% | C1: alfentanil 10 µg/kg I1: alfentanil 20 µg/kg I2: alfentanil 25 µg/kg I3: alfentanil 30 µg/kg | None | C1: 1/25 I1: 4/25 I2: 5/25 I3: 2/25 | ns | C1: 8/25 I1: 12/25 I2: 13/25 I3: 9/25 | |
| C1: d‐tubocurarine 3 mg and succinylcholine 1 mg/kg | 140/140 | C1: thiopental 4 mg/kg I1: etomidate 0.3 mg/kg I2: etomidate 0.3 mg/kg I3: propofol 2 mg/kg I4: propofol 2 mg/kg I5: thiopental 4 mg/kg I6: thiopental 4 mg/kg | C1: none I1: alfentanil 40 µg/kg I2: alfentanil 40 µg/kg I3: alfentanil 40 µg/kg I4: alfentanil 40 µg/kg I5: alfentanil 40 µg/kg I6: alfentanil 40 µg/kg | C1: none I1: none I2: lidocaine 1 mg/kg I3: none I4: lidocaine 1 mg/kg I5: none I6: lidocaine1 mg/kg | C1: 1/20 I1: 3/20 I2: 1/20 I3: 3/20 I4: 2/20 I5: 8/20 I6: 3/20 | C1: 0/20 I1: 0/20 I2: 0/20 I3: 0/20 I4: 0/20 I5: 0/20 I6: 0/20 | ns | |
| C1: vecuronium 1 mg + succinylcholine 1 mg/kg C2: vecuronium 1 mg + succinylcholine 1 mg/kg | 100/100 | C1: thiopental 5.5 mg/kg C2: propofol 2.2 mg/kg I1: propofol 2.4 mg/kg I2: propofol 2.2 mg/kg | C1: fentanyl 0.1 mg C2: fentanyl 0.1 mg I1: fentanyl 0.1 mg I2: fentanyl 0.2 mg | 2 mL lidocaine 1% IV | C1: 1/25 C2: 1/25 I1: 3/25 I2: 5/25 | C1: 2/25 C2: 1/25 I1: 1/28 I2: 4/25 | ns | |
| C1: succinylcholine 1 mg/kg | 120/120 | C1: propofol 3.0 mg/kg I1: propofol 2.6 mg/kg I2: propofol 2.6 mg/kg I3: propofol 3.1 mg/kg | C1: none I1: alfentanil 15 μg/kg I2: alfentanil 30 μg/kg I3: none | None | C1: 0/30 I1: 1/30 I2: 0/30 I3: 6/30 | ns | ns | |
| C1: atracurium 0.5 mg/kg | 66/66 | C1: propofol 2.5 mg/kg I1: propofol 2.5 mg/kg | C1: none I1: remifentanil 2 μg/kg | None | C1: 4/31 I1: 14/35 | ns | ns | |
| ns = not specified | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difficult tracheal intubation: low risk of bias vs high or uncertain risk of bias Show forest plot | 34 | 3565 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [3.49, 7.15] |
| 1.1 Low risk of bias | 4 | 508 | Risk Ratio (M‐H, Random, 95% CI) | 13.27 [8.19, 21.49] |
| 1.2 High or uncertain risk of bias | 30 | 3057 | Risk Ratio (M‐H, Random, 95% CI) | 4.14 [2.92, 5.87] |
| 2 Difficult tracheal intubation: depolarizing vs non‐depolarizing NMBA Show forest plot | 32 | 3413 | Risk Ratio (M‐H, Random, 95% CI) | 5.25 [3.61, 7.63] |
| 2.1 Depolarizing NMBA | 16 | 1540 | Risk Ratio (M‐H, Random, 95% CI) | 5.79 [2.64, 12.72] |
| 2.2 Non‐depolarizing NMBA | 16 | 1873 | Risk Ratio (M‐H, Random, 95% CI) | 4.72 [3.17, 7.02] |
| 3 Difficult tracheal intubation: remifentanil vs no remifentanil Show forest plot | 26 | 3008 | Risk Ratio (M‐H, Random, 95% CI) | 5.64 [3.82, 8.31] |
| 3.1 Remifentanil | 4 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 15.86 [4.43, 56.71] |
| 3.2 No remifentanil | 22 | 2636 | Risk Ratio (M‐H, Random, 95% CI) | 5.23 [3.54, 7.74] |
| 4 Difficult tracheal intubation: alfentanil vs no alfentanil Show forest plot | 26 | 2618 | Risk Ratio (M‐H, Random, 95% CI) | 4.77 [3.25, 7.01] |
| 4.1 Alfentanil | 6 | 511 | Risk Ratio (M‐H, Random, 95% CI) | 4.46 [1.66, 11.98] |
| 4.2 No alfentanil | 20 | 2107 | Risk Ratio (M‐H, Random, 95% CI) | 5.10 [3.34, 7.79] |
| 5 Difficult tracheal intubation: local anaesthesia vs no local anaesthesia Show forest plot | 31 | 3184 | Risk Ratio (M‐H, Random, 95% CI) | 5.04 [3.48, 7.29] |
| 5.1 Local anaesthesia | 5 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.14, 3.18] |
| 5.2 No local anaesthesia | 26 | 2877 | Risk Ratio (M‐H, Random, 95% CI) | 6.26 [4.15, 9.44] |
| 6 Difficult tracheal intubation: excluded anticipated DTI vs included anticipated DTI Show forest plot | 34 | 3564 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [3.50, 7.16] |
| 6.1 Exclusion of patients with anticipated difficult intubation | 25 | 2886 | Risk Ratio (M‐H, Random, 95% CI) | 5.32 [3.54, 8.00] |
| 6.2 No exclusion of patients with anticipated difficult intubation | 9 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 4.40 [1.71, 11.29] |
| 7 Difficult tracheal intubation: "best‐case scenario" Show forest plot | 34 | 2410 | Risk Ratio (M‐H, Random, 95% CI) | 5.99 [3.46, 10.38] |
| 8 Difficult tracheal intubation excluding dose‐finding studies Show forest plot | 16 | 1536 | Risk Ratio (M‐H, Random, 95% CI) | 3.40 [1.63, 7.10] |
| 9 Difficult tracheal intubation: funding from pharmaceutical industry Show forest plot | 34 | 3565 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [3.49, 7.15] |
| 9.1 No funding from pharmaceutical industry | 24 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 5.33 [3.16, 8.98] |
| 9.2 Funding from pharmaceutical industry | 10 | 1015 | Risk Ratio (M‐H, Random, 95% CI) | 4.10 [2.67, 6.31] |
| 10 One or more events of upper airway discomfort or injury: low risk of bias vs high or uncertain risk of bias Show forest plot | 7 | 844 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.08, 1.71] |
| 10.1 Low risk of bias | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 2.74 [1.21, 6.21] |
| 10.2 High or uncertain risk of bias | 6 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.08, 1.58] |
| 11 One or more events of upper airway discomfort or injury: depolarizing vs non‐depolarizing NMBA Show forest plot | 7 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.09, 1.74] |
| 11.1 Depolarizing NMBA | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.83, 2.65] |
| 11.2 Non‐depolarizing NMBA | 5 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.97, 1.94] |
| 12 One or more events of upper airway discomfort or injury: remifentanil vs no remifentanil Show forest plot | 7 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.09, 1.74] |
| 12.1 Remifentanil | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.61, 2.08] |
| 12.2 No remifentanil | 5 | 653 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.16, 1.75] |
| 13 One or more events of upper airway discomfort or injury: alfentanil vs no alfentanil Show forest plot | 5 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.85, 2.53] |
| 13.1 No alfentanil | 5 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.85, 2.53] |
| 14 One or more events of upper airway discomfort or injury: excluded anticipated DTI vs included anticipated DTI Show forest plot | 7 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.09, 1.74] |
| 14.1 Excluded anticipated DTI | 6 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.05, 1.79] |
| 14.2 Included anticipated DTI | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.39, 10.31] |
| 15 Difficult laryngoscopy: low risk of bias vs high or uncertain risk of bias Show forest plot | 13 | 1308 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.53, 4.21] |
| 15.1 Low risk of bias | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.47, 34.20] |
| 15.2 High or uncertain risk of bias | 12 | 1230 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.47, 4.16] |