سوماتریپتان (همه راههای تجویز) در مدیریت بالینی حملات میگرنی حاد در بزرگسالان ‐ بررسی اجمالی مرورهای کاکرین
Appendices
Appendix 1. Additional data from placebo‐controlled studies
Use of rescue, or additional, medication
Rescue medication (usually a different analgesic, or in some studies a second dose of test medication) was available to participants whose symptoms were not adequately controlled in the vast majority of studies included in the four reviews. Participants were asked to wait, usually for two hours, before taking rescue medication in order to give the test medication enough time to have an effect. Ideally, the number of participants requiring rescue medication because of failure of the initial dose of test medication should be recorded soon after the first primary efficacy time point (two hours) (Tfelt‐Hansen 2012). Delay beyond six hours in recording this outcome risks conflating the use of rescue medication and treatment of recurrence of the headache. In practice, most of the studies recorded it at 24 hours, without always clearly differentiating between primary failure of the test medication and recurrence following initial response. Despite this shortcoming, we felt that use of additional medication within 24 hours remained a useful measure of treatment failure if one considers treatment success to be adequate pain relief that is sustained for 24 hours. Use of rescue medication at or after a defined time point is, therefore, a useful measure of treatment failure (lack of efficacy).
Pooled analyses were performed on five doses, route of administration, and baseline pain severity combinations for which sufficient data were available (Summary of results H). Four treatments were administered to participants with moderate or severe baseline pain, while one (oral 50 mg) was specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results H: Use of additional medication within 24 hours of dosing in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNTp (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 4 | 2079 | 266/1339 | 309/740 | 20 | 42 | 0.77 (0.68 to 0.87) | 4.6 (3.8 to 5.6) |
| Oral | 100 | 6 | 2810 | 621/1877 | 543/933 | 33 | 58 | 0.57 (0.52 to 0.62) | 4.0 (3.5 to 4.7) |
| Subcutaneous | 6 | 5 | 987 | 168/621 | 176/366 | 27 | 48 | 0.52 (0.45 to 0.60) | 4.8 (3.7 to 6.7) |
| Intranasal | 20 | 2 | 642 | 136/422 | 108/220 | 32 | 49 | 0.66 (0.55 to 0.79) | 5.9 (4.0 to 11) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 2 | 384 | 66/221 | 94/163 | 30 | 58 | 0.54 (0.43 to 0.69) | 3.6 (2.7 to 5.5) |
All dose and route combinations of sumatriptan resulted in significantly fewer participants needing additional medication than after placebo. When baseline pain was moderate or severe, calculated NNTps ranged from 12 with the lowest dose of intranasal sumatriptan, to 4 with the highest dose of oral sumatriptan. The proportion of participants requiring additional medication ranged from 20% to 33% with sumatriptan, compared with 42% to 58% with placebo. For both the oral and intranasal routes of administration, where more than one dose was analysed, the higher dose appeared to produce a lower (better) NNTp. The significant overlap between the 95% confidence intervals does not suggest any clinically important dose response relationship.
The 50 mg dose of oral sumatriptan administered to participants with mild baseline pain did not result in a significantly different NNTp when compared with the same dose administered to participants with moderate or severe pain. The calculated NNTp was 3.6 after treatment of mild pain, compared with 4.6 after treatment of moderate or severe pain.
Relief of headache‐associated symptoms
In addition to relief of headache pain, relief of headache‐associated symptoms is an important part of any anti‐migraine treatment. The majority of studies do not comment on the severity of associated symptoms, and relief is therefore defined as the complete resolution of any symptom present at baseline by a defined time after administration. Since it is common for individual migraine sufferers to regularly experience the same associated symptom(s), while others do not, we have chosen to express the proportion of participants experiencing relief as a fraction of participants with the symptom at baseline rather than as a fraction of the total treated population. This increases the relevance of the information to those patients who regularly suffer from associated symptoms.
Nausea
Pooled analyses were performed on eight dose, route of administration, and baseline pain severity combinations for which sufficient data were available (Summary of results I). Six treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results I: Relief of nausea within two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 4 | 550 | 172/357 | 66/193 | 48 | 34 | 1.5 (1.2 to 1.9) | 7.2 (4.5 to 18) |
| Oral | 50 | 7 | 973 | 268/596 | 123/377 | 45 | 33 | 1.4 (1.2 to 1.7) | 8.1 (5.4 to 16) |
| Oral | 100 | 14 | 2996 | 880/1955 | 317/1041 | 45 | 30 | 1.5 (1.4 to 1.7) | 6.9 (5.5 to 9.1) |
| Subcutaneous | 6 | 5 | 667 | 276/364 | 103/303 | 76 | 34 | 2.2 (1.9 to 2.6) | 2.4 (2.1 to 2.9) |
| Intranasal | 5 | 2 | 476 | 140/294 | 58/182 | 48 | 32 | 1.5 (1.2 to 1.9) | 6.4 (4.1 to 15) |
| Intranasal | 20 | 5 | 1272 | 484/825 | 153/447 | 59 | 34 | 1.7 (1.5 to 2.0) | 4.1 (3.3 to 5.3) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 3 | 280 | 78/145 | 10/135 | 54 | 7 | 6.9 (3.8 to 13) | 2.2 (1.8 to 2.7) |
| Oral | 100 | 3 | 265 | 58/130 | 10/135 | 45 | 7 | 5.9 (3.2 to 11) | 2.7 (2.1 to 3.6) |
Figure 5 shows the calculated NNTs for relief of nausea at two hours for four of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for rectal 25 mg for this outcome).
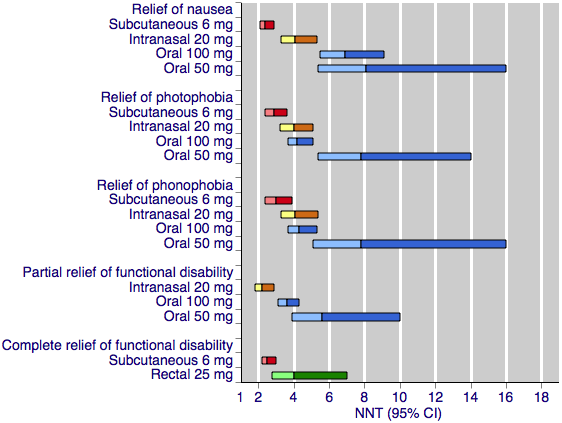
Sumatriptan versus placebo. Calculated NNTs for relief of migraine‐associated symptoms and functional disability after two hours, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.
All dose and route combinations provided superior relief of nausea compared with placebo. For participants with moderate or severe baseline pain, calculated NNTs were about 7 to 8 for the oral doses, 4 to 6 for the intranasal doses, and 2.4 for the subcutaneous dose. The proportion of participants with relief of nausea within two hours after oral sumatriptan was about 45% to 50%, about 50% to 60% after intranasal sumatriptan, and 76% after subcutaneous sumatriptan. Placebo response rates were consistently around 30% to 35% across all routes of administration.
The two doses of oral sumatriptan administered to participants with mild baseline pain also provided significant relief of nausea. Calculated NNTs were 2.2 and 2.7 for the 50 and 100 mg doses respectively. The proportion of participants with relief of nausea after treatment with sumatriptan was similar to that seen after sumatriptan treatment in participants with moderate or severe baseline pain; however, the proportion of placebo‐treated participants reporting relief of nausea was much lower amongst participants treating mild baseline pain. We did not perform statistical comparisons between the treatment effects in mild and moderate or severe baseline pain for relief of associated symptoms due to important differences between the two groups of participants. Participants treating mild baseline pain are less likely to have headache‐associated symptoms before treatment, and this significant difference in baseline incidence is likely to affect the relief obtained by these participants. In addition, any associated symptoms experienced by participants treating mild baseline pain are likely to be less severe than those experienced by participants treating moderate or severe attacks. Since we do not take into consideration the severity of symptoms when calculating relief, it is not meaningful to compare the relief in these two very different starting populations.
Photophobia
Pooled analyses were performed on seven dose, route of administration, and baseline pain intensity combinations for which sufficient data were available (Summary of results J). Five treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results J: Relief of photophobia within two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 3 | 411 | 97/240 | 35/171 | 40 | 20 | 1.8 (1.3 to 2.5) | 5.0 (3.5 to 8.9) |
| Oral | 50 | 6 | 1144 | 284/638 | 160/506 | 45 | 32 | 1.4 (1.2 to 1.7) | 7.8 (5.4 to 14) |
| Oral | 100 | 9 | 2494 | 834/1703 | 201/791 | 49 | 25 | 1.9 (1.6 to 2.1) | 4.2 (3.7 to 5.1) |
| Subcutaneous | 6 | 3 | 631 | 245/343 | 105/288 | 71 | 36 | 1.9 (1.6 to 2.2) | 2.9 (2.4 to 3.6) |
| Intranasal | 20 | 3 | 1021 | 314/643 | 89/378 | 49 | 24 | 2.1 (1.7 to 2.5) | 4.0 (3.2 to 5.1) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 3 | 483 | 125/237 | 44/246 | 53 | 18 | 3.0 (2.2 to 4.0) | 2.9 (2.3 to 3.7) |
| Oral | 100 | 3 | 475 | 131/229 | 44/246 | 57 | 18 | 3.2 (2.4 to 4.3) | 2.5 (2.1 to 3.2) |
Figure 5 shows the calculated NNTs for relief of photophobia at two hours for four of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for rectal 25 mg for this outcome).
All dose and route combinations provided superior relief of photophobia compared with placebo. For participants with moderate or severe baseline pain, calculated NNTs were about 4 to 8 for the oral and intranasal doses, and 3 for the subcutaneous dose. The proportion of participants with relief of nausea within two hours after oral sumatriptan was about 40% to 50%, compared with about 35% to 50% after intranasal sumatriptan, and 71% after subcutaneous sumatriptan. Placebo response rates were around 20% to 35% across all routes of administration.
The two doses of oral sumatriptan administered to participants with mild baseline pain also provided significant relief of photophobia. Calculated NNTs were 2.9 and 2.5 for the 50 and 100 mg doses, respectively. About 50% to 60% of participants experienced relief of photophobia after treatment with sumatriptan compared with about 20% after treatment with placebo. As discussed previously, statistical comparisons between the treatment effects in mild and moderate or severe baseline pain were not performed for relief of headache‐associated symptoms.
Phonophobia
Pooled analyses were performed on six dose, route of administration, and baseline pain severity combinations for which sufficient data were available (Summary of results K). Four treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results K: Relief of phonophobia within two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 4 | 852 | 244/490 | 134/362 | 50 | 37 | 1.4 (1.2 to 1.6) | 7.8 (5.1 to 16) |
| Oral | 100 | 7 | 2118 | 736/1492 | 164/626 | 49 | 26 | 1.8 (1.6 to 2.1) | 4.3 (3.7 to 5.3) |
| Subcutaneous | 6 | 3 | 572 | 223/310 | 101/262 | 72 | 39 | 1.8 (1.5 to 2.2) | 3.0 (2.4 to 3.9) |
| Intranasal | 20 | 3 | 933 | 309/594 | 93/339 | 52 | 27 | 1.9 (1.6 to 2.3) | 4.1 (3.3 to 5.4) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 3 | 413 | 105/202 | 37/211 | 52 | 18 | 3.0 (2.2 to 4.2) | 2.9 (2.3 to 3.9) |
| Oral | 100 | 3 | 400 | 120/189 | 37/211 | 63 | 18 | 3.7 (2.7 to 5.1) | 2.2 (1.8 to 2.7) |
Figure 5 shows the calculated NNTs for relief of phonophobia at two hours for four of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for rectal 25 mg for this outcome).
All dose and route combinations provided superior relief of phonophobia compared with placebo. For participants with moderate or severe baseline pain, calculated NNTs were about 4 to 8 for the oral doses, 4 to 7 for the intranasal doses, and 3.0 for the subcutaneous dose. The proportion of participants with relief of nausea within two hours after oral sumatriptan was about 50%, about 40% to 50% after intranasal sumatriptan, and 72% after subcutaneous sumatriptan. Placebo response rates were around 25% to 40% across all routes of administration.
The two doses of oral sumatriptan administered to participants with mild baseline pain also provided significant relief of phonophobia. Calculated NNTs were 2.9 and 2.2 for the 50 and 100 mg doses respectively. About 50% to 60% of participants experienced relief of phonophobia after treatment with sumatriptan, compared with about 20% after treatment with placebo. As discussed previously, statistical comparisons between the treatment effects in mild and moderate or severe baseline pain were not performed for relief of headache‐associated symptoms.
Relief of functional disability
Functional disability provides a measure of the impact of a migraine on the capacity of the sufferer to work and carry out normal daily activities. It is typically assessed on a 4‐point scale, as follows: able to work and function normally (0 = none), working ability impaired to some degree (1 = mild), working ability severely impaired (2 = moderate), or bed rest required (4 = severe).
Relief of functional disability was defined in different ways by the studies included in each of the reviews. Some required complete relief of any functional disability (i.e. any disability at baseline reduced to none by two hours), while others required only partial relief (i.e. moderate or severe disability at baseline reduced to mild or none by two hours).
Partial relief of functional disability
Pooled analyses were performed on four dose and route of administration combinations for which sufficient data were available (Summary of results L). All of these treatments were administered to participants with moderate or severe baseline pain.
| Summary of results L: Partial relief of functional disability at two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 3 | 381 | 107/220 | 51/161 | 49 | 32 | 1.4 (1.1 to 1.8) | 5.9 (3.7 to 14) |
| Oral | 50 | 4 | 607 | 186/378 | 72/229 | 49 | 31 | 1.5 (1.2 to 1.8) | 5.6 (3.9 to 10) |
| Oral | 100 | 6 | 1827 | 651/1113 | 220/714 | 58 | 31 | 1.9 (1.7 to 2.1) | 3.6 (3.1 to 4.3) |
| Intranasal | 20 | 2 | 225 | 89/144 | 13/81 | 62 | 16 | 3.8 (2.3 to 6.4) | 2.2 (1.8 to 2.9) |
Figure 5 shows the calculated NNTs for partial relief of functional disability at two hours for three of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for subcutaneous 6 mg or rectal 25 mg for this outcome).
All dose and route combinations provided superior relief of functional disability compared with placebo. Calculated NNTs ranged from 5.9 to 3.6 with oral administration, to 2.2 with intranasal treatment. The proportion of sumatriptan‐treated participants with partial relief of functional disability at two hours ranged from about 50% to 60% with the low and high doses, respectively. The proportion of placebo‐treated participants with the same outcome was about 30% with the three doses of oral sumatriptan, and half that (16%) with intranasal sumatriptan. In general, higher doses of sumatriptan resulted in lower (better) NNTs, but the differences between NNTs were not statistically significant (overlapping confidence intervals), suggesting that any dose response relationship in not clinically significant.
Complete relief of functional disability
Pooled analyses were performed on two dose and route of administration combinations for which sufficient data were available (Summary of results M). Both these treatments were administered to participants with moderate or severe baseline pain.
| Summary of results M: Complete relief of functional disability at two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Subcutaneous | 6 | 3 | 750 | 213/377 | 62/373 | 56 | 17 | 3.4 (2.7 to 4.4) | 2.5 (2.2 to 3.0) |
| Rectal | 25 | 2 | 238 | 60/145 | 15/93 | 41 | 16 | 2.6 (1.6 to 4.3) | 4.0 (2.8 to 7.0) |
Figure 5 shows the calculated NNTs for complete relief of functional disability at two hours for these dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for oral 100 mg or 50 mg, or for intranasal 20 mg for this outcome).
Both dose and route combinations provided superior relief of functional disability compared with placebo. Calculated NNTs were 4.0 with the rectal administration, and 2.5 with the subcutaneous treatment, with 41% and 56% of participants respectively achieving this outcome with sumatriptan, and 16% and 17% with placebo.
Appendix 2. Summary tables for sumatriptan versus active comparators
Pain‐free at two hours
Pooled analyses were performed on 12 dose and route of administration combinations for which sufficient data were available to evaluate the pain‐free response at two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Pain‐free at two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | Rizatriptan 5 mg | 2 | 2210 | 310/1117 | 363/1093 | 28 | 33 | 0.84 (0.74 to 0.95) | ‐18 (‐11 to ‐62) |
| Oral | 25 | Rizatriptan 10 mg | 2 | 2231 | 310/1117 | 440/1114 | 28 | 39 | 0.70 (0.62 to 0.79) | ‐8.5 (‐6.4 to ‐13) |
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 726 | 116/359 | 97/367 | 32 | 26 | 1.2 (0.97 to 1.5) | Not calculated |
| Oral | 50 | Rizatriptan 5 mg | 2 | 2209 | 394/1116 | 363/1093 | 35 | 33 | 1.1 (0.94 1.2) | Not calculated |
| Oral | 50 | Rizatriptan 10 mg | 2 | 2230 | 394/1116 | 440/1114 | 35 | 39 | 0.89 (0.80 to 0.99) | ‐24 (‐12 to ‐560) |
| Oral | 50 | Eletriptan 40 mg | 2 | 721 | 64/362 | 86/359 | 18 | 24 | 0.74 (0.55 to 0.99) | ‐16 (‐8.2 to ‐270) |
| Oral | 50 | Eletriptan 80 mg | 2 | 706 | 64/362 | 104/344 | 18 | 30 | 0.58 (0.44 to 0.76) | ‐8.0 (‐5.3 to ‐ 16) |
| Oral | 100 | Almotriptan 12.5 mg | 2 | 754 | 129/387 | 102/367 | 33 | 28 | 1.2 (0.97 to 1.5) | Not calculated |
| Oral | 100 | Eletriptan 40 mg | 3 | 2263 | 271/1130 | 366/1133 | 24 | 32 | 0.74 (0.65 to 0.85) | ‐12 (‐8.3 to ‐22) |
| Oral | 100 | Eletriptan 80 mg | 2 | 604 | 55/299 | 103/305 | 18 | 34 | 0.54 (0.41 to 0.72) | ‐6.5 (‐4.5 to ‐12) |
| Oral | 100 | Rizatriptan 10 mg | 2 | 936 | 143/460 | 178/476 | 31 | 37 | 0.82 (0.69 to 0.98) | ‐16 (‐8.1 to ‐410) |
| Oral | 100 | ASA 900 mg + MCP 10 mg | 2 | 575 | 71/275 | 48/300 | 26 | 16 | 1.6 (1.2 to 2.3) | 10 (6.1 to 31) |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin; MCP ‐ metoclopramide | ||||||||||
Pain‐free at one hour
Pooled analyses were performed on three dose and route of administration combinations for which sufficient data were available to evaluate the pain‐free response at one hour. All treatments were administered to participants with moderate or severe baseline pain.
| Pain‐free at one hour in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 726 | 19/359 | 20/367 | 5 | 5 | 0.97 (0.53 to 1.8) | Not calculated |
| Oral | 100 | Eletriptan 40 mg | 3 | 2263 | 59/1130 | 75/1133 | 5 | 7 | 0.79 (0.57 to 1.1) | Not calculated |
| Oral | 100 | Eletriptan 80 mg | 2 | 604 | 19/299 | 40/305 | 6 | 13 | 0.48 (0.28 to 0.81) | ‐15 (‐8.7 to ‐48) |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin | ||||||||||
Sustained pain‐free during the 24 hours postdose
Pooled analyses were performed on one dose and route of administration combination for which sufficient data were available to evaluate the 24‐hour sustained pain‐free response. The treatments were administered to participants with moderate or severe baseline pain.
| Sustained pain‐free during the 24 hours postdose in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 100 | Almotriptan 12.5 mg | 2 | 754 | 111/387 | 110/367 | 29 | 30 | 0.96 (0.77 to 1.2) | Not calculated |
Headache relief at two hours
Pooled analyses were performed on 13 dose and route of administration combinations for which sufficient data were available to evaluate the headache relief response at two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Headache relief at two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | Rizatriptan 5 mg | 2 | 2210 | 669/1117 | 731/1093 | 60 | 67 | 0.90 (0.84 to 0.96) | ‐14 (‐9.1 to ‐34) |
| Oral | 25 | Rizatriptan 10 mg | 2 | 2231 | 669/1117 | 780/1114 | 60 | 70 | 0.86 (0.81 to 0.91) | ‐9.9 (‐7.1 to ‐16) |
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 726 | 191/359 | 153/367 | 53 | 42 | 1.3 (1.1 to 1.5) | 8.7 (5.3 to 23) |
| Oral | 50 | Zolmitriptan 2.5 mg | 2 | 1609 | 543/814 | 523/795 | 67 | 66 | 1.0 (0.94 to 1.1) | Not calculated |
| Oral | 50 | Zolmitriptan 5 mg | 2 | 1633 | 543/814 | 537/819 | 67 | 66 | 1.0 (0.95 to 1.1) | Not calculated |
| Oral | 50 | Rizatriptan 5 mg | 3 | 2911 | 949/1469 | 951/1442 | 65 | 66 | 0.98 (0.93 to 1.0) | Not calculated |
| Oral | 50 | Rizatriptan 10 mg | 2 | 2227 | 710/1113 | 780/1114 | 64 | 70 | 0.91 (0.86 to 0.96) | ‐16 (‐9.9 to ‐43) |
| Oral | 50 | Eletriptan 40 mg | 2 | 721 | 186/362 | 217/359 | 51 | 60 | 0.85 (0.75 to 0.97) | ‐11 (‐6.1 to ‐54) |
| Oral | 50 | Eletriptan 80 mg | 2 | 706 | 186/362 | 226/344 | 51 | 66 | 0.78 (0.69 to 0.88) | ‐7.0 (‐4.7 to ‐14) |
| Oral | 100 | Eletriptan 40 mg | 3 | 2263 | 622/1130 | 706/1133 | 55 | 62 | 0.88 (0.82 to 0.94) | ‐14 (‐8.8 to ‐31) |
| Oral | 100 | Eletriptan 80 mg | 2 | 604 | 151/299 | 198/305 | 51 | 65 | 0.78 (0.68 to 0.90) | ‐6.9 (‐4.5 to ‐15) |
| Oral | 100 | Paracetamol 1000 mg + MCP 10 mg | 2 | 1035 | 233/514 | 225/521 | 45 | 43 | 1.1 (0.92 to 1.2) | Not calculated |
| Oral | 100 | ASA 900 mg + MCP 10 mg | 2 | 575 | 137/275 | 138/300 | 50 | 46 | 1.1 (0.92 to 1.3) | Not calculated |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin; MCP ‐ metoclopramide | ||||||||||
Headache relief at one hour
Pooled analyses were performed on 12 dose and route of administration combinations for which sufficient data were available to evaluate the headache relief response at one hour. All treatments were administered to participants with moderate or severe baseline pain.
| Headache relief at one hour in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | Rizatriptan 5 mg | 2 | 2210 | 375/1117 | 404/1093 | 34 | 37 | 0.91 (0.81 to 1.0) | Not calculated |
| Oral | 25 | Rizatriptan 10 mg | 2 | 2231 | 375/1117 | 456/1114 | 34 | 41 | 0.82 (0.74 to 0.91) | ‐14 (‐8.8 to ‐30) |
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 726 | 86/359 | 113/367 | 24 | 31 | 0.78 (0.61 to 0.99) | ‐15 (‐7.5 to ‐270) |
| Oral | 50 | Zolmitriptan 2.5 mg | 2 | 1609 | 330/814 | 318/795 | 41 | 40 | 1.0 (0.90 to 1.1) | Not calculated |
| Oral | 50 | Zolmitriptan 5 mg | 2 | 1633 | 330/814 | 320/819 | 41 | 39 | 1.0 (0.92 to 1.2) | Not calculated |
| Oral | 50 | Rizatriptan 5 mg | 2 | 2209 | 409/1116 | 404/1093 | 37 | 37 | 0.99 (0.89 to 1.1) | Not calculated |
| Oral | 50 | Rizatriptan 10 mg | 2 | 2230 | 409/1116 | 456/1114 | 37 | 41 | 0.90 (0.81 to 1.0) | Not calculated |
| Oral | 50 | Eletriptan 40 mg | 2 | 721 | 90/362 | 90/359 | 25 | 25 | 0.99 (0.77 to 1.3) | Not calculated |
| Oral | 50 | Eletriptan 80 mg | 2 | 706 | 90/362 | 119/344 | 25 | 35 | 0.72 (0.57 to 0.91) | ‐10 (‐6.1 to ‐33) |
| Oral | 100 | Eletriptan 40 mg | 3 | 2263 | 282/1130 | 368/1133 | 25 | 32 | 0.77 (0.68 to 0.88) | ‐13 (‐8.9 to ‐26) |
| Oral | 100 | Eletriptan 80 mg | 2 | 604 | 68/299 | 106/305 | 23 | 35 | 0.65 (0.50 to 0.84) | ‐8.3 (‐5.2 to ‐21) |
| Oral | 100 | Rizatriptan 10 mg | 2 | 936 | 120/460 | 163/476 | 26 | 34 | 0.76 (0.62 to 0.93) | ‐12 (‐7.1 to ‐43) |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin | ||||||||||
Sustained headache relief during the 24 hours postdose
Pooled analyses were performed on one dose and route of administration combination for which sufficient data were available to evaluate the 24‐hour sustained headache relief response. The treatments were administered to participants with moderate or severe baseline pain.
| Sustained headache relief during the 24 hours postdose in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 100 | Eletriptan 40 mg | 2 | 1998 | 340/1001 | 430/997 | 34 | 43 | 0.79 (0.71 to 0.88) | ‐11 (‐7.5 to ‐20) |
Any adverse event during within 24 hours
Pooled analyses were performed on nine dose and route of administration combinations for which sufficient data were available to evaluate the incidence of adverse events within 24 hours of treatment. All treatments were administered to participants with moderate or severe baseline pain.
| Any adverse event within 24 hours in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative harm | NNH (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | Rizatriptan 5 mg | 2 | 1169 | 250/587 | 238/582 | 43 | 41 | 1.0 (0.91 to 1.2) | Not calculated |
| Oral | 25 | Rizatriptan 10 mg | 2 | 1186 | 250/587 | 276/599 | 43 | 46 | 0.92 (0.81 to 1.1) | Not calculated |
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 730 | 64/361 | 55/369 | 18 | 15 | 1.2 (0.85 to 1.6) | Not calculated |
| Oral | 50 | Zolmitriptan 2.5 mg | 2 | 1771 | 290/893 | 283/878 | 32 | 32 | 1.0 (0.88 to 1.2) | Not calculated |
| Oral | 50 | Zolmitriptan 5 mg | 2 | 1790 | 290/893 | 322/897 | 32 | 36 | 0.91 (0.80 to 1.0) | Not calculated |
| Oral | 50 | Rizatriptan 5 mg | 2 | 1160 | 276/578 | 238/582 | 48 | 41 | 1.2 (1.0 to 1.3) | Not calculated |
| Oral | 50 | Rizatriptan 10 mg | 2 | 1177 | 276/578 | 276/599 | 48 | 46 | 1.0 (0.92 to 1.2) | Not calculated |
| Oral | 100 | Rizatriptan 10 mg | 2 | 856 | 217/421 | 203/435 | 52 | 47 | 1.1 (0.96 to 1.3) | Not calculated |
| Oral | 100 | ASA 900 mg + MCP 10 mg | 2 | 621 | 112/300 | 78/321 | 37 | 24 | 1.5 (1.2 to 2.0) | 7.7 (4.9 to 17 |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin; MCP ‐ metoclopramide | ||||||||||
Use of rescue medication
Pooled analyses were performed on two dose and route of administration combinations for which sufficient data were available to evaluate the use of rescue medication during the 24 hours postdose. All treatments were administered to participants with moderate or severe baseline pain.
| Use of rescue medication during the 24 hours postdose in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNTp (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 100 | Eletriptan 40 mg | 2 | 1918 | 261/960 | 203/958 | 27 | 21 | 1.3 (1.1 to 1.5) | ‐17 (‐10 to ‐46) |
| Oral | 100 | Paracetamol 1000 mg + MCP 10 mg | 2 | 1243 | 198/606 | 245/637 | 33 | 38 | 0.86 (0.74 to 1.0) | Not calculated |
| Footnotes: MCP ‐ metoclopramide | ||||||||||
Relief of migraine‐associated symptoms
Nausea
Pooled analyses were performed on three dose and route of administration combinations for which sufficient data were available to evaluate the relief of nausea within two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Relief of nausea within two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 100 | Eletriptan 40 mg | 3 | 1478 | 352/719 | 420/759 | 49 | 55 | 0.87 (0.79 to 0.96) | ‐16 (‐8.7 to ‐77) |
| Oral | 100 | Eletriptan 80 mg | 2 | 408 | 100/204 | 123/204 | 49 | 60 | 0.83 (0.69 to 0.99) | ‐8.9 (‐4.8 to ‐60) |
| Oral | 100 | ASA 900 mg + MCP 10 mg | 2 | 410 | 60/192 | 76/218 | 31 | 35 | 0.91 (0.69 to 1.2) | Not calculated |
| Footnotes: MCP ‐ metoclopramide | ||||||||||
Photophobia
Pooled analyses were performed on four dose and route of administration combinations for which sufficient data were available to evaluate the relief of photophobia within two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Relief of photophobia within two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 50 | Eletriptan 40 mg | 2 | 528 | 107/261 | 132/267 | 41 | 49 | 0.83 (0.69 to 1.0) | Not calculated |
| Oral | 50 | Eletriptan 80 mg | 2 | 508 | 107/261 | 142/247 | 41 | 57 | 0.72 (0.60 to 0.86) | ‐6.1 (‐4.0 to ‐13) |
| Oral | 100 | Eletriptan 40 mg | 3 | 1692 | 438/855 | 500/837 | 51 | 60 | 0.85 (0.78 to 0.93) | ‐12 (‐7.6 to ‐26) |
| Oral | 100 | Eletriptan 80 mg | 2 | 457 | 110/232 | 142/225 | 47 | 63 | 0.76 (0.64 to 0.90) | ‐6.4 (‐4.1 to ‐15) |
Phonophobia
Pooled analyses were performed on three dose and route of administration combinations for which sufficient data were available to evaluate the relief of phonophobia within two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Relief of phonophobia within two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 50 | Eletriptan 40 mg | 2 | 517 | 120/257 | 139/260 | 47 | 53 | 0.87 (0.73 to 1.0) | Not calculated |
| Oral | 50 | Eletriptan 80 mg | 2 | 508 | 120/257 | 145/251 | 47 | 58 | 0.81 (0.69 to 0.96) | ‐9.0 (‐5.1 to ‐41) |
| Oral | 100 | Eletriptan 40 mg | 2 | 1361 | 352/691 | 405/670 | 51 | 60 | 0.84 (0.76 to 0.92) | ‐11 (‐6.8 to ‐24) |
Relief of functional disability
Partial relief of functional disability
Pooled analyses were performed on four dose and route of administration combinations for which sufficient data were available to evaluate the partial relief of functional disability within two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Partial relief of functional disability within two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 50 | Eletriptan 40 mg | 2 | 590 | 153/298 | 180/292 | 51 | 62 | 0.83 (0.72 to 0.96) | ‐9.7 (‐5.5 to ‐43) |
| Oral | 50 | Eletriptan 80 mg | 2 | 570 | 153/298 | 168/272 | 51 | 62 | 0.84 (0.73 to 0.97) | ‐9.6 (‐5.4 to ‐43) |
| Oral | 100 | Eletriptan 40 mg | 3 | 1880 | 553/936 | 645/944 | 59 | 68 | 0.86 (0.80 to 0.92) | ‐11 (‐7.4 to ‐20) |
| Oral | 100 | Eletriptan 80 mg | 2 | 516 | 129/255 | 173/261 | 51 | 66 | 0.77 (0.66 to 0.89) | ‐6.4 (‐4.2 to ‐14) |
Complete relief of functional disability
There were insufficient data to perform any pooled analyses for the complete relief of functional disability.
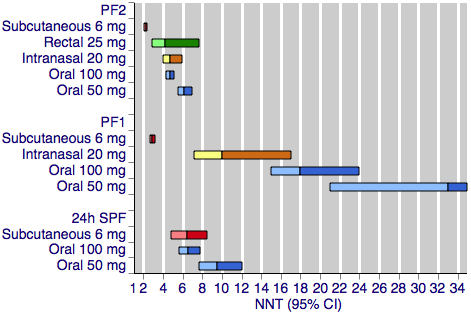
Sumatriptan versus placebo. Calculated NNTs for a pain‐free response after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
PF2: pain‐free at two hours; PF1: pain‐free at one hour; 24h SPF: 24‐hour sustained pain‐free.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.

Sumatriptan versus placebo. Calculated NNTs for headache relief after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
HR2 (headache relief at two hours); HR1 (headache relief at one hour); 24h SHR (24‐hour sustained headache relief).
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.
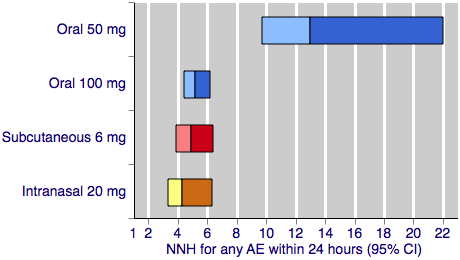
Sumatriptan versus placebo. Calculated NNHs for any adverse event within 24 hours of dosing, in participants treating moderate or severe migraine pain. Results for four of the five most commonly used dose and route of administration combinations (adverse event information for rectal sumatriptan not available), listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, and intranasal doses are shown with yellow bars.
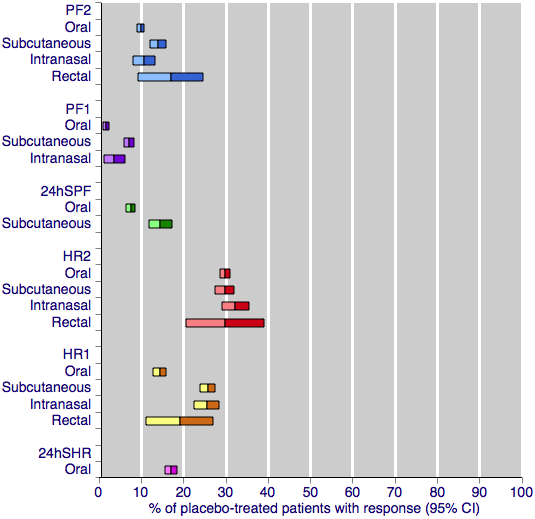
Placebo response rates for the primary efficacy outcomes, by route of administration. Response rates of each outcome are grouped by colour to facilitate comparison between different routes of administration. Proportion of placebo‐treated participants pain‐free at two hours are shown with blue bars, pain‐free at one hour with purple bars, 24‐h sustained pain‐free with green bars, headache relief at two hours with red bars, headache relief at one hour with yellow bars, and 24‐h sustained headache relief with a pink bar.

Sumatriptan versus placebo. Calculated NNTs for relief of migraine‐associated symptoms and functional disability after two hours, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.

