Propofol versus tiopental sódico para el tratamiento del estado epiléptico resistente
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009202.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Epilepsia
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
-
Conceiving the review: Hemanshu Prabhakar (HP).
-
Co‐ordinating the review: HP.
-
Undertaking manual searches: HP.
-
Screening search results: HP.
-
Organising retrieval of papers: HP.
-
Screening retrieved papers against inclusion criteria: HP.
-
Appraising quality of papers: HP.
-
Extracting data from papers: HP.
-
Writing to authors of papers for additional information: HP.
-
Providing additional data about papers: HP.
-
Obtaining and screening data on unpublished studies: HP.
-
Data management for the review: HP, Mani Kalaivani (MK).
-
Entering data into Review Manager (RevMan 2014): HP.
-
RevMan statistical data: HP, MK.
-
Other statistical analysis not using RevMan: HP, MK.
-
Double entry of data: (data entered by person one: HP; data entered by person two: MK).
-
Interpretation of data: HP, MK.
-
Statistical inferences: HP, MK.
-
Writing the review: HP.
-
Guarantor for the review (one author): HP.
-
Person responsible for reading and checking review before submission: HP, MK.
Sources of support
Internal sources
-
All India Institute of Medical Sciences, New Delhi, India.
External sources
-
National Institute of Health Research (NIHR), UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Declarations of interest
-
Hemanshu Prabhakar: None known.
-
Mani Kalaivani: None known.
Acknowledgements
We wish to thank the Cochrane Epilepsy Group for their continuous support in helping us prepare the review. We would like to thank Professor A Rossetti for providing the additional data and information on the included study. We wish to thank the South Asian Cochrane Network and Centre, CMC, Vellore, India, who conducted the workshop at the Prof. BV Moses and ICMR Center for Advanced Research and Training in Evidence‐Informed Healthcare, where this review was completed. We wish to acknowledge Gyaninder Pal Singh and Ashish Bindra who contributed to the original version of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 03 | Propofol versus thiopental sodium for the treatment of refractory status epilepticus | Review | Hemanshu Prabhakar, Mani Kalaivani | |
| 2015 Jun 25 | Propofol versus thiopental sodium for the treatment of refractory status epilepticus | Review | Hemanshu Prabhakar, Mani Kalaivani | |
| 2012 Aug 15 | Propofol versus thiopental sodium for the treatment of refractory status epilepticus | Review | Hemanshu Prabhakar, Ashish Bindra, Gyaninder Pal Singh, Mani Kalaivani | |
| 2011 Jul 06 | Propofol versus thiopental sodium for the treatment of refractory status epilepticus | Protocol | Hemanshu Prabhakar, Ashish Bindra, Gyaninder Pal Singh, Mani Kalaivani | |
Differences between protocol and review
The primary outcome in our protocol 'total control of seizures' is now defined as total control of seizures after the first course of the study drug.
By 'mortality' we now mean only in‐hospital mortality of the patients receiving study drugs. It does not include deaths after patients have been discharged from hospital.
A Google Scholar database search has not been conducted and so it has been removed from the list.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO
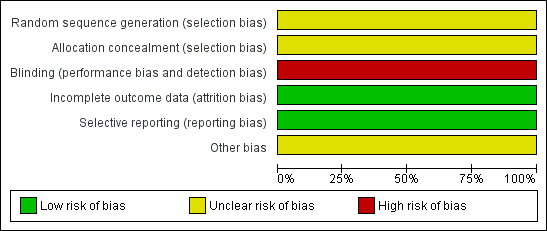
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

Study flow diagram.

Comparison 1 Propofol versus thiopental sodium, Outcome 1 Total control of seizures.

Comparison 1 Propofol versus thiopental sodium, Outcome 2 In‐hospital mortality.

Comparison 1 Propofol versus thiopental sodium, Outcome 3 Adverse events.
| Study | Propofol group | Thiopentone sodium group |
| Rossetti 2011 | Median: 4 days | Median: 17 days |
| Rossetti 2011 | Range: 2 to 28 days | Range: 5 to 70 days |
Comparison 1 Propofol versus thiopental sodium, Outcome 4 Duration of mechanical ventilation.

Comparison 1 Propofol versus thiopental sodium, Outcome 5 Long‐term outcomes.
| Propofol compared to Thiopental sodium for the treatment of refractory status epilepticus | ||||||
| Patient or population: patients with the treatment of refractory status epilepticus | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Thiopental sodium | Propofol | |||||
| Total control of seizures | Study population | RR 1.5 | 21 | ⊕⊕⊝⊝ | ||
| 286 per 1000 | 429 per 1000 | |||||
| In‐hospital mortality | Study population | RR 1.5 | 21 | ⊕⊕⊝⊝ | ||
| 143 per 1000 | 214 per 1000 | |||||
| Length of intensive care unit (ICU) stay | Not reported | Not reported | NA | NA | NA | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Single blinded study: we downgraded one level for risk of bias | ||||||
| Study ID | Interventions | Screened (n) | Randomised (n) | Safety analysis (n) | ITT (n) | Finishing study (n) | [%] of randomised participants |
| I1 Propofol I2 Barbiturate (thiopental (n = 7) and pentobarbital (n = 3)) | I1 14 I2 10 | I1 14 I2 10 | I1 14 I2 10 | I1 14 I2 10 | I1 14 I2 9 | I1 100 I2 90 | |
| I1: intervention 1; I2: intervention 2; ITT: intention‐to‐treat; n: number. | |||||||
| Characteristic | |
| I1 I2 | Propofol Thiopental |
| Participants who died (n) Epilepsy‐related I1 Propofol I2 Thiopental | 0 0 |
| Participants who died (n) All causes I1 Propofol I2 Thiopental | 3 1 |
| Adverse events (n) I1 Propofol I2 Thiopental | 14 11 |
| Serious adverse events (n) I1 Propofol I2 Thiopental | 1 1 |
| Duration of ICU stay | Not reported |
| Duration of mechanical ventilation (median (range)) I1 Propofol I2 Thiopental | 17 days (5 to 70 days) 4 days (2 to 28 days) |
| Duration of hospitalisation | Not reported |
| Neurological deficits | Not reported |
| Cognitive deficits | Not reported |
| Haematological toxicity | Not reported |
| Liver toxicity | Not reported |
| Hypersensitivity or drug allergy | Not reported |
| Bronchopneumonia | Not reported |
| Other side effects | Not reported |
| I1: intervention 1; I2: intervention 2; ICU: intensive care unit; n: number. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total control of seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 In‐hospital mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infection | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.41] |
| 3.2 Hypotension | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.38, 2.00] |
| 3.3 Other serious complications | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 6.86] |
| 4 Duration of mechanical ventilation Show forest plot | Other data | No numeric data | ||
| 5 Long‐term outcomes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

