Intervenciones para aumentar la adherencia a los fármacos para la dependencia del tabaco
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009164.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 23 febrero 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Tabaquismo
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Draft the protocol: All authors
Develop the search strategy: GJH, MM
Search for trials: GJH, MM, FV, AF
Obtain copies of trials: GJH
Select which studies to include: GJH, MM
Extract data from studies: GJH, MM, FV, AF, NL
Enter data into RevMan: GJH, MM
Carry out the analysis: GJH
Interpret the analysis: All authors
Draft the final review: All authors
Update the review: GJH
Sources of support
Internal sources
-
King's College London, UK.
Database access
-
University of Cambridge, UK.
Computer use, database access
External sources
-
No sources of support supplied
Declarations of interest
Gareth Hollands and Paul Aveyard are authors of one study included in this review. All other authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank Monaz Mehta (Managing Editor), Lindsay Stead (Managing Editor, who ran searches of the Tobacco Addiction Group Specialized Register), and all at the Cochrane Tobacco Addiction Group. We also greatly appreciate the input of the peer reviewers who have commented on this review throughout its development.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Aug 16 | Interventions to increase adherence to medications for tobacco dependence | Review | Gareth J Hollands, Felix Naughton, Amanda Farley, Nicola Lindson, Paul Aveyard | |
| 2015 Feb 23 | Interventions to increase adherence to medications for tobacco dependence | Review | Gareth J Hollands, Máirtín S McDermott, Nicola Lindson‐Hawley, Florian Vogt, Amanda Farley, Paul Aveyard | |
| 2011 Jun 15 | Interventions to increase adherence to medications for tobacco dependence | Protocol | Gareth J Hollands, Florian Vogt, Máirtín McDermott, Amanda C Parsons, Paul Aveyard | |
Differences between protocol and review
The criteria for eligible interventions was refined between the protocol and the review. The original primary intention of the review was to examine the effect of interventions to increase adherence where this was the clearly intended focus of those intervening. However, this primary intention was not adequately reflected in the original criteria. As such, a large number of studies of interventions that could in theory alter adherence but where this was not the researchers' intention would have been relevant for inclusion. Furthermore, this lack of clarity meant that most extant studies that featured any intervention in smokers would have to be examined at the full‐text screening stage because a clear focus on increasing adherence (which can typically be derived from the title and abstract screening process) was not necessary for consideration for inclusion.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Benzazepines [therapeutic use];
- Bupropion [therapeutic use];
- Drug Therapy, Combination [methods];
- Medication Adherence [*statistics & numerical data];
- Nicotinic Agonists [*therapeutic use];
- Nortriptyline [therapeutic use];
- Quinoxalines [therapeutic use];
- Randomized Controlled Trials as Topic;
- Smoking Cessation [*methods];
- Smoking Prevention;
- *Tobacco Use Cessation Devices;
- Tobacco Use Disorder [*drug therapy];
Medical Subject Headings Check Words
Humans;
PICO
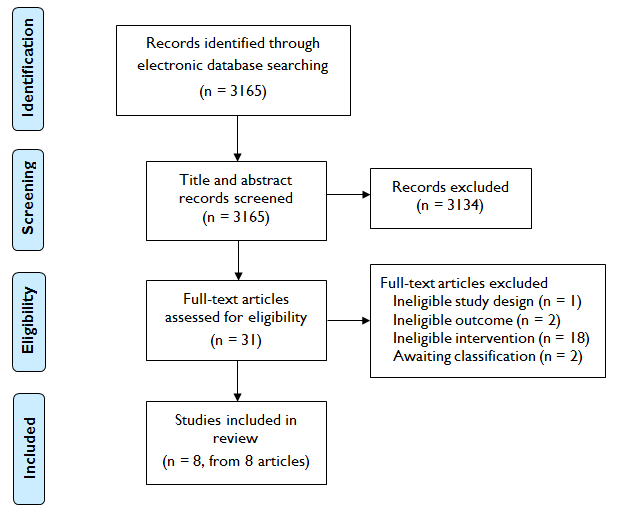
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Primary outcome (adherence), Outcome 1 Adherence ‐ Dichotomous outcomes.
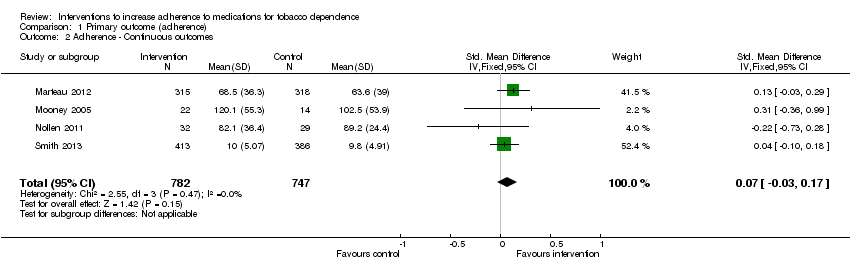
Comparison 1 Primary outcome (adherence), Outcome 2 Adherence ‐ Continuous outcomes.

Comparison 2 Secondary outcomes, Outcome 1 Short‐term abstinence < 6 months.
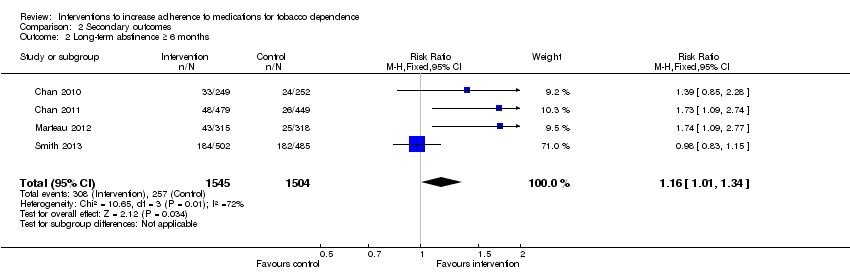
Comparison 2 Secondary outcomes, Outcome 2 Long‐term abstinence ≥ 6 months.
| Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking | |||||
| Patient or population: Adult smokers | |||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Interventions to increase adherence | ||||
| Adherence to medications for tobacco dependence (dichotomous outcomes) | RR 1.14 | Study population | 1630 | ⊕⊕⊕⊝ | |
| 368 per 1000 achieve a specified satisfactory level of adherence | 419 per 1000 (375 to 471) achieve a specified satisfactory level of adherence | ||||
| Adherence to medications for tobacco dependence (continuous outcomes) | SMD 0.07 (‐0.03 to 0.17) | The mean level of adherence is 0 | The mean level of adherence is 0.07 standard deviations higher (0.03 lower to 0.17 higher) | 1529 | ⊕⊕⊝⊝ |
| Short‐term abstinence from smoking (<6 months) | RR 1.07 | Study population | 1755 | ⊕⊕⊝⊝ | |
| 363 per 1000 achieve abstinence | 389 per 1000 (345 to 439) achieve abstinence | ||||
| Long‐term abstinence from smoking (≥6 months) | RR 1.16 | Study population | 3049 | ⊕⊕⊝⊝ | |
| 171 per 1000 achieve abstinence | 198 per 1000 (173 to 229) achieve abstinence | ||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | |||||
| GRADE Working Group grades of evidence | |||||
| 1All studies are judged to be at high or unclear risk of bias which lowers confidence in estimate of effect 2Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from very small harm to small benefit 3Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from small harm to substantial benefit 4Substantial heterogeneity with inconsistency in point estimates and limited overlap of confidence intervals | |||||
| Study | Brief description of specific intervention components intended to increase adherence* | Additional contact time relative to standard care? | Medication for which adherence was targeted |
| Chan 2010 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Chan 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Marteau 2012 | Tailored and communicated about NRT dosage using a more potent rationale (genotype versus phenotype) | No | NRT |
| Mooney 2005 | Personalised feedback of questionnaire responses regarding medication | No | NRT |
| Mooney 2007 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Nollen 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | Varenicline |
| Schmitz 2005 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Smith 2013 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| * For further details see Characteristics of Included Studies | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adherence ‐ Dichotomous outcomes Show forest plot | 5 | 1630 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.28] |
| 2 Adherence ‐ Continuous outcomes Show forest plot | 4 | 1529 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.03, 0.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term abstinence < 6 months Show forest plot | 4 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 2 Long‐term abstinence ≥ 6 months Show forest plot | 4 | 3049 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.01, 1.34] |

