Maßnahmen, um die Therapietreue bei Tabakabhängigkeit zu steigern
Appendices
Appendix 1. Taxonomy of possible interventions (adapted from Haynes 2008)
a) more instruction for patients, e.g. verbal, written, or visual material; programmed learning; and formal education sessions;
b) counselling about the patients’ target condition, the importance of therapy and compliance with therapy, the possible side‐effects, patient empowerment, couple‐focused therapy to increase social support;
c) automated telephone, computer‐assisted patient monitoring and counselling;
d) manual telephone follow‐up;
e) family intervention;
f ) various ways to increase the convenience of care, e.g. provision at the worksite or at home;
g) simplified dosing;
h) involving patients more in their care through self‐monitoring;
i) reminders, e.g. programmed devices, and tailoring the regimen to daily habits;
j) special ’reminder’ medication packaging;
k) dose‐dispensing units of medication and medication charts;
l) appointment and prescription refill reminders;
m) reinforcement or rewards for both improved adherence and treatment response, e.g. reduced frequency of visits;
n) different medication formulations, such as tablet versus syrup ;
o) crisis intervention conducted when necessary;
p) direct observation of treatments (DOTS) by health workers or family members;
q) lay health mentoring;
r) augmented pharmacy services;
s) psychological therapy, e.g. cognitive behaviour therapy, multisystemic therapy;
t) mailed communications;
u) group meetings.
Appendix 2. MEDLINE (Ovid SP) search strategy
1 exp medication adherence/ 7730
2 exp smoking cessation/ 20345
3 (adhere* or complian* or concord*).tw. 222406
4 or/1‐3 244332
5 (NRT or nicotine replacement therap* or bupropion or wellbutrin or zyban or voxra or budeprion or aplenzin or amfebutamone or varenicline or chantix or champix).tw. 5254
6 (nicotine adj7 (patch* or gum* or inhaler* or inhalator* or lozenge* or microtab* or tablet* or spray*)).tw. 2247
7 5 or 6 6925
8 randomised controlled trial.pt. 379042
9 controlled clinical trial.pt. 88839
10 clinical trial.pt. 489753
11 random*.tw. 656627
12 placebo.tw. 151719
13 trial.tw. 341515
14 groups.tw. 1279987
15 or/8‐14 2179105
16 4 and 7 and 15 1705
17 limit 16 to humans 1700
Appendix 3. Embase (Ovid SP) search strategy
1 exp medication adherence/ 4398
2 exp smoking cessation/ 37433
3 (adhere* or complian* or concord*).tw. 310641
4 or/1‐3 348019
5 (NRT or nicotine replacement therap* or bupropion or wellbutrin or zyban or voxra or budeprion or aplenzin or amfebutamone or varenicline or chantix or champix).tw. 8998
6 (nicotine adj7 (patch* or gum* or inhaler* or inhalator* or lozenge* or microtab* or tablet* or spray*)).tw. 2677
7 5 or 6 10951
8 randomised controlled trial/ 345939
9 single blind procedure/ or double blind procedure/ 131682
10 crossover procedure/ 39531
11 random*.tw. 884924
12 placebo*.tw. 199266
13 ((singl* or doubl*) adj (blind* or mask*)).tw. 157668
14 (cross over or crossover or factorial* or latin square).tw. 93570
15 (assign* or allocat* or volunteer*).tw. 488528
16 or/8‐15 1411900
17 4 and 7 and 16 1897
18 limit 17 to human 1836
Appendix 4. PsycINFO (Ovid SP) search strategy
1 exp medical regimen compliance/ 11128
2 exp smoking cessation/ 9156
3 (adhere* or complian* or concord*).tw. 46968
4 or/1‐3 58112
5 (NRT or nicotine replacement therap* or bupropion or wellbutrin or zyban or voxra or budeprion or aplenzin or amfebutamone or varenicline or chantix or champix).tw. 2854
6 (nicotine adj7 (patch* or gum* or inhaler* or inhalator* or lozenge* or microtab* or tablet* or spray*)).tw. 1221
7 5 or 6 3739
8 random*.ti,ab,hw,id. 132134
9 trial*.ti,ab,hw,id. 123964
10 placebo*.ti,ab,hw,id. 31234
11 ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id. 22030
12 (cross over or crossover or factorial* or latin square).ti,ab,hw,id. 21908
13 (assign* or allocat* or volunteer*).ti,ab,hw,id. 119014
14 treatment effectiveness evaluation/ 16887
15 mental health program evaluation/ 1870
16 exp experimental design/ 47784
17 "2000".md. 27392
18 or/8‐17 379651
19 4 and 7 and 18 1030
20 limit 19 to human 1016
Appendix 5. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 (adhere* or complian* or concord*):ti,ab,kw 24410
#2 (NRT or nicotine replacement therap* or bupropion or wellbutrin or zyban or voxra or budeprion or aplenzin or amfebutamone or varenicline or chantix or champix):ti,ab,kw or (nicotine adj7 (patch* or gum* or inhaler* or inhalator* or lozenge* or microtab* or tablet* or spray*)):ti,ab,kw 1589
#3 (#1 AND #2) in Trials 147
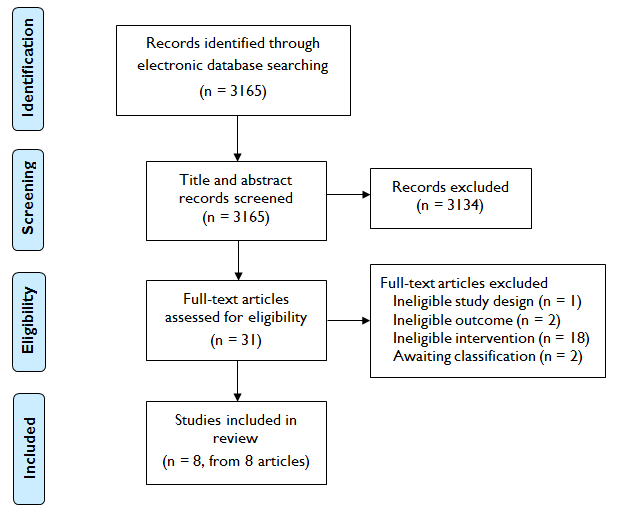
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Primary outcome (adherence), Outcome 1 Adherence ‐ Dichotomous outcomes.
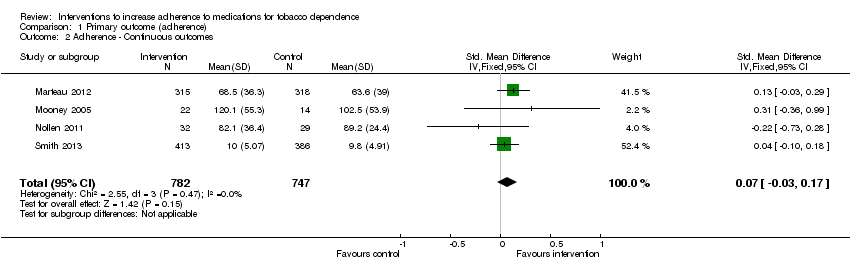
Comparison 1 Primary outcome (adherence), Outcome 2 Adherence ‐ Continuous outcomes.

Comparison 2 Secondary outcomes, Outcome 1 Short‐term abstinence < 6 months.
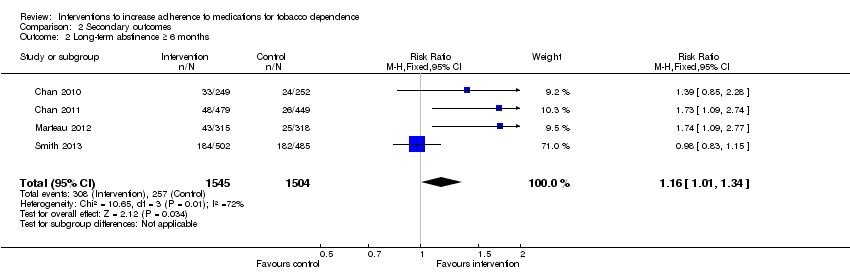
Comparison 2 Secondary outcomes, Outcome 2 Long‐term abstinence ≥ 6 months.
| Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking | |||||
| Patient or population: Adult smokers | |||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Interventions to increase adherence | ||||
| Adherence to medications for tobacco dependence (dichotomous outcomes) | RR 1.14 | Study population | 1630 | ⊕⊕⊕⊝ | |
| 368 per 1000 achieve a specified satisfactory level of adherence | 419 per 1000 (375 to 471) achieve a specified satisfactory level of adherence | ||||
| Adherence to medications for tobacco dependence (continuous outcomes) | SMD 0.07 (‐0.03 to 0.17) | The mean level of adherence is 0 | The mean level of adherence is 0.07 standard deviations higher (0.03 lower to 0.17 higher) | 1529 | ⊕⊕⊝⊝ |
| Short‐term abstinence from smoking (<6 months) | RR 1.07 | Study population | 1755 | ⊕⊕⊝⊝ | |
| 363 per 1000 achieve abstinence | 389 per 1000 (345 to 439) achieve abstinence | ||||
| Long‐term abstinence from smoking (≥6 months) | RR 1.16 | Study population | 3049 | ⊕⊕⊝⊝ | |
| 171 per 1000 achieve abstinence | 198 per 1000 (173 to 229) achieve abstinence | ||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | |||||
| GRADE Working Group grades of evidence | |||||
| 1All studies are judged to be at high or unclear risk of bias which lowers confidence in estimate of effect 2Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from very small harm to small benefit 3Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from small harm to substantial benefit 4Substantial heterogeneity with inconsistency in point estimates and limited overlap of confidence intervals | |||||
| Study | Brief description of specific intervention components intended to increase adherence* | Additional contact time relative to standard care? | Medication for which adherence was targeted |
| Chan 2010 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Chan 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Marteau 2012 | Tailored and communicated about NRT dosage using a more potent rationale (genotype versus phenotype) | No | NRT |
| Mooney 2005 | Personalised feedback of questionnaire responses regarding medication | No | NRT |
| Mooney 2007 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Nollen 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | Varenicline |
| Schmitz 2005 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Smith 2013 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| * For further details see Characteristics of Included Studies | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adherence ‐ Dichotomous outcomes Show forest plot | 5 | 1630 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.28] |
| 2 Adherence ‐ Continuous outcomes Show forest plot | 4 | 1529 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.03, 0.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term abstinence < 6 months Show forest plot | 4 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 2 Long‐term abstinence ≥ 6 months Show forest plot | 4 | 3049 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.01, 1.34] |

