Аэробные физические упражнения у взрослых пациентов с гематологическими злокачественными новообразованиями
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
| |
| Participants | Eligibility criteria
Participants (N = 64)
Mean age
Stage/type of disease
Country
| |
| Interventions | Exercise group
Control group
| |
| Outcomes | Reported and relevant for this review
Reported and not relevant for this review
| |
| Notes | Authors report no conflicts of interest, funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization was achieved....using computer‐generated numbers" |
| Allocation concealment (selection bias) | Low risk | "randomization was achieved....using computer‐generated numbers" |
| Blinding of participants and personnel (performance bias) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome OS in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | "All 64 randomized patients represented the intent‐to‐treat population." |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Not reported |
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
| |
| Participants | Eligibility criteria
Participants (N = 24)
Mean age
Stage of disease
Country
| |
| Interventions | Exercise group
Control group
All participants received cytarabine plus idarubicin | |
| Outcomes | Reported and analysed in this review
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Two patients out of 24 (9%) dropped out because of severe complications. It is unclear whether these complications are related to the intervention Quote: "One patient dropped out of each group due to severe complications" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Gender distribution unbalanced between arms |
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
| |
| Participants | Eligibility criteria
Participants (N = 24)
Mean age
Stage of disease
Country
| |
| Interventions | Exercise group
Control group
All participants received high‐dose chemotherapy including tandem transplantation. Half of them were randomised to receive thalidomide during induction, posttransplantation consolidation, and maintenance therapy | |
| Outcomes | Reported and analysed in this review
Reported but not relevant for this review
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (patient‐reported outcomes) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Study was finalised before the last 6 participants were enrolled due to funding constraints |
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
| |
| Participants | Eligibility criteria
Participants (N = 187)
Mean age
Stage of disease
Therapy
Country
| |
| Interventions | Exercise group
Control group
| |
| Outcomes | Reported and analysed in this review
Reported but not relevant for this review
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | 15 participants who dropped out were not analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol are reported |
| Other bias | High risk | 50% of participants received thalidomide. In the study analysis this drug administration was not considered and no subgroup data were provided for participants receiving or not receiving thalidomide |
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
| |
| Participants | Eligibility criteria
Participants (N = 122)
Mean age
Stage of disease
Country
| |
| Interventions | Exercise group
Control group
| |
| Outcomes | Reported and analysed in this review
Reported but not relevant for this review
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After completing baseline tests, participants were stratified by major disease type and current treatment status and were randomly assigned to aerobic exercise training or usual care by using a computer‐generated program. The allocation sequence was generated independently and concealed in opaque envelopes from the study coordinator who assigned participants to groups." |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was generated independently and concealed in opaque envelopes from the study coordinator who assigned participants to groups." |
| Blinding of participants and personnel (performance bias) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | High risk | "Outcomes assessors were not always blinded to group assignment but were trained in standardising testing procedures" |
| Incomplete outcome data (attrition bias) | Low risk | All participants were considered in study analysis in conformity with their randomised group assignment |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Study supported by Lance Armstrong Foundation. Any bias due to this support is not expected. No other sources of potential bias were reported |
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
| |
| Participants | Eligibility criteria
Participants (N = 100)
Mean age
Stage/type of disease
Country
| |
| Interventions | Exercise group
Control group
| |
| Outcomes | Reported and analysed in this review
Reported but not relevant for this review
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome OS in this unblinded trial is unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessor (patient‐reported outcomes) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Low risk | 100‐day assessment was performed in a clinic that was separate from the hospital so the physician was unaware of the randomised assignment |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Not reported |
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
| |
| Participants | Eligibility criteria
Participants (N = 131)
Mean age
Stage/type of disease
Country
| |
| Interventions | Exercise group
Control group
| |
| Outcomes | Reported and analysed in this review
| |
| Notes | Financially supported by Zürcher Krebsliga ("Zurich cancer league") and Eidgenössische Sportkommission (Federal Authorities of the Swiss Confederation, Federal Department of Defence, Civil Protection and Sport) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "minimization procedure was used to achieve an optimal balance between groups for the factors age, sex and type of transplantation" |
| Allocation concealment (selection bias) | Low risk | "opaque envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | "was carried out on intention to treat analysis" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Not reported |
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
| |
| Participants | Eligibility criteria
Participants (N = 61)
Mean age
Stage of disease
Country
| |
| Interventions | Exercise group
Control group
| |
| Outcomes | Reported and analysed in this review
Reported but not relevant for this review
| |
| Notes | Financially supported by a grant by AMGEN | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "carried out by an independent randomization office" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | "Intention to treat strategies for substituting missing values" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Study stopped early, due to low recruitment. "At this point, we considered physiological parameters much more relevant to evaluate the intervention than QoL, hence the study was stopped early" Statistically significant baseline imbalances for QoL, favouring the control arm |
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
| |
| Participants | Eligibility criteria
Participants (N = 105)
Mean age
Disease
Country
| |
| Interventions | Exercise group
Control group
All participants received allogeneic stem cell transplantation | |
| Outcomes | Reported and relevant for this review
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised by the minimisation procedure stratified by age, disease, and sex for each centre to an exercise or a control group“ |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome OS in this unblinded trial is unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | High risk | "The testers were not blinded to randomisation but not involved in the therapeutic supervision of the patients.“ |
| Incomplete outcome data (attrition bias) | High risk | 112 participants were randomly assigned to both study arms. In study analysis only 105 participants were included. Moreover, it is not reported, how many patients in the control arm performed exercise |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Not reported |
ALL: acute lymphoblastic leukaemia; AML: acute myeloid leukaemia; B‐NHL: B‐cell non‐Hodgkin's lymphoma; CLL: chronic lymphoblastic leukaemia; CML: chronic myeloid leukaemia; DCEP: dexamethasone, cyclophosphamide, etoposide, and cisplatin; EPO: erythropoietin; MDS: myelodysplastic syndrome; NHL: non‐Hodgkin's lymphoma; OS: overall survival; T‐NHL: T‐cell non‐Hodgkin's lymphoma; QoL: quality of life
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study investigated influence of Tibetan yoga intervention on psychological adjustment and sleep quality. This intervention does not correspond to our prescribed exercise intervention | |
| Investigated endpoints are muscle protein status and turnover. These outcomes do not correspond to our prescribed outcomes | |
| Exercise intervention only consisted of strength training. This intervention does not correspond to our predefined types of exercise intervention | |
| Study included participants up to the age of 18 years | |
| Control group received standard treatment care and physiotherapy. Intervention consisted of physical exercise together with psycho‐education and progressive relaxation | |
| Study explored changes in lymphocyte count and T‐cell subsets. These outcomes do not correspond to our prescribed outcomes | |
| Study included participants up to the age of 18 years | |
| Investigated endpoint is muscular strength. This outcome does not correspond to our prescribed outcomes | |
| Study included participants up to the age of 18 years | |
| Study did not compare an exercise group with a standard care group. In this study both groups conducted physical exercise intervention | |
| Study included participants up to the age of 18 years | |
| In this study participants suffering from different cancer types (lymphomas, breast, gynaecologic or testicular cancer) were included, no subgroup analyses for those with haematological malignancies were provided |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Design of the Exercise Intervention after stem cell transplantation (EXIST) study: a randomised controlled trial to evaluate the effectiveness and cost‐effectiveness of an individualised high intensity physical exercise program on fitness and fatigue in patients with multiple myeloma or (non‐) Hodgkin’s lymphoma treated with high dose chemotherapy and autologous stem cell transplantation |
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
| Participants | Eligibility criteria
Participants (N = 120)
Mean age
Stage of disease
Country
|
| Interventions | Exercise group
Control group
|
| Outcomes | Reported
Not reported but relevant
Reported but not relevant
Primary Outcome:
|
| Starting date | Not reported |
| Contact information | Department of Hematology, Academic Medical Center, University of Amsterdam, Netherlands |
| Notes |
ASCT: HDC: WBC: white blood cell
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.59, 1.47] |
| Analysis 1.1 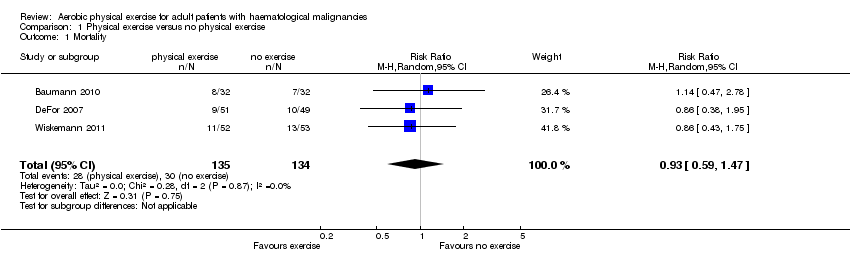 Comparison 1 Physical exercise versus no physical exercise, Outcome 1 Mortality. | ||||
| 2 Quality of life (QoL) Show forest plot | 4 | 352 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.15, 0.45] |
| Analysis 1.2  Comparison 1 Physical exercise versus no physical exercise, Outcome 2 Quality of life (QoL). | ||||
| 3 QoL sensitivity analysis Show forest plot | 3 | 291 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.03, 0.49] |
| Analysis 1.3 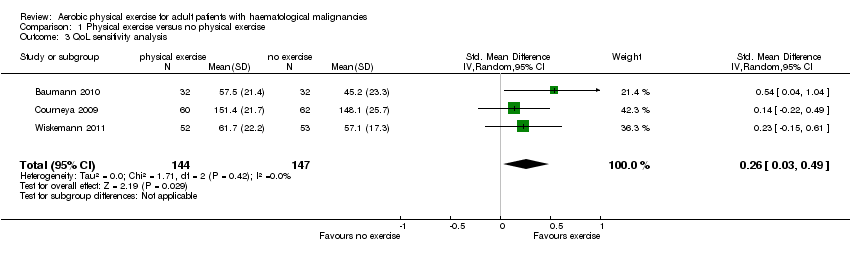 Comparison 1 Physical exercise versus no physical exercise, Outcome 3 QoL sensitivity analysis. | ||||
| 4 QoL SCT versus no SCT Show forest plot | 4 | 352 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.15, 0.45] |
| Analysis 1.4 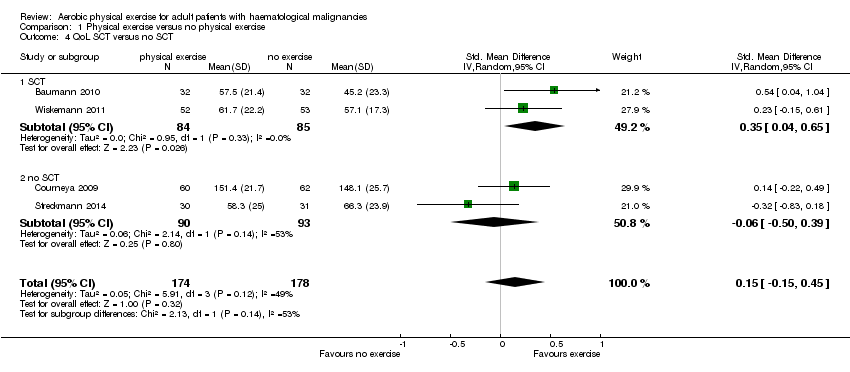 Comparison 1 Physical exercise versus no physical exercise, Outcome 4 QoL SCT versus no SCT. | ||||
| 4.1 SCT | 2 | 169 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.04, 0.65] |
| 4.2 no SCT | 2 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.50, 0.39] |
| 5 Physical functioning/QoL Show forest plot | 4 | 422 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.13, 0.52] |
| Analysis 1.5 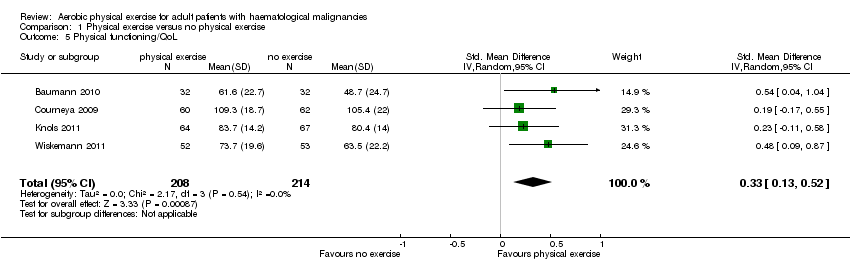 Comparison 1 Physical exercise versus no physical exercise, Outcome 5 Physical functioning/QoL. | ||||
| 6 Depression/QoL Show forest plot | 3 | 249 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.00, 0.50] |
| Analysis 1.6  Comparison 1 Physical exercise versus no physical exercise, Outcome 6 Depression/QoL. | ||||
| 7 Anxiety/QoL Show forest plot | 3 | 249 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.64, 0.28] |
| Analysis 1.7  Comparison 1 Physical exercise versus no physical exercise, Outcome 7 Anxiety/QoL. | ||||
| 8 Fatigue Show forest plot | 7 | 692 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| Analysis 1.8  Comparison 1 Physical exercise versus no physical exercise, Outcome 8 Fatigue. | ||||
| 9 Fatigue SCT versus no SCT Show forest plot | 7 | 692 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| Analysis 1.9  Comparison 1 Physical exercise versus no physical exercise, Outcome 9 Fatigue SCT versus no SCT. | ||||
| 9.1 SCT | 4 | 487 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.06, 0.55] |
| 9.2 no SCT | 3 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.12, 0.43] |
| 10 Weight Show forest plot | 2 | 253 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐4.08, 4.68] |
| Analysis 1.10  Comparison 1 Physical exercise versus no physical exercise, Outcome 10 Weight. | ||||
| 11 Lean body mass Show forest plot | 2 | 253 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐1.34, 4.02] |
| Analysis 1.11  Comparison 1 Physical exercise versus no physical exercise, Outcome 11 Lean body mass. | ||||
| 12 Serious adverse events (SAEs) Show forest plot | 3 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.96, 2.18] |
| Analysis 1.12  Comparison 1 Physical exercise versus no physical exercise, Outcome 12 Serious adverse events (SAEs). | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Physical exercise versus no physical exercise, outcome: 1.1 Mortality.

Forest plot of comparison: 1 Physical exercise versus no physical exercise, outcome: 1.3 QoL sensitivity analysis.
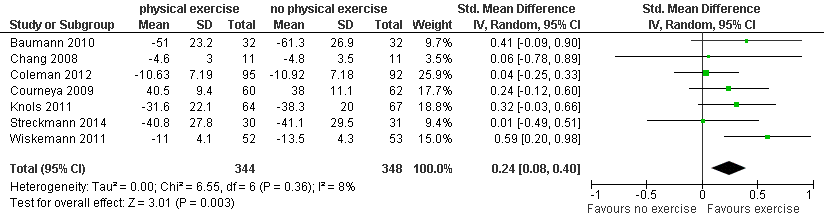
Forest plot of comparison: 1 Physical exercise versus no physical exercise, outcome: 1.8 Fatigue.

Comparison 1 Physical exercise versus no physical exercise, Outcome 1 Mortality.

Comparison 1 Physical exercise versus no physical exercise, Outcome 2 Quality of life (QoL).
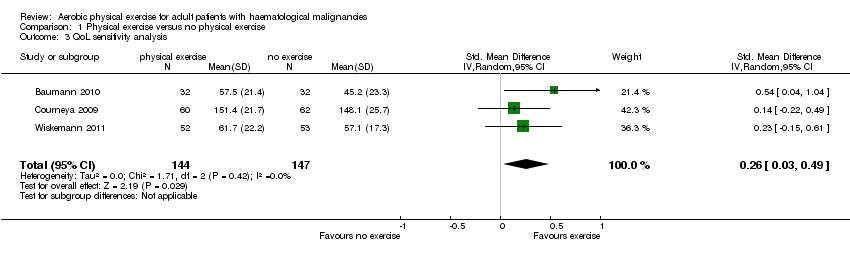
Comparison 1 Physical exercise versus no physical exercise, Outcome 3 QoL sensitivity analysis.

Comparison 1 Physical exercise versus no physical exercise, Outcome 4 QoL SCT versus no SCT.

Comparison 1 Physical exercise versus no physical exercise, Outcome 5 Physical functioning/QoL.

Comparison 1 Physical exercise versus no physical exercise, Outcome 6 Depression/QoL.

Comparison 1 Physical exercise versus no physical exercise, Outcome 7 Anxiety/QoL.
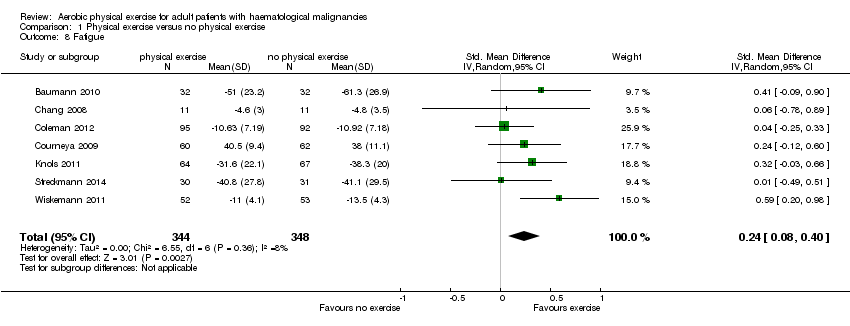
Comparison 1 Physical exercise versus no physical exercise, Outcome 8 Fatigue.

Comparison 1 Physical exercise versus no physical exercise, Outcome 9 Fatigue SCT versus no SCT.

Comparison 1 Physical exercise versus no physical exercise, Outcome 10 Weight.

Comparison 1 Physical exercise versus no physical exercise, Outcome 11 Lean body mass.
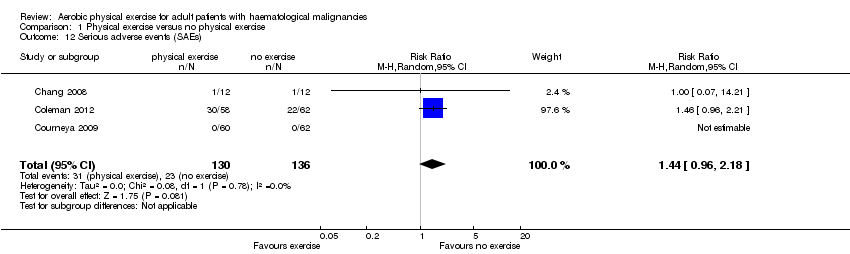
Comparison 1 Physical exercise versus no physical exercise, Outcome 12 Serious adverse events (SAEs).
| Physical exercise versus no physical exercise for adults with haematological malignancies | ||||||
| Patient or population: Adults with haematological malignancies | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control group without exercise | Physical exercise | |||||
| Overall survival Mortality | 224 per 1000 | 208 per 1000 | RR 0.93 | 269 | ⊕⊕⊕⊝ | Overall survival not reported, number of participants deceased during study or first 100 days |
| Quality of Life | The mean QoL in the intervention group was | SMD 0.26 | 291 | ⊕⊕⊝⊝ | ||
| Physical functioning/QoL with 1 indicating best outcome | The mean physical functioning/Qol in the intervention groups was | SMD 0.33 | 422 | ⊕⊕⊕⊝ | ||
| Depression/QoL with 1 indicating best outcome | The mean depression/qol in the intervention groups was | SMD 0.25 | 249 | ⊕⊕⊝⊝ | ||
| Anxiety/QoL with 1 indicating best outcome | The mean anxiety/qol in the intervention groups was | SMD ‐0.18 | 249 | ⊕⊕⊝⊝ | ||
| Fatigue with 1 indicating best outcome | The mean fatigue in the intervention groups was | SMD 0.24 | 692 | ⊕⊕⊕⊝ | ||
| Physical performance | see comment | see comment | see comment | see comment | see comment | Due to various outcome definitions and measuring instruments no meta‐analysis possible |
| Serious adverse events | 169 per 1000 | 244 per 1000 | RR 1.44 | 266 (3 studies) | ⊕⊕⊝⊝ | |
| Adverse events | 10 per 1000 | 72 per 1000 | RR 7.23 | 122 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Small number of participants and events, wide confidence interval 2Outcome assessor (participant) not blinded in participant‐reported outcome (QoL questionnaires) 3Baseline imbalances, especially usage of erythropoietin and thalidomide unknown in both intervention arms 4Very small number of participants and events, very wide confidence interval | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.59, 1.47] |
| 2 Quality of life (QoL) Show forest plot | 4 | 352 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.15, 0.45] |
| 3 QoL sensitivity analysis Show forest plot | 3 | 291 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.03, 0.49] |
| 4 QoL SCT versus no SCT Show forest plot | 4 | 352 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.15, 0.45] |
| 4.1 SCT | 2 | 169 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.04, 0.65] |
| 4.2 no SCT | 2 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.50, 0.39] |
| 5 Physical functioning/QoL Show forest plot | 4 | 422 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.13, 0.52] |
| 6 Depression/QoL Show forest plot | 3 | 249 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.00, 0.50] |
| 7 Anxiety/QoL Show forest plot | 3 | 249 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.64, 0.28] |
| 8 Fatigue Show forest plot | 7 | 692 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 9 Fatigue SCT versus no SCT Show forest plot | 7 | 692 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 9.1 SCT | 4 | 487 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.06, 0.55] |
| 9.2 no SCT | 3 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.12, 0.43] |
| 10 Weight Show forest plot | 2 | 253 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐4.08, 4.68] |
| 11 Lean body mass Show forest plot | 2 | 253 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐1.34, 4.02] |
| 12 Serious adverse events (SAEs) Show forest plot | 3 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.96, 2.18] |


