سوماتریپتان (همه راههای تجویز) در مدیریت بالینی حملات میگرنی حاد در بزرگسالان ‐ بررسی اجمالی مرورهای کاکرین
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009108.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 27 mayo 2014see what's new
- Tipo:
-
- Overview
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All authors contributed to writing the protocol. CD collated data from the individual reviews and entered it into RevMan. SD checked the data. All authors were involved with writing the full review.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
Institutional support
External sources
-
Lifting The Burden: the Global Campaign against Headache, UK.
Funding for administrative costs associated with editorial review of the protocol
-
International Headache Society, UK.
Funding for administrative costs associated with editorial and peer review of the full review
Declarations of interest
The authors of this overview are also the authors of the four included individual reviews.
SD and RAM have received research support from charities, government, and industry sources at various times. RAM has consulted for various pharmaceutical companies and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. CD has no interests to declare. Support for this review came from the Oxford Pain Relief Trust.
Acknowledgements
We would like to acknowledge the helpful comments and editorial expertise of Timothy Steiner, Douglas McCory, and Rebecca Gray.
Lifting The Burden: the Global Campaign against Headache and the International Headache Society provided financial support for the editorial process (see Sources of support).
Version history
| Published | Title | Stage | Authors | Version |
| 2014 May 27 | Sumatriptan (all routes of administration) for acute migraine attacks in adults ‐ overview of Cochrane reviews | Review | Christopher J Derry, Sheena Derry, R Andrew Moore | |
| 2011 May 11 | Sumatriptan (all routes of administration) for acute migraine attacks in adults: an overview of Cochrane reviews | Protocol | R Andrew Moore, Christopher J Derry, Sheena Derry | |
Notes
This overview is part of a series on sumatriptan for acute migraine attacks in adults (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d) which replaces an earlier Cochrane review of oral sumatriptan (McCrory 2003).
At December 2016, this overview has been stabilised following discussion with the authors and editors. If appropriate, we will update the overview if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
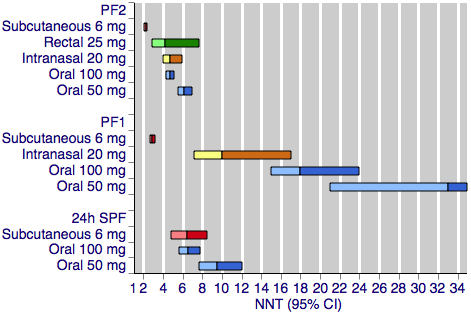
Sumatriptan versus placebo. Calculated NNTs for a pain‐free response after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
PF2: pain‐free at two hours; PF1: pain‐free at one hour; 24h SPF: 24‐hour sustained pain‐free.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.

Sumatriptan versus placebo. Calculated NNTs for headache relief after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
HR2 (headache relief at two hours); HR1 (headache relief at one hour); 24h SHR (24‐hour sustained headache relief).
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.
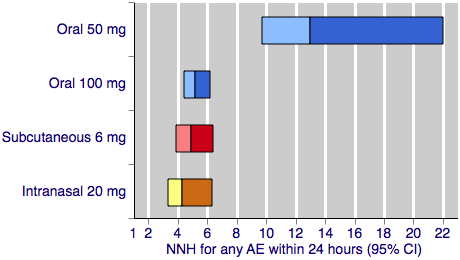
Sumatriptan versus placebo. Calculated NNHs for any adverse event within 24 hours of dosing, in participants treating moderate or severe migraine pain. Results for four of the five most commonly used dose and route of administration combinations (adverse event information for rectal sumatriptan not available), listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, and intranasal doses are shown with yellow bars.
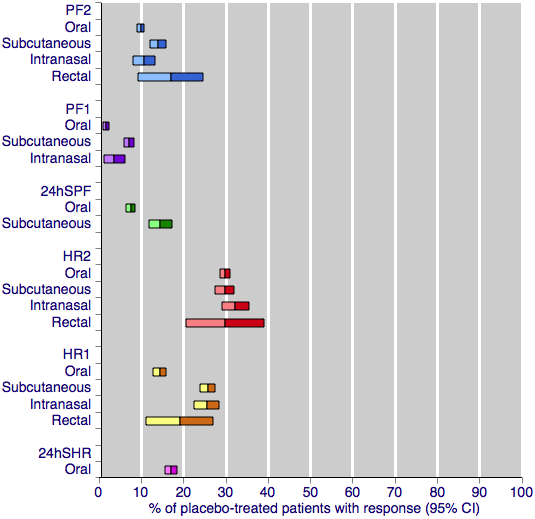
Placebo response rates for the primary efficacy outcomes, by route of administration. Response rates of each outcome are grouped by colour to facilitate comparison between different routes of administration. Proportion of placebo‐treated participants pain‐free at two hours are shown with blue bars, pain‐free at one hour with purple bars, 24‐h sustained pain‐free with green bars, headache relief at two hours with red bars, headache relief at one hour with yellow bars, and 24‐h sustained headache relief with a pink bar.

Sumatriptan versus placebo. Calculated NNTs for relief of migraine‐associated symptoms and functional disability after two hours, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.

