Metformina durante la inducción de la ovulación con gonadotrofinas seguida del coito programado o la inseminación intrauterina para la subfertilidad asociada con el síndrome de ovario poliquístico
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009090.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 enero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Ginecología y fertilidad
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MC and LT proposed developing a Cochrane review on this topic and developed the title. MvW and MN developed and wrote the draft of the protocol and the intended methods of the review. All review authors agreed and helped to respond to peer reviewers' comments on the protocol.
EB, MvW and MN performed literature searches for the review, selected relevant trials, procured data and information about studies, assessed validity and checked data extracted for each trial; these review authors entered all study information, data and text into RevMan; performed analyses; wrote the abstract, background, methods, results and conclusion sections of the review; and approved the final version.
MC, FV, LT and BM took part in writing the abstract, background, methods, results and conclusion sections of the review and approved the final version.
Sources of support
Internal sources
-
Internal support, Netherlands.
Partly internally funded
External sources
-
None, Other.
No external support
Declarations of interest
MC has received travel costs and honoraria from Merck Sharp and Dohme (MSD) and Merck Serono to attend ART Scientific Meetings to present papers on topics not related to this review. He is a shareholder at a private fertility clinic and undertakes private practice within that facility. FV has received occasional payment for lectures for ESHRE unrelated to the topic of this review. BM and his institution have received payment for consultancy from ObsEva Geneva. BM has received payment for review preparation from the European Journal of Obstetrics and Gynaecology, and has received travel/accommodation/meeting expenses for various non‐commercial scientific meetings. MW has received travel/accommodation/meeting expenses from ESHRE or Oxford Press for attendance at scientific meetings.
EB and LT have no interests to declare.
Acknowledgements
We would like to acknowledge the Cochrane Gynaecology and Fertility Group for extending to us the opportunity to write a review on this topic. We thank Marian Showell for preparing the search strategy.
Review authors of the 2016 update thank Dr Jur Oosterhuis for his contributions to previous versions of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 24 | Metformin during ovulation induction with gonadotrophins followed by timed intercourse or intrauterine insemination for subfertility associated with polycystic ovary syndrome | Review | Esmée M Bordewijk, Marleen Nahuis, Michael F Costello, Fulco Van der Veen, Leopoldo O Tso, Ben Willem J Mol, Madelon van Wely | |
| 2011 May 11 | Metformin co‐administration during follicle stimulating hormone ovulation induction with timed intercourse or intra‐uterine insemination for subfertility associated with polycystic ovary syndrome | Protocol | Marleen Nahuis, Michael F Costello, Fulco Van der Veen, L O Tso, Jur Oosterhuis, Ben Willem J Mol, Madelon van Wely | |
Differences between protocol and review
In the protocol, review authors planned to pool process outcomes. However, as all data were reported per cycle, we could not pool results. We provided data on ovulation rate per started cycle, cycle cancellation rate per started cycle, mean duration of stimulation days per cycle and FSH dose per cycle per study without pooling.
In the protocol, review authors intended to pool ongoing pregnancy and live birth rates when an ongoing pregnancy was defined as pregnancy of at least 20 weeks' gestation. However, we defined ongoing pregnancy as a pregnancy of at least 12 weeks' gestation. We present both live birth and ongoing pregnancy data.
We adjusted protocol search strategies and refined them for the review.
After receiving referee comments, we decided to make multiple pregnancy the primary safety outcome. Nowadays, OHSS is a rare event that follows ovulation induction with gonadotrophins in PCOS. Ovulation induction with gonadotrophins is always monitored.
We decided to use the term 'gonadotrophins' rather than 'FSH', as ovulation induction is also possible with HP‐HMG. FSH is present in recombinant FSH, in urinary‐derived highly purified FSH and in highly purified human menopausal gonadotrophins. These FSH‐containing gonadotrophins are usually referred to as gonadotrophins.
Notes
None.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Coitus;
- Combined Modality Therapy [methods];
- Drug Therapy, Combination [methods];
- Fertility Agents, Female [*therapeutic use];
- Fertilization in Vitro [*methods];
- Follicle Stimulating Hormone [*therapeutic use];
- Gonadotropins [therapeutic use];
- Infertility, Female [etiology, *therapy];
- Live Birth;
- Metformin [*therapeutic use];
- Ovulation Induction [*methods];
- Polycystic Ovary Syndrome [*complications];
- Pregnancy Outcome;
- Pregnancy Rate;
- Pregnancy, Multiple;
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Study flow diagram.
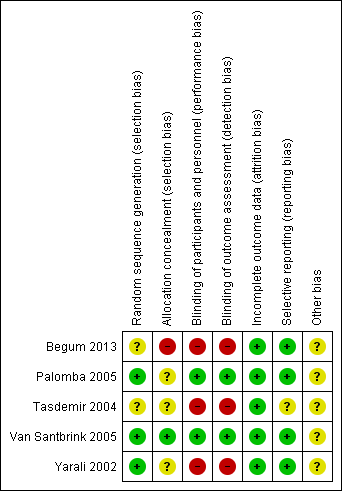
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
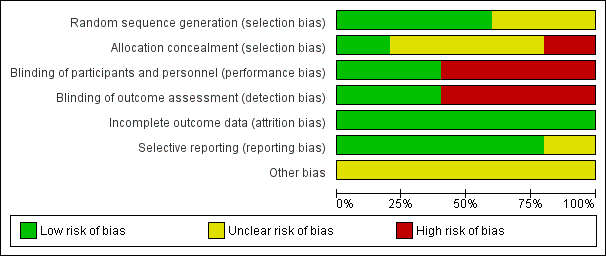
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
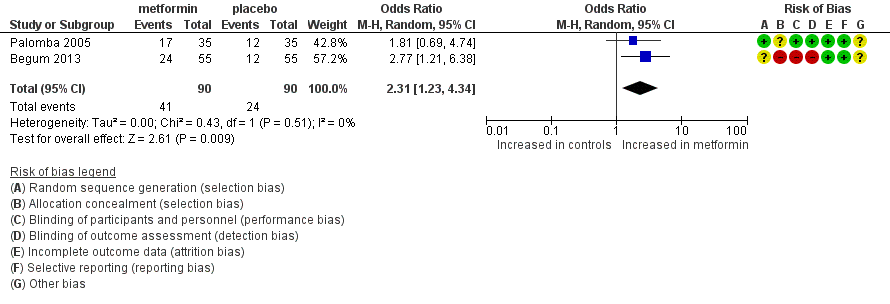
Forest plot of comparison: 1 dichotomous outcome, outcome: 1.1 live birth rate (per woman).

Forest plot of comparison: 1 Metformin versus placebo co‐treatment for assisted reproduction, outcome: 1.2 Multiple pregnancy rate (per woman).

Forest plot of comparison: 1 Metformin versus placebo co‐treatment for assisted reproduction, outcome: 1.3 Ongoing pregnancy rate (per woman).
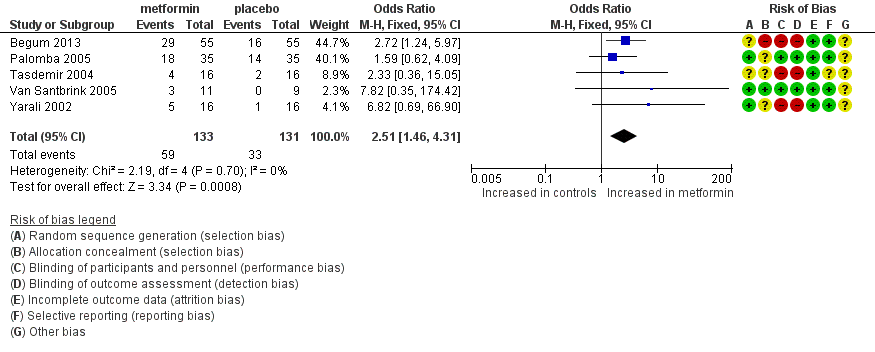
Forest plot of comparison: 1 Metformin versus placebo co‐treatment for assisted reproduction, outcome: 1.4 Clinical pregnancy rate.

Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 1 Live birth rate (per woman).
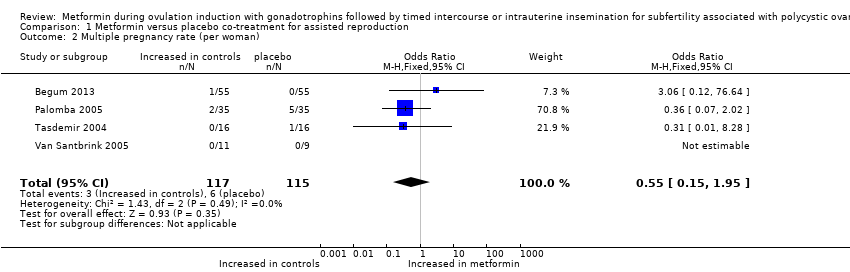
Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 2 Multiple pregnancy rate (per woman).

Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 3 Ongoing pregnancy rate (per woman).
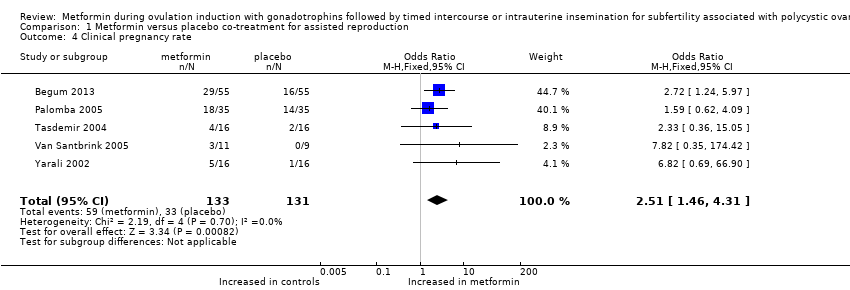
Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 4 Clinical pregnancy rate.

Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 5 Miscarriage rate (per clinical pregnancy).
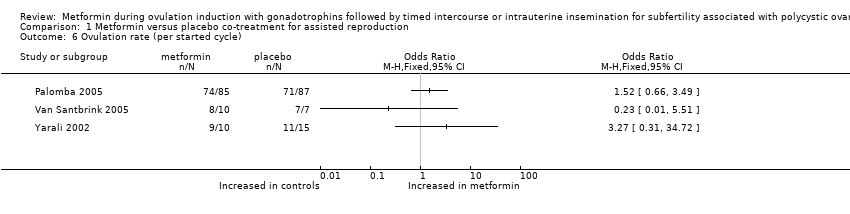
Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 6 Ovulation rate (per started cycle).
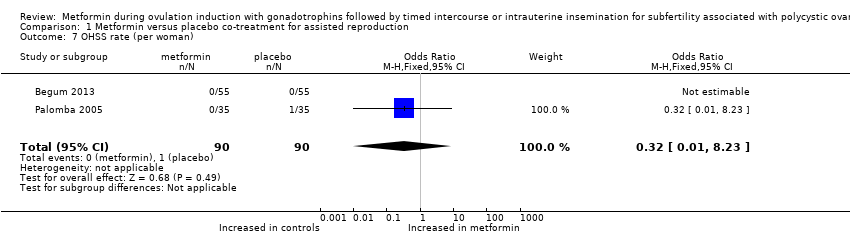
Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 7 OHSS rate (per woman).

Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 8 Cycle cancellation rate (per started cycle).

Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 9 Adverse effects.
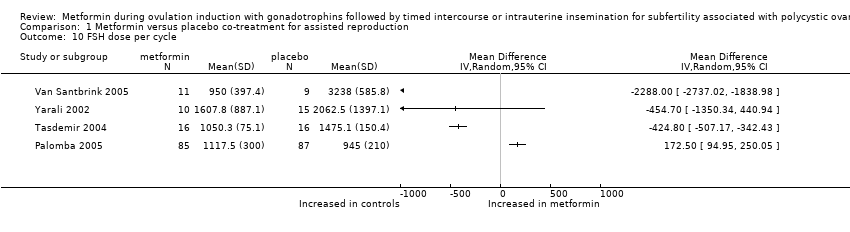
Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 10 FSH dose per cycle.

Comparison 1 Metformin versus placebo co‐treatment for assisted reproduction, Outcome 11 Duration of stimulation days per cycle.
| Metformin versus placebo co‐treatment for assisted reproduction for subfertility associated with polycystic ovary syndrome | ||||||
| Patient or population: patients with subfertility associated with polycystic ovary syndrome | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo co‐treatment | Metformin co‐treatment | |||||
| Live birth rate (per woman) | 267 per 1000 | 457 per 1000 190 more per 1000 (42 to 345) | OR 2.31 | 180 | ⊕⊕⊝⊝ | |
| Multiple pregnancy rate (per woman) | 52 per 1000 | 26 per 1000 23 fewer per 1000 (44 fewer to 25 more) | OR 0.55 (0.15 to 1.95) | 232 | ⊕⊕⊝⊝ | |
| Ongoing pregnancy rate (per woman) | 217 per 1000 | 393 per 1000 189 more per 1000 (57 to 336) | OR 2.46 | 232 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate (per woman) | 252 per 1000 | 444 per 1000 206 more per 1000 (78 to 340) | OR 2.51 | 264 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aLimited sample size, limited precision, lack of blinding of participants and of outcome assessors. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate (per woman) Show forest plot | 2 | 180 | Odds Ratio (M‐H, Random, 95% CI) | 2.31 [1.23, 4.34] |
| 2 Multiple pregnancy rate (per woman) Show forest plot | 4 | 232 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 1.95] |
| 3 Ongoing pregnancy rate (per woman) Show forest plot | 4 | 232 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.36, 4.46] |
| 4 Clinical pregnancy rate Show forest plot | 5 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.46, 4.31] |
| 5 Miscarriage rate (per clinical pregnancy) Show forest plot | 3 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.19, 2.01] |
| 6 Ovulation rate (per started cycle) Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 OHSS rate (per woman) Show forest plot | 2 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.23] |
| 8 Cycle cancellation rate (per started cycle) Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Adverse effects Show forest plot | 2 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.39, 8.09] |
| 10 FSH dose per cycle Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Duration of stimulation days per cycle Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

