Intervenciones para aumentar la adherencia a los fármacos para la dependencia del tabaco
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: Randomised controlled trial Country: Hong Kong, China Recruitment methods: Mass media publicity and referrals from hospitals/clinics and physicians Setting: No information other than a non‐clinical setting | |
| Participants | Inclusion criteria: Male; Chinese; 18+ years old; Self‐reported erectile dysfunction; Smoked at least 1 cigarette per day; Intended to quit smoking within 7 days of first contact; Willing to use NRT; Not following any other smoking cessation regime Exclusion criteria: Psychologically or physically unable to communicate; Taking regular psychotropic medications; Serious health problems preventing use of NRT Participants randomised: 501 participants in eligible groups (mean age = 48.8 years (s.d.=11.5); 0% female; 100% Chinese) | |
| Interventions | Aim of intervention: To increase adherence to NRT and smoking cessation Nature of intervention: Additional counselling component focused on medication adherence, delivered by trained male counsellor. Patient centred approach, utilising motivational interviewing techniques and the 4R approach. The NRT adherence intervention was developed from WHO guidelines on adherence interventions which emphasize the importance of adhering to the prescribed dosage, assessed and discussed ways to overcome barriers and delivered problem‐oriented interventions to improve adherence. Participants received 15 minutes face‐to‐face smoking cessation counselling and 3 minutes NRT adherence counselling, plus 1 week of free NRT (gum or patch) at first contact. They were tested for carbon monoxide (CO) and given a self‐help quitting pamphlet. They also received a telephone hotline number of a counsellor. There was further counselling and CO testing at 1 week and 4 weeks, plus 1 week of NRT at 1 week. At 1 week, NRT usage was checked and additional adherence counselling was given. At 4 weeks NRT usage was checked and additional counselling given as needed. Nature of control: The control group received the same content apart from the NRT adherence counselling at baseline and the NRT checking and adherence counselling at week 1. | |
| Outcomes | Primary adherence outcome (dichotomous data): Continuous use of NRT for 4 weeks, assessed at 3 months (ITT data). Checked by self‐report via telephone contact and possibly pill counts of medication also used, although procedure unclear. Other adherence outcomes: 8‐week NRT adherence rate at 3 months. Checked by telephone call at 3 months. This outcome relates to adherence beyond the treatment period with no NRT being supplied. Secondary outcomes: Self‐reported 7‐day point prevalent abstinence, assessed at 6 months; Biochemically validated quit rate, assessed at 6 months (selected as abstinence outcome by review authors); Self‐reported reduction (≥50%) in cigarette consumption, assessed at 6 months | |
| Notes | An additional 218 participants were randomised to a third arm which was ineligible for this review. Abstinence outcome not reported by arm and so not useable data in report. Study authors were contacted and supplied data 4/2014, confirming that the biochemically validated quit rate for group A1 and group A2 was 13.3% (33/249) and 9.5% (24/252), respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given to enable judgement beyond stating that it was randomised (pg252, para 7). |
| Allocation concealment (selection bias) | Unclear risk | No details given to enable judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details given to enable judgement. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient details of procedure to enable judgement although longer‐term follow‐up by telephone was conducted by staff blinded to assignment (pg253, para 2) |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was used and reported (pg253, para 7). There were no differences in attrition between arms (pg253, paragraph 8). |
| Selective reporting (reporting bias) | Low risk | Trial was pre‐registered ISRCTN13070778 with specified outcomes remaining consistent for the study report. |
| Validity and reliability of outcome measures | Unclear risk | Adherence outcomes may have included pill counts of medication used as well as self‐report by telephone but procedure unclear (pg253, para 1). Abstinence outcome was biochemically validated (pg253, para 3). |
| Baseline comparability | Low risk | No reported differences between arms in baseline demographic and smoking characteristics (pg253, para 7). |
| Consistency in intervention delivery | Unclear risk | No details given to enable judgement. |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
| Methods | Design: Randomised controlled trial Country: Hong Kong, China Recruitment methods: Local media publicity and by contacting previous cohorts of smokers who had cessation counselling but failed to quit Setting: No information but appears to be smoking cessation clinic. | |
| Participants | Inclusion criteria: 18+ years old; Chinese; Smoked at least 2 cigarettes per day; No intention to quit in the next 4 weeks but interested in reducing smoking; Not following any other smoking cessation regime; No contraindication to NRT Exclusion criteria: Psychologically or physically unable to communicate; Taking regular psychotropic medications; Serious health problems preventing use of NRT; Pregnant / intending to become pregnant in next 6 months Participants randomised: 928 participants in eligible groups (mean age = 41.9 years (s.d.=10.3); 19.4% female; 100% Chinese) | |
| Interventions | Aim of intervention: To increase adherence to NRT, and smoking reduction and cessation. Nature of intervention: Additional counselling component focused on medication adherence, delivered by trained smoking cessation counsellor. Patient centred approach, utilising motivational interviewing techniques and the 5R approach. The NRT adherence intervention was developed from WHO guidelines on adherence interventions which emphasise the importance of adhering to the prescribed dosage, assessed and discussed ways to overcome barriers and delivered problem‐oriented interventions to improve adherence. Participants received 15 minutes face‐to‐face smoking reduction intervention, including information on the health consequences of smoking and counselling emphasising achieving the goal of cessation by focusing on reduction before quitting, highlighting how reduction is effective when quitting is difficult and how to reduce their smoking. They also received 3 minutes NRT adherence counselling plus 1 week of free NRT (gum or patch) at first contact. They were tested for carbon monoxide (CO) and given a self‐help quitting pamphlet. There was further smoking reduction and adherence counselling and CO testing at 1 week, plus administration of a further 3 weeks of NRT. NRT usage was also checked. At 4 weeks, participants received a similar intervention as at 1 week. Nature of control: The control group received the same content apart from the NRT adherence counselling at baseline, week 1 and week 4. | |
| Outcomes | Primary adherence outcome (dichotomous data): Continuous use of NRT over 8 weeks, assessed at 3 months (ITT data). Checked by self‐report via telephone contact but possibly also by pill counts and procedure not clear. Other adherence outcomes: Continuous use of NRT over 4 weeks, assessed at 3 months. Secondary outcomes: Self‐reported 7‐day point prevalent abstinence, assessed at 6 months; Biochemically validated quit rate, assessed at 6 months (selected as abstinence outcome by review authors); Self‐reported 7‐day point prevalent abstinence, assessed at 3 months; Self‐reported reduction (≥50%) in cigarette consumption, assessed at 6 months | |
| Notes | An additional 226 participants were randomised to a third arm which was ineligible for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random numbers generated by a computer prior to participant recruitment (pg1156, para 6) |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was determined by a research assistant not conducting the intervention. Assignment was by opening sealed, opaque envelopes and followed informed consent (pg1156, para 6) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Counsellors were inevitably not blind to the intervention but it is not clear that this is likely to influence the treatment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Research assistants contacting participants at follow‐up were blinded to arm allocation (pg1157, para 2) but it is not clear that this was the only means by which the primary outcome was assessed. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was used and reported with non‐respondents at follow‐up treated conservatively as non‐adherent and continuing smokers (pg1158, para 1). There were no differences in attrition between arms (pg1158, para 2). |
| Selective reporting (reporting bias) | Low risk | Trial was pre‐registered ISRCTN05172176 with specified outcomes remaining consistent for the study report. |
| Validity and reliability of outcome measures | Unclear risk | Adherence outcome was seemingly checked by self‐report but combination of pill counts of medication used and self‐report may have been used and procedure not clear (pg1156, paragraph 6; pg1157 para 2). Abstinence outcome was biochemically validated (pg1157, para 5). |
| Baseline comparability | Unclear risk | Reported difference between arms in baseline CO level and it is not mentioned if this is adjusted for in the analysis (pg253, para 7). |
| Consistency in intervention delivery | Low risk | Some sessions conducted by each of the counsellors were recorded and validated by an experienced nurse supervisor. |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
| Methods | Design: Randomised controlled trial Country: UK Recruitment methods: Participants were recruited through NHS primary care practices. Smokers were identified through practice registers and sent a letter offering assistance to quit and an invitation to participate in the trial. Setting: Smoking cessation clinics in primary care | |
| Participants | Inclusion criteria: Smoking at least 10 cigarettes per day; Wanting to quit smoking; 18+ years old Exclusion criteria: None stated Participants randomised: 633 participants (mean age = 47.3 years (s.d.=13.3); 54.3% female; 90.2% white | |
| Interventions | Aim of intervention: To increase adherence to NRT by informing participants that their oral dose is tailored based on an analysis of their genotype, rather than their phenotype (FTND score) Nature of intervention: Communicating different means of tailoring prescribed medication, delivered by trained research nurses. Behavioural support (based on withdrawal orientated therapy) and nicotine patches were provided (with the patch dose tailored in relation to cigarettes per day) to all participants. Participants were also prescribed an oral NRT product of their choice. The dose of oral NRT in the intervention arm was tailored based on gene variant. Participants were given both forms of NRT one day pre‐quit and told the basis for their dosage. They were also provided with a personalised booklet and an appointment card documenting the dose of NRT to use daily and giving the reason for the dose. The rationale for the dose was reiterated at each subsequent clinic. Behavioural support was offered twice prior to quit day, weekly afterwards for 4 weeks and then at 8 weeks. The quit day was set two weeks and a day after baseline. Support sessions lasted 10‐30 minutes, depending on progress and stage of quit attempt. Nature of control: The control group received the same content apart from the dose of oral NRT and the corresponding communication of the rationale was tailored based on FTND score. | |
| Outcomes | Primary adherence outcome (continuous data): Proportion of all prescribed NRT taken over 28 days, assessed at 28 days of treatment period (ITT data). Checked by pill counts of medication used. Other adherence outcomes: Proportion of all prescribed NRT taken over 7 days; Proportion of participants showing no use of NRT; Proportion of participants showing use of NRT beyond 28 days Secondary outcomes: Biochemically validated prolonged abstinence at 28 days; Biochemically validated prolonged abstinence at 6 months; Anxiety assessed using the short‐form Spielberger State‐Trait Anxiety Inventory (STAI‐6) | |
| Notes | Phenotype arm is regarded as control arm as it is more similar to standard care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer generated (pg4, para 2) |
| Allocation concealment (selection bias) | Low risk | Allocation was conducted from a central isolated location, separate from trial co‐ordination and participant recruitment (pg4, para 2). The randomisation sequence was revealed sequentially and concealed from the trial team, nurses and participants (pg4, para 3). |
| Blinding of participants and personnel (performance bias) | Unclear risk | After assignment, nurses were inevitably not blind to the intervention but it is not clear that this is likely to influence the treatment (pg4, para 3) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors for primary outcome were not blinded, but because pill counts were used it is unclear if this constitutes a clear risk of bias. Outcome assessors for longer‐term follow‐up were blinded to allocation (pg4, para 3) |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was used and reported with non‐respondents at follow‐up treated conservatively as non‐adherent and continuing smokers (pg4, para 11). There were no differences in attrition between arms (pg7, para 3). |
| Selective reporting (reporting bias) | Low risk | Study was pre‐registered including specified outcomes and these were unchanged in study report (ISRCTN14352545). This is also clear in a published protocol. |
| Validity and reliability of outcome measures | Low risk | Primary adherence outcome was checked by pill counts of medication used (pg3, para 5). Abstinence outcomes were biochemically validated (pg3, para 13). |
| Baseline comparability | Low risk | No reported differences between arms in baseline demographic and smoking characteristics (pg6, Table 1). |
| Consistency in intervention delivery | Low risk | A standardised script was used, detailed in the published protocol (pg3, para 3) |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
| Methods | Design: Randomised controlled trial Country: USA Recruitment methods: Recruited from community via radio, newspaper and handbill advertisements Setting: Research clinic at tobacco research centre | |
| Participants | Inclusion criteria: Aged 18‐65; Physically healthy; Smoking 15‐50 cigarettes per day for at least one year; No untreated major mental illness; No contraindications for nicotine gum use; No concurrent use of other nicotine or tobacco products; Have experienced past nicotine withdrawal syndrome according to DSM Exclusion criteria: Pregnancy Participants randomised: 63 participants (mean age = 34.6 years (s.d.=10.9); 55.6% female; 87.3% Caucasian) | |
| Interventions | Aim of intervention: A brief low‐cost intervention to increase compliance to NRT Nature of intervention: Additional personalised feedback component focused on medication use / adherence, delivered by smoking cessation counsellors. Participants initially received a presentation on the benefits of quitting, a review of coping skills and support and encouragement. Personalised feedback was then delivered that addressed the effectiveness, safety and necessity of nicotine replacement. First, facts were presented about NRT followed by personalised feedback based on responses to three questionnaires completed at visit 1‐ the beliefs about medicines questionnaire, the attitudes about nicotine replacement questionnaire, and the perceived risks of nicotine replacement questionnaire. Tailored scripts were used to reinforce correct knowledge and pro‐medication beliefs. In contrast incorrect knowledge, negative or ambivalent positions were raised using nonconfrontational language that allowed for engagement, reflection and clarification. A clarifying statement would then be offered. The broader goal was to define the pros and cons of treatment and shift the decisional balance toward adequate use of gum. The intervention was a single session of approximately 20 minutes. Nature of control: Participants received a presentation on the benefits of quitting, a review of coping skills and support and encouragement. A smoking history section reviewed general smoking experiences. This section was intended as a 'placebo' topic with some face relevance but little probable influence on gum use. | |
| Outcomes | Primary adherence outcomes (dichotomous and continuous data): Rates of gum compliance of 12 pieces per day (for those who received medication and started the treatment phase, not ITT); Total gum use (in participants completing the treatment phase, not ITT). These two outcomes were selected as primary outcomes by review authors as most stringent dichotomous data and continuous data, respectively. Checked by pill counts of medication used. Assessed for days 1‐15. Other adherence outcomes: Daily gum use Secondary outcomes: Biochemically validated point‐prevalent abstinence at 1 week; Biochemically validated point‐prevalent abstinence at 2 weeks (selected by the review authors as most stringent and consistent with adherence outcome timepoint); Self‐reported point‐prevalent abstinence at 4,5,6 and 7 weeks; NHLBI defined abstinence at 3 and 6 weeks; Additional secondary outcome measures for which the data are not reported were as follows: Three measures of attitudes and knowledge about nicotine replacement therapy at weeks 1, 6 and 7 ‐ BMQ, ANRT‐12, PRNR; The Minnesota Nicotine Withdrawal Scale. Adverse events relating to nicotine toxicity and nicotine gum were also assessed. | |
| Notes | An additional 34 participants were randomised to an additional arm not eligible for inclusion in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given to enable judgement. |
| Allocation concealment (selection bias) | Unclear risk | No details given to enable judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Counsellors were inevitably not blind to the intervention but it is not clear that this is likely to influence the treatment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear that outcome assessors were blinded, but because pill counts were used it is unclear if this constitutes a clear risk of bias |
| Incomplete outcome data (attrition bias) | High risk | There were no significant differences in attrition over time across all three arms (pg571, para 4), but the two arms of interest had substantial and differing attrition levels over the treatment period of 31% (intervention) and 55% (control). Data reported for the primary outcome does not refer to all randomised participants and reasons for dropout are not detailed. |
| Selective reporting (reporting bias) | Unclear risk | Unable to find any trial registration or published protocol |
| Validity and reliability of outcome measures | Low risk | Primary adherence outcome was checked by pill counts of medication used (pg569, para 5). Abstinence outcomes were biochemically validated (pg570, para 3). |
| Baseline comparability | Low risk | No differences were observed at baseline (pg571, para 3). |
| Consistency in intervention delivery | Low risk | A standardised script and checklist was used (pg568 para 7) |
| Summary risk of bias | High risk | Summary risk of bias assessed as high. |
| Methods | Design: Randomised controlled trial Country: USA Recruitment methods: Not reported. Setting: Outpatient research clinic, located at a university medical centre. | |
| Participants | Inclusion criteria: Female; Aged 20‐65; Physically healthy; Smoking a minimum of 10 cigarettes per day; No current DSM‐IV Axis 1 disorder Exclusion criteria: Pregnancy / nursing; Current treatment with bupropion or other smoking cessation medication Participants randomised: 55 participants (mean age = 42.1 years (s.d.=10); 100% female; 61.8% Caucasian) | |
| Interventions | Aim of intervention: To provide feedback on medication use (using electronic Medication Event Monitoring Systems (MEMS) to increase bupropion compliance Nature of intervention: Provision of additional feedback on adherence levels, given by a CBT therapist. Following baseline assessment all participants began 7 weeks of open‐label treatment with bupropion SR (300mg) dispensed in Medication Event Monitoring bottles (containing a computer chip that records the times when bottle opening occurs). In addition, all participants received individual weekly CBT sessions for smoking cessation, focusing on identification of high risk situation for smoking, coping skills training and lapse recovery strategies. In the intervention condition the weekly CBT was increased in duration by 10 minutes a session, during which time the MEMS feedback was given in graphical form and the treatment regimen was clarified. Problem solving techniques were used to help the participant to tailor the regime to their schedule by associating medication taking with regular activities or routines. Secondly potential barriers to compliance were identified and strategies for removing barriers discussed. Third participants were encouraged to self‐monitor pill consumption on daily diaries reviewed at the next therapy session. Nature of control: As above but without the extra 10 minutes added to each session for enhanced therapy | |
| Outcomes | Primary adherence outcome (dichotomous data): Rates of full compliance i.e. two doses taken per day in an optimal schedule (ITT data). Assessed daily over 7‐week treatment period, objectively using Medication Event Monitoring bottles. Other adherence outcomes: Rates of dose compliance i.e. two doses taken per day over 7‐week treatment period. Secondary outcomes: Biochemically validated abstinence at week 6 (selected as abstinence outcome by review authors. as most consistent with adherence outcome timepoint but there is no useable data in the report); Biochemically validated abstinence at week 3 | |
| Notes | Authors contacted to attempt to obtain data for secondary abstinence outcome but no response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given to enable judgement. |
| Allocation concealment (selection bias) | Unclear risk | No details given to enable judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Therapists were inevitably not blind to the intervention but it is not clear that this is likely to influence the treatment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear that outcome assessors were blinded, but because MEMS monitoring data used it is unlikely that this constitutes a clear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | There were no differences in attrition between arms (pg878, para 2). Data reported for the primary outcome appears to refer to all randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | Unable to find any trial registration or published protocol |
| Validity and reliability of outcome measures | Low risk | Primary adherence outcome was only measured objectively using MEMS monitoring data. Abstinence biochemically validated. |
| Baseline comparability | Unclear risk | No details given to enable judgement |
| Consistency in intervention delivery | Unclear risk | No details given to enable judgement |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
| Methods | Design: Randomised controlled trial Country: USA Recruitment methods: Not detailed. Setting: Community‐based clinic serving a predominantly black population. | |
| Participants | Inclusion criteria: Black; ≥ 18 years of age; smoking >10 cpd; wanting to quit; willing to take varenicline. Exclusion criteria: Planning to move from the area within three months; had contraindications to the use of varenicline, including a cardiovascular event in the month prior to enrolment, renal impairment, taking insulin for diabetes but unwilling to closely monitor blood sugar, or history of clinically significant allergic reactions to varenicline; a major depressive disorder in the past year requiring treatment; history of alcohol or drug dependency in the past year; history of psychosis, panic disorder, bipolar disorder, or any eating disorders; current breast feeding, pregnancy, or plans to get pregnant in the next three months. Participants randomised: 72 participants (mean age = 46.8 years (SD=11.3); 62.5% female; 100% black) | |
| Interventions | Aim of intervention: To improve varenicline use. Nature of intervention: The intervention arm received standard components which were also received by the control arm, plus additional adherence support counselling. These were delivered by study counsellors although their disciplinary backgrounds/training are not detailed. The standard components comprised i) A culturally targeted quit smoking guide addressing the health consequences of smoking, benefits of quitting, and strategies to promote abstinence; ii) a one‐month supply of varenicline in a monthly pill box. Participants were verbally instructed on how to take the medication. Participants were encouraged to initiate varenicline on Day 1, set a quit date on Day 8 and to not smoke cigarettes during the 3‐month treatment phase. Participants returned to the clinic at the end of months 1 and 2 for medication refills; iii) Standard counselling: All participants met with a study counsellor during the randomisation visit to develop a plan for quitting on day 8. Counsellors followed semi‐structured scripts to provide information about the risks of continued smoking, benefits of quitting, discuss strategies for coping with withdrawal and assist participants in developing a quit plan. The additional adherence support counselling comprised five additional counselling sessions on days 8, 12, 20, 30, and 60 of the treatment period. Using the Information‐Motivation‐Behavioural skills model of adherence behaviour change, counsellors provided information to enhance participants’ motivation in their ability to take the medication as prescribed (e.g., consequences of adherence/nonadherence) and behavioural skills for managing side effects (e.g., nausea) and remembering to take their medication (e.g., timing doses with daily activities). Nature of control: The control arm received the three standard components only. | |
| Outcomes | Primary adherence outcome (continuous data): Percentage of prescribed varenicline doses taken at three months (for those remaining engaged to provide data). Assessed during monthly medication refill clinic visits by research staff with pill counts. Other adherence outcomes: Percentage of prescribed varenicline doses taken at one month; Percentage of prescribed varenicline doses at two months Secondary outcomes: Biochemically validated 7‐day point‐prevalence abstinence at three months, verified by salivary cotinine (selected as abstinence outcome by review authors as most consistent with adherence outcome timepoint); biochemically validated 7‐day point‐prevalence abstinence at one month, verified by CO; biochemically validated 7‐day point‐prevalence abstinence at two months, verified by CO. Reduction in self‐reported cigarettes per day from baseline, assessed at three months. Adverse events were assessed. | |
| Notes | Participants numbers per arm are not given for primary outcome in published paper. We contacted the authors for clarification and they confirmed that n=29 for control arm, and n=32 for intervention arm (8/2014). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of allocation sequence not detailed. |
| Allocation concealment (selection bias) | Low risk | Allocation was determined by drawing a sealed envelope with preassigned randomisation numbers, at the randomisation visit (pg869, para 4) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Counsellors were inevitably not blind to the intervention but it is not clear that this is likely to influence the treatment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear that outcome assessors were blinded, but because pill counts were used it is unclear if this constitutes a clear risk of bias. |
| Incomplete outcome data (attrition bias) | Unclear risk | The overall level of attrition is moderate across the treatment period (15‐21%) but reasons for dropout are not detailed. No reported differences in attrition by arm (pg870, Results para 1). Data reported for the primary outcome does not appear to refer to all randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | Unable to find any trial registration or published protocol |
| Validity and reliability of outcome measures | Low risk | Primary adherence outcome was by pill counts. Abstinence was biochemically validated. |
| Baseline comparability | Low risk | No significant differences at baseline were reported |
| Consistency in intervention delivery | Low risk | Standard counselling was delivered according to semi‐structured scripts. Adherence counselling was delivered based on a model of adherence behaviour change. All counselling sessions were audiotaped and integrity of protocols was checked by weekly supervision of audiotaped sessions. |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
| Methods | Design: Randomised controlled trial Country: USA Recruitment methods: Advertisements in local papers and radio announcements. Setting: Outpatient research clinic, located at a university medical centre. | |
| Participants | Inclusion criteria: English‐speaking; Female; Aged 30‐70; Physically healthy; Smoking a minimum of 10 cigarettes per day Exclusion criteria: Dependence on other substances; Evidence of psychotic, depressive or anxiety disorders; Pregnancy / nursing; Serious medical problems Participants randomised: 97 participants (mean age = 49 (s.d.=9.9); 100% female; 72% Caucasian) | |
| Interventions | Aim of intervention: To determine whether pill taking instructions and personalised feedback using MEMS (Medication Event Monitoring System) enhances bupropion compliance Nature of intervention: Provision of additional feedback on adherence levels, given by a clinic nurse. Participants received written and verbal instructions on proper administration of bupropion. All doses were administered in MEMS bottles (containing a computer chip that records the times when bottle opening occurs) in the morning and one in the evening with at least 8hrs (but not more than 12hr) between. Participants in the intervention group were told about the recording device in the bottle cap ‐ specifically that the cap would record the time and date that they took the medication. MEMS feedback was given in graphical form weekly with repeated instructions to increase compliance and a check of side effects. Feedback sessions lasted approximately 5‐10 mins. The treatment regime was 7 weeks in duration with weekly counselling visits. Nature of control: Participants did not receive any particular information, direction or feedback beyond the standard dosing instructions. Participants met briefly with nurse for a weekly check of side effects. The control arm was designed to typify usual care in a medical setting. | |
| Outcomes | Primary adherence outcome (dichotomous data): Rates of full compliance i.e. two doses taken per day in an optimal schedule (ITT data). Assessed daily over 7‐week treatment period, objectively using Medication Event Monitoring bottles. Other adherence outcomes: Rates of dose compliance i.e. two doses taken per day over 7‐week treatment period. Secondary outcomes: None reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given to enable judgement. |
| Allocation concealment (selection bias) | Unclear risk | No details given to enable judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Nurses were inevitably not blind to the intervention but it is not clear that this is likely to influence the treatment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear that outcome assessors were blinded, but because MEMS monitoring data used it is unlikely that this constitutes a clear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | There were no differences in attrition between arms (pg142, para 7). We assume that data reported refers to all randomised participants (given wording used and consistent with reported degrees of freedom for F‐tests). |
| Selective reporting (reporting bias) | Unclear risk | Unable to find any trial registration or published protocol |
| Validity and reliability of outcome measures | Low risk | Primary adherence outcome was only measured objectively using MEMS monitoring data. Abstinence biochemically validated |
| Baseline comparability | Low risk | No reported differences between arms in baseline demographic and smoking characteristics (pg142, para 5). |
| Consistency in intervention delivery | Unclear risk | No details given to enable judgement |
| Summary risk of bias | Unclear risk | Summary risk of bias assessed as unclear. |
| Methods | Design: Randomised controlled trial. The study was a 2 x 2 x 2 factorial design examining three manipulations, only one of which is relevant to this review. Country: USA Recruitment methods: Participants were recruited from people who called the Wisconsin Tobacco Quit Line (WTQL), who were invited to participate in the study. There was no additional advertising or targeted recruitment. Setting: Counselling intervention conducted over the telephone. | |
| Participants | Inclusion criteria: Age ≥18 years; English speaking; smoking ≥10 cigarettes/day; willing to set a quit date within the next 30 days. Exclusion criteria: Pregnant or lactating; medical contraindications for study medications (e.g., past 30 days, heart attack or stroke; past 6 months, serious or worsening angina, very rapid or irregular heartbeat requiring medication); unwillingness to use study medications Participants randomised: 987 participants (mean age = 41.9 years (s.d.=13.0); 57.6% female; 76.4% Caucasian) | |
| Interventions | Aim of intervention: To address problematic beliefs or knowledge about NRT that might adversely affect appropriate use of the pharmacotherapies. Nature of intervention: All study participants received a standard quit guide in the mail, access to recorded medication information (via phone), and access to an online cessation program maintained by the quitline. They could make ad hoc calls to the quitline for additional assistance. They received standard cessation counselling. During call 1, quitline counsellors discussed smoking history, prior quit attempts, problem‐solving and coping strategies, social support, and appropriate use of cessation medications; also, a target quit date was set during this first call. Call 2 occurred on or close to the quit date and focused on management of withdrawal symptoms, appropriate use of medications, strategies to maintain abstinence in high‐risk situations, and early relapse prevention. Calls 3 and 4 also addressed relapse prevention but counselling was tailored to address concerns and questions raised by the participant. In addition, intervention participants received medication adherence counselling (MAC) during all standard counselling calls. The MAC protocol was developed by study investigators and involved the following: (a) prequit assessment of beliefs that might undermine NRT adherence, (b) ongoing medication adherence assessment by counsellors, and (c) tailored coaching based on the ongoing assessments. Nature of control: Control participants received the standard quit materials and standard counselling only. | |
| Outcomes | Primary adherence outcome (continuous data): Self‐reported number of days of nicotine patch use in the first 2 weeks in those remaining engaged at this timepoint (this was the most relevant outcome given the factorial design because all participants irrespective of randomised arm received nicotine patches for at least 2 weeks). Other adherence outcomes: Self‐reported number of days of gum use in the first 2 weeks; Self‐reported number of weeks of nicotine patch use in the first 6 weeks; Self‐reported number of weeks of gum use in the first 6 weeks. Secondary outcomes; 30‐day PPA at 6 weeks postquit (selected as timepoint most relevant to adherence outcome), 30‐day PPA at 12 weeks postquit, 30‐day PPA at 26 weeks postquit (selected as longest timepoint). 7‐day PPA at 2 weeks postquit; 7‐day PPA at 6 weeks postquit; 7‐day PPA at 12 weeks postquit; 7‐day PPA at 26 weeks postquit. Abstinence outcomes were assessed by self‐report. | |
| Notes | The study uses a factorial design in order to examine the effect of three different enhancements to quitline treatment: i) patch only versus combination (patch plus oral) nicotine replacement therapy (NRT); ii) shorter versus longer duration of NRT; iii) standard counselling versus counselling to increase NRT adherence. We are only interested in the effect of the latter, with data for this comparison collapsing the other factor conditions. Study authors contacted and responded 8/2014 in seeking exact number of participants by arm for primary outcome. Their response indicated that there were 386 participants in the standard counselling group and 413 participants in the adherence counselling group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to the eight treatment combinations via a list of randomised numbers generated by SAS Proc Plan (SAS Institute Inc., Cary, NC) (pg719) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details to determine that allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Counsellors were responsible for randomisation and subsequent pre‐quit counselling and so were inevitably not blinded to condition (pg719, para 7), but it is not clear that this would influence the treatment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome data were collected by university‐based research staff not affiliated with the quitline, but it is unclear if they were blinded (pg720, para 5). |
| Incomplete outcome data (attrition bias) | Low risk | The level of attrition is moderate (18‐20%) and not different between arms (pg721, para 5) and reasons for dropout are given. Not intention to treat (pg720, Analysis plan and statistical methods para 1). |
| Selective reporting (reporting bias) | Low risk | Study was pre‐registered including specified primary outcomes and these were unchanged in study report (NCT01087905). |
| Validity and reliability of outcome measures | High risk | Primary adherence outcome was only measured by self‐report via phone. Abstinence measures were not biochemically validated. |
| Baseline comparability | Low risk | No reported differences between arms in baseline demographic and smoking characteristics (pg721, para 4). |
| Consistency in intervention delivery | Unclear risk | No details given to enable judgement although seemed to (if not clearly stated) follow a basic protocol in terms of outlining the intended focus of each call. |
| Summary risk of bias | High risk | Summary risk of bias assessed as high. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence ‐ the protocol for the behavioural support interventions "did not specify the nature of the support offered". Adherence outcome was of use / not use for specific time periods ‐ assessing "whether NRT was being used in general and not the degree of adherence". | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control include a focus on medication use | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence ‐ it is not suggested that this is an aim for the study or that the intervention is being employed to encourage increased adherence in participants | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Participants in both the intervention and control arms "recommended the use of NRT and contained information about such products and their use". | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control include a focus on medication use and for the intervention arm the component focused on medication use was one of multiple elements relating to smoking cessation | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control include a focus on medication use | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Intervention and control conditions were two different formats both "designed to provide motivation for cessation and patch use through attention to the participants’ own assessment of their reasons to quit, tools needed to quit, and goal‐setting around quitting or reducing smoking". | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. For the intervention materials the adherence component was one of multiple elements ‐ "emphasis was placed upon a range of behavioral coping mechanisms of which gum was simply one major strategy for combating urges to smoke". | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control include a focus on medication use | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control include a focus on medication use | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Intervention is seemingly focused on both smoking cessation and adherence components with smoking cessation being the primary outcome. | |
| Not an eligible study design ‐ historical cohort study | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Intervention is focused on both smoking cessation and adherence components with smoking cessation being the focus of the stated aim and the stated primary outcome. | |
| No eligible adherence outcome was assessed | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. The stated aim of the intervention is to evaluate the efficacy of tailored and untailored materials as supplements to nicotine replacement therapy. The specified primary outcomes are rates of continuous abstinence. Prompts to comply with the medication are one of multiple reported elements of the intervention. | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. Both intervention and control include a focus on medication use | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. All arms include a focus on medication use | |
| Intervention is not principally focused on increasing adherence to medications for tobacco dependence. The stated aim of the intervention was "to evaluate the efficacy of the nicotine sublingual tablet or placebo combined with either low or high behavioral support for smoking cessation in COPD patients after 6 months and 12 months" with specified primary and secondary outcomes being smoking cessation, smoking reduction and quality of life. The intervention is described as "counselling on smoking cessation... and subjects were also given take‐home material with tips on smoking cessation". Participants were "recommended to use study medication" as one of multiple reported elements of the counselling intervention but it is not reported that this is administered differentially to intervention and control arms. | |
| No eligible adherence outcome was assessed ‐ includes a measure of use vs not use of medication |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | (from abstract) "A secure web program was created to properly dose cigarette smokers to gum strength (2 vs. 4 mg) and dosing program (# of pieces/day [PPD]). The program then sends SMS text messaging to the user's cellular telephone to prompt medication use at regular intervals. We then conducted a randomised trial examining tailored text messaging (TTM) to support text messaging (STM) in 110 cigarette smokers attempting to quit smoking while using nicotine gum." |
| Participants | The sample was 53% male, 63% White, 43 + 11 years of age, and smoked 19 + 7.6 cigarettes per day (CPD). There were no differences between groups at baseline for CPD, gum dosing, and recommended PPD." |
| Interventions | Tailored text messaging (TTM) to support text messaging (STM) |
| Outcomes | Outcome variables included self‐reported seven day recalls of nicotine gum use and cigarette smoking at 7, 28, and 56 days post quit date. |
| Notes | Requires assessment of full text to confirm eligibility but only an abstract is seemingly available. Lead author unable to be contacted, although member of author team who was able to be contacted (5/2013) indicated that the study was conducted by a company and had not been written up for publication. Abstract presents results as follows: On an intent‐to‐treat basis, independent‐sample t‐tests revealed that subjects in the TTM condition reported chewing more nicotine gum than subjects in the STM condition, (6.5 PPD vs. 4.5 PPD, respectively, P=.003). No significant differences were found at 4 weeks or 8 weeks, or for cigarette use variables. |
| Methods | Design: Randomised controlled trial. Country: China. |
| Participants | Smokers willing to make a quit attempt |
| Interventions | Participants were randomly assigned to either: i) varenicline combined with a mobile phone text messaging smoking cessation programme. The programme comprised motivational messages, support for behavioural change and 'medicine attention'. ii) a control group that received varenicline only |
| Outcomes | The primary outcomes were varenicline usage for 12 weeks and self‐reported continuous smoking abstinence, biochemically verified by exhaled CO test at 3 and 6 months. |
| Notes | Only an abstract is available. It is not clear from this whether the principal focus of the intervention was on increasing adherence, although this seems unlikely from the abstract content. We have been unable to contact the authors to receive more information. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Evaluation of Treatments to Improve Smoking Cessation Medication Adherence |
| Methods | Randomised trial |
| Participants | 544 smokers |
| Interventions | Medication Duration during quit attempt; Counselling; Automated Medication Adherence Calls; Electronic Medication Monitoring Device + Feedback; Cognitive Adherence Intervention |
| Outcomes | Medication Adherence assessed for 26 weeks (depending on the condition) after the target quit day |
| Starting date | June 2010 |
| Contact information | Tanya R Schlam, PhD: [email protected] |
| Notes | Identifier: NCT01120704 |
| Trial name or title | Improving Adherence to Smoking Cessation Medication Among PLWHA (HIV) |
| Methods | Randomised trial |
| Participants | 190 smokers from HIV/AIDS clinics |
| Interventions | Standard Care (SC); SC + text message reminders; SC + text message reminders + cell phone‐delivered adherence‐focused behavioral therapy (ABT) |
| Outcomes | Adherence to varenicline and biochemically validated smoking abstinence at 12 weeks and 3‐month follow‐up from the time of study enrolment |
| Starting date | March 2013 |
| Contact information | Principal Investigator: Donna Shelley, NYU School of Medicine; Contact: Tuo‐[email protected] |
| Notes | Identifier: NCT01898195 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adherence ‐ Dichotomous outcomes Show forest plot | 5 | 1630 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.28] |
| Analysis 1.1  Comparison 1 Primary outcome (adherence), Outcome 1 Adherence ‐ Dichotomous outcomes. | ||||
| 2 Adherence ‐ Continuous outcomes Show forest plot | 4 | 1529 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.03, 0.17] |
| Analysis 1.2 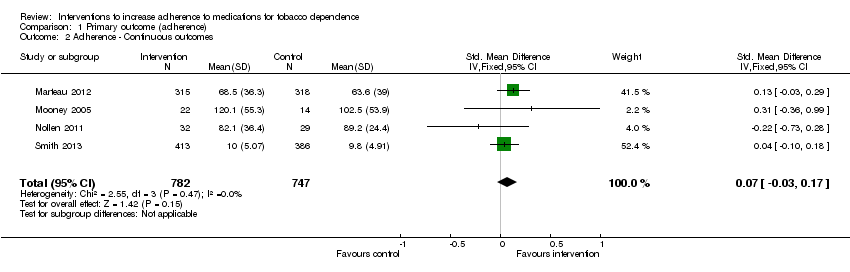 Comparison 1 Primary outcome (adherence), Outcome 2 Adherence ‐ Continuous outcomes. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term abstinence < 6 months Show forest plot | 4 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| Analysis 2.1  Comparison 2 Secondary outcomes, Outcome 1 Short‐term abstinence < 6 months. | ||||
| 2 Long‐term abstinence ≥ 6 months Show forest plot | 4 | 3049 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.01, 1.34] |
| Analysis 2.2  Comparison 2 Secondary outcomes, Outcome 2 Long‐term abstinence ≥ 6 months. | ||||
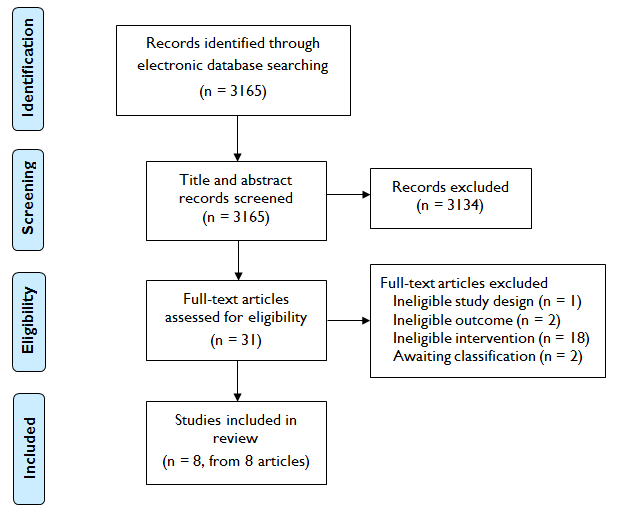
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Primary outcome (adherence), Outcome 1 Adherence ‐ Dichotomous outcomes.
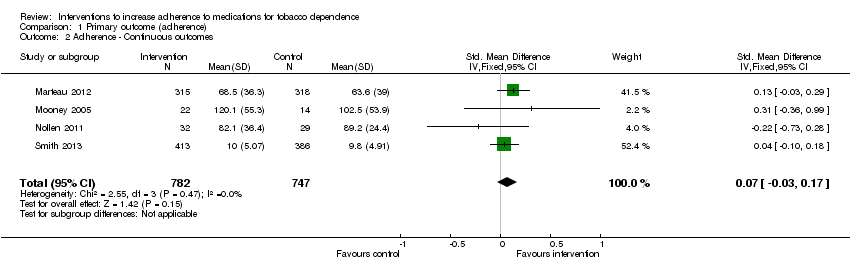
Comparison 1 Primary outcome (adherence), Outcome 2 Adherence ‐ Continuous outcomes.

Comparison 2 Secondary outcomes, Outcome 1 Short‐term abstinence < 6 months.
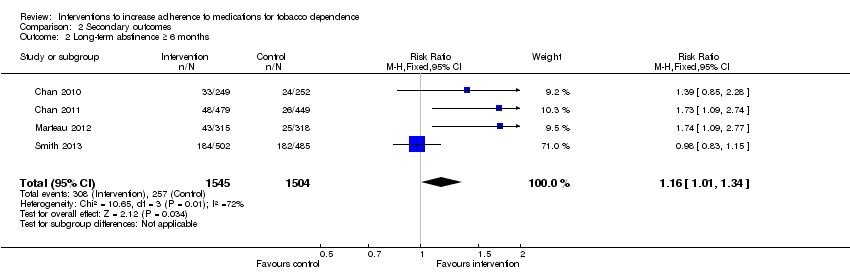
Comparison 2 Secondary outcomes, Outcome 2 Long‐term abstinence ≥ 6 months.
| Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking | |||||
| Patient or population: Adult smokers | |||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Interventions to increase adherence | ||||
| Adherence to medications for tobacco dependence (dichotomous outcomes) | RR 1.14 | Study population | 1630 | ⊕⊕⊕⊝ | |
| 368 per 1000 achieve a specified satisfactory level of adherence | 419 per 1000 (375 to 471) achieve a specified satisfactory level of adherence | ||||
| Adherence to medications for tobacco dependence (continuous outcomes) | SMD 0.07 (‐0.03 to 0.17) | The mean level of adherence is 0 | The mean level of adherence is 0.07 standard deviations higher (0.03 lower to 0.17 higher) | 1529 | ⊕⊕⊝⊝ |
| Short‐term abstinence from smoking (<6 months) | RR 1.07 | Study population | 1755 | ⊕⊕⊝⊝ | |
| 363 per 1000 achieve abstinence | 389 per 1000 (345 to 439) achieve abstinence | ||||
| Long‐term abstinence from smoking (≥6 months) | RR 1.16 | Study population | 3049 | ⊕⊕⊝⊝ | |
| 171 per 1000 achieve abstinence | 198 per 1000 (173 to 229) achieve abstinence | ||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | |||||
| GRADE Working Group grades of evidence | |||||
| 1All studies are judged to be at high or unclear risk of bias which lowers confidence in estimate of effect 2Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from very small harm to small benefit 3Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from small harm to substantial benefit 4Substantial heterogeneity with inconsistency in point estimates and limited overlap of confidence intervals | |||||
| Study | Brief description of specific intervention components intended to increase adherence* | Additional contact time relative to standard care? | Medication for which adherence was targeted |
| Chan 2010 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Chan 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Marteau 2012 | Tailored and communicated about NRT dosage using a more potent rationale (genotype versus phenotype) | No | NRT |
| Mooney 2005 | Personalised feedback of questionnaire responses regarding medication | No | NRT |
| Mooney 2007 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Nollen 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | Varenicline |
| Schmitz 2005 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Smith 2013 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| * For further details see Characteristics of Included Studies | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adherence ‐ Dichotomous outcomes Show forest plot | 5 | 1630 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.28] |
| 2 Adherence ‐ Continuous outcomes Show forest plot | 4 | 1529 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.03, 0.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term abstinence < 6 months Show forest plot | 4 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 2 Long‐term abstinence ≥ 6 months Show forest plot | 4 | 3049 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.01, 1.34] |

