Hormona de crecimiento humana recombinante para el tratamiento de las quemaduras y los sitios donantes
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT, double‐blind | |
| Participants | 94 children (mean age 7.5 years) admitted to a US burn centre, ages between 1 and 18 years, n = 60 male. Burns > 40% TBSA + > 10% full‐thickness (third‐degree). Admitted within 3 days of injury. At least 1 donor site required. | |
| Interventions | rhGH 0.2 mg/kg/day (n = 45) or saline as a placebo (n = 49) administered by subcutaneous injection for the entire acute‐phase hospital stay (mean = 34.5 days, SD = 52.3) | |
| Outcomes | Burn Scar Rating Scale (Yeong 1997), % of people requiring reconstruction, number of plastic surgery operations in the first 2 years, time from injury to reconstructive operations in months. Burn scars were assessed by 3 experienced burn surgeons. | |
| Funding | Not reported | |
| Notes | Kappa interrater agreement was 0.78 for surface, 0.80 for border height, 0.72 for thickness, 0.81 for colour difference Only medians and ranges are given for Burn Scar Rating Scale categories, operations per patient and time from injury to reconstruction Ranges should not be used to estimate standard deviations (Higgins 2011b) The contacted authors could not provide additional data. Only data on percentage of people requiring reconstruction were included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Observers were blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | Patient follow‐up was completed for 95% of the participants |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Study protocol was not available. |
| Methods | RCT | |
| Participants | 219 adults enrolled in the study, from a burn centre in China. 12 people were lost; 207 people were analysed. Mean age: 36 years; 168 males, 39 females. Mean TBSA 61.5%; mean TBSA second‐degree burn 32%; mean TBSA third‐degree burn 19.6%. Included scalds, flame burns, chemical burns and electric burns. people with severe associated injuries were excluded. | |
| Interventions | rhGH 0.19 IU/kg/day (Gene Science®) (n = 112) or placebo saline (n = 95) were administered daily by subcutaneous injection morning or night beginning after a mean of 7.3 (SD = 2.8) days after injury and continuing for 10 to 16 days | |
| Outcomes | Mortality, hyperglycaemia (fasting blood‐glucose ≥ 10 mmol/L), septicaemia | |
| Funding | ||
| Notes | Article in Chinese. Informed consent for the study was obtained from participants or relatives. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised blocks", no further details reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Method of blinding not reported |
| Incomplete outcome data (attrition bias) | Low risk | 219 adults were enrolled in the study, 12 participants were lost or rejected and 207 participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Study protocol was not available. |
| Methods | RCT, double‐blind. | |
| Participants | 62 children (mean age: 8.6 years) who were admitted to a US burn centre. Ages between 2 to 18 years, n = 37 male. The children had burns > 40% TBSA with second‐ or third‐degree facial burns. The participants were treated with autografts during the acute phase and pressure garments after discharge. | |
| Interventions | rhGH 0.05 mg/kg/day (n = 30) or placebo (n = 32) were administered by subcutaneous injection from the patient's discharge date until 1 year after the burn. 6 of the 30 participants received 0.1 mg/kg/day rhGH. | |
| Outcomes | Seattle Scar Scale (Yeong 1997), Hamilton Scar Scale (Crowe 1998) and Vancouver Scar Scale (Sullivan 1990; Baryza 1995) at 6, 9, 12 and 18 to 24 months post‐burn were administered by 3 observers. The Seattle and Hamilton Scar Scales were scored by evaluating photographs of faces and scars. The Vancouver Scar Scale was used for the clinical evaluation of participants. | |
| Funding | The rhGH was provided by Eli Lilly and Company | |
| Notes | Increased levels of IGF‐1 were found in the treatment group (for assessment of compliance) No mean scores and standard deviations were provided in the publication. Data were provided by the authors. The 3 scar assessment observers were blinded to the treatment. Informed consent for the study was obtained from the participants or relatives. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | The 3 scar assessment observers were blinded to the treatment |
| Incomplete outcome data (attrition bias) | Low risk | No missing data for the Seattle, Hamilton and Vancouver Scar Scales |
| Selective reporting (reporting bias) | Unclear risk | Unclear. The study protocol was not available. |
| Methods | RCT, not blinded | |
| Participants | 36 adults admitted to a US burn centre in 1996 and 1997. Mean age: 48 years. Mean TBSA: 40%; mean full‐thickness: 29%; inhalation injury: 43%. | |
| Interventions | rhGH 0.1 mg/kg/day by intramuscular injection (n = 20) or 20 mg oral oxandrolone (n = 16) were administered once daily beginning between Days 7 and 10 post‐burn until the patient was ready for discharge to a rehabilitation centre | |
| Outcomes | Initial donor site healing time in days, as indicated by the atraumatic removal of the xeroform gauze. Net weight loss (kg) and nitrogen loss (g/day). | |
| Funding | ||
| Notes | The control group (n = 24) was not randomised (convenience sample), so the data of comparing rhGH with oxandrolone was included in the review, but the comparison with the control group was not. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Not possible because the rhGH was administered parenterally and oxandrolone was administered orally |
| Incomplete outcome data (attrition bias) | Low risk | No missing data for the healing time of donor sites and weight loss |
| Selective reporting (reporting bias) | Unclear risk | Unclear. The study protocol was not available. |
| Methods | RCT, double‐blind | |
| Participants | 46 children (mean age 7.8 years) admitted to a US burn centre. Ages were between 2 and 18 years; n = 33 male, n = 13 female. Flame or scald burns, > 40% TBSA + > 20% full‐thickness (third‐degree). Excision (except face and perineum) and grafting were completed within 48 hours of admission. Donor sites were harvested with electric dermatome and dressed with Scarlet Red®‐impregnated fine mesh gauze. | |
| Interventions | rhGH 0.2 mg/kg/day (n = 20) or placebo (n = 26) were administered by subcutaneous or intramuscular injection within 8 days of the burn on the morning of excision and until the burn wound was 95% closed or the initial donor site was healed | |
| Outcomes | The initial donor site's healing time in days, as indicated by the atraumatic removal of the Scarlet Red® gauze and assessed by 1 of 2 evaluators | |
| Funding | Supported by Genentech, Inc., San Francisco, California | |
| Notes | No standard deviations from the length of hospital stay in days. In this study, data from participants, who received rhGH for therapeutic reasons were also presented, but these data are not included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method was not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Participants, physicians and nurses were blinded to the contents of the injection vials, which were provided by the manufacturer |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data for the healing time of donor sites |
| Selective reporting (reporting bias) | Low risk | The healing time of donor sites was reported for the 46 participants included in the RCT. Only one outcome measure was reported in this study. Study protocol was not available. |
| Methods | RCT. Double‐blind, 2‐phase study. Randomisation was balanced for age, cause and extent of burn injury. | |
| Participants | 40 children (mean age: 8.6 years; range: 2 to 18 years) admitted to a US burn centre. Flame or scald burns, > 40% TBSA + > 20% full‐thickness (third‐degree). Excision (except face and perineum) and grafting occurred within 48 hours of admission. The donor sites were harvested with electric dermatome and dressed with Scarlet Red®‐impregnated fine mesh gauze. participants with severe associated injuries were excluded. | |
| Interventions | rhGH 0.1 mg/kg/day subcutaneous (n = 12, phase 1) or 0.2 mg/kg/day intramuscular (n = 10, phase 2) or placebo saline (total placebo n = 18; Phase 1: n = 12; Phase 2: n = 6) was administered by injection beginning at admission and continued throughout the hospitalisation period | |
| Outcomes | Healing time in days of the initial donor site as indicated by atraumatic removal of the Scarlet Red® gauze. Healing time for harvest 1, 2 and 3. Length of hospital stay per % TBSA burn. Hyperglycaemia, defined as elevated glucose levels necessitating exogenous insulin. Healing time of donor sites for harvest 1 reported for the 10 participants from phase 2 receiving 0.2 mg/kg/day rhGH and for the 17 participants of the combined placebo group used for this review. Length of hospital stay in days per % TBSA. | |
| Funding | Supported by Genentech, Inc., San Francisco, California | |
| Notes | This was a 2‐phase study. The placebo participants from the 2 phases were pooled and those data could not be split. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers chart" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Participants, physicians and nurses were blinded to the contents of the injection vials, which were numbered by the drug company. Laboratory studies were conducted by independent laboratories to ensure that blinding was maintained. |
| Incomplete outcome data (attrition bias) | Low risk | 1 patient from the control group had missing outcome data for healing time of donor sites for study Phase 2. No participants were missing data for length of hospital stay. |
| Selective reporting (reporting bias) | High risk | Length of hospital stay was not a pre‐specified outcome. No study protocol was available. |
| Methods | RCT, double‐blind | |
| Participants | 28 children (mean age: 5.4 years; range: 1 to 16 years) admitted to a US burn centre; 17 males. > 40% TBSA + > 10% full‐thickness (third‐degree). Mean TBSA: 60%; mean third‐degree burn area: 50%. | |
| Interventions | rhGH 0.2 mg/kg/day (n = 13) or placebo (saline, n = 15) by subcutaneous injection within 3 days after injury and for at least 25 days afterward. Mean length of rhGH therapy: 34 days. | |
| Outcomes | Mortality. Acute phase proteins, constitutive hepatic proteins, cytokines and IGF‐1. | |
| Funding | ||
| Notes | Additional data were provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Method of blinding not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing data for mortality |
| Selective reporting (reporting bias) | Low risk | All outcome data were presented in a pre‐specified way. Only data for the outcome mortality were used for meta‐analysis. The study protocol was not available. |
| Methods | RCT, double‐blind | |
| Participants | 24 adults (mean age: 36.7 years; range: 18 to 65 years) admitted to a burn centre in Spain; 19 males. Flame or scald burns; > 40% TBSA and > 15% full‐thickness (third‐degree). Escharectomy and first grafting took place after a mean of 16.2 days. The donor sites were harvested with electric dermatome and dressed with hydrocolloid cellulose. participants with multiple traumas were excluded. | |
| Interventions | rhGH 0.15 mg/kg/day (Humatrope©) (n = 13) or placebo (n = 11) were administered by intramuscular injection in 2 equal doses beginning on the day of the first autograft and continuing until hospital discharge | |
| Outcomes | Donor site healing time. The donor site was classified as healed when it was adequate for re‐harvesting as a new autograft donor site. Mean number of skin grafts per patient Time admitted to the burn unit in absolute days or in relation to % TBSA or % full‐thickness | |
| Funding | The study was supported by Lilly, S.A. | |
| Notes | The donor site area was evaluated daily by the same person Adverse effect: 1 patient had hyperglycaemia that required insulin therapy. Informed consent was obtained for the study from the patient or relatives. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Method of blinding not reported |
| Incomplete outcome data (attrition bias) | Low risk | Data were missing for 1 control group patient |
| Selective reporting (reporting bias) | High risk | Mortality and septicaemia were not pre‐specified outcomes. The study protocol was not available. |
| Methods | RCT | |
| Participants | 60 adults, mean age 38.6 years, admitted in burn centre in China from March 2000 to June 2003. TBSA 30% to 80%; mean TBSA: 61.5%; > 20% third‐degree; mean third‐degree burn area: 33.1%. No participants had severe inhalation injury. Deep burns were treated with escharotomy and skin grafting. | |
| Interventions | 3 groups of n = 20 each. Control group: glycine orally as placebo (0.5 g/kg/day); glutamine + rhGH group: glutamine orally (0.5 g/kg/day) with 0.2 IU/kg/day rhGH by daily subcutaneous injection; glutamine group: only glutamine orally (0.5 g/kg/day). Treatment was administered from the 1st to 14th post‐burn day. | |
| Outcomes | Wound healing rate in % on 30th post‐burn day. Wound healing rate was not defined. Total hospital stay in days. | |
| Funding | ||
| Notes | Article in Chinese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | The blinding method was not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No data were missing for wound healing rate and length of hospital stay |
| Selective reporting (reporting bias) | Low risk | All presented outcome data were pre‐specified, but the study protocol was not available |
| Methods | RCT | |
| Participants | 20 adults, (15 males, 5 females) admitted to a burn centre in China from July 1998 to July 1999. Mean age: 30.5 years; intervention group mean age: 32 years (SD = 4); control group mean age: 29 years (SD = 6). Mean 61% TBSA, mean 27% third‐degree TBSA. All participants underwent eschar excision < 4 days and skin autografting. | |
| Interventions | rhGH 0.5 IU/kg/day subcutaneously (n = 10) or normal saline subcutaneously from the 3rd to 17th post‐burn day | |
| Outcomes | Healing time of deep partial‐thickness burns and donor sites in days. Wound healing rate was not defined. Length of hospital stay in days. Hyperglycaemia (blood sugar > 12 mmol/L for 3 consecutive days). Mortality was zero in both groups, therefore these data with no events were not used in this meta‐analysis. | |
| Funding | ||
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing data for the healing times of burn wounds and donor sites and duration of hospital stay |
| Selective reporting (reporting bias) | Low risk | All presented outcome data were pre‐specified, but the study protocol was not available |
| Methods | RCT, double‐blind | |
| Participants | 49 adults (mean age: 38.3 years; range: 18 to 60 years; 44 males) admitted to a burn centre in Germany, between 1995 and 1997. Abbreviated Burn Severity Index 7 to 11; > 20% TBSA. Early excision and autografting with mesh were performed. Donor sites were harvested with electric dermatome and dressed with Scarlet Red® ‐impregnated fine mesh gauze. participants with multiple injuries were excluded. | |
| Interventions | rhGH 0.5 IU/kg/day (Genotropin©; n = 26) or placebo, water with m‐cresol (n = 23), were administered by intramuscular injection in 2 equal doses beginning on the second day after trauma and continuing for 28 days | |
| Outcomes | The Wound Closure Index (WCI; Scott‐Conner 1986a) of the transplanted and un operated wounds. Wound healing was defined as complete epithelisation. Donor site healing time in days, as indicated by the atraumatic removal of the Scarlet Red® gauze. Wound healing was measured daily clinically and with photographs by the same person, who was blinded to treatment. | |
| Funding | Not reported | |
| Notes | 1 patient in the rhGH group was withdrawn because of hyperglycaemia; 4 in the rhGH group and 3 in the placebo group died (1 additional patient in the placebo group died 1 day after the study stopped). 4 participants withdrew their permission (2 in each group). 19 participants in the rhGH group and 18 in the placebo group were used for analysis. A WCI of 1 means a healing rate of 1% per day As‐treated or per‐protocol analyses were performed by the authors of the study No standard deviations of WCI and of healing time of donor sites in days were given, so this study was not used for the analysis of the healing time. Informed consent for the study was obtained from patient or relatives. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was provided by pharmaceutical firm |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | The contents of the injection vials were masked. Wound healing was measured clinically and with photographs daily by the same person who was blinded to the treatment. |
| Incomplete outcome data (attrition bias) | Low risk | 7 participants were withdrawn in the treatment group and 5 were withdrawn in the placebo group. 1 patient in the rhGH group was withdrawn because of hyperglycaemia; 4 in the rhGH group and 3 in the placebo group died (1 additional patient in the placebo group died 1 day after the study stopped). 4 participants withdrew their permission (2 in each group). 19 participants in the rhGH group and 18 in the placebo group were used for analysis. |
| Selective reporting (reporting bias) | Low risk | All of the presented outcome data were pre‐specified, but the study protocol was not available |
| Methods | RCT, double‐blind | |
| Participants | 44 children aged 19 or younger (mean age: 8 years; 30 males) were enrolled study between 1999 and 2004 for an additional year of examinations after 1 year of rhGH treatment post‐discharge from a US burn centre. TBSA > 40%; mean 56% TBSA; mean 47% third‐degree burns. The children were randomised upon discharge. | |
| Interventions | rhGH 0.05 mg/kg/day (n = 19) or placebo (n = 25) by subcutaneous injection from discharge date until 1 year after burn. The injections started on the day of discharge (equal to the time point at which wounds were 95% healed). After another year without rhGH treatment, 14 participants in the rhGH group and 18 in the placebo group completed the study. | |
| Outcomes | Outcomes were estimated one day before discharge and after 12 and 24 months post‐burn. Need for reconstructive operations. Hyperglycaemia. Lean body mass estimated with dual‐energy X‐ray absorptiometry (DEXA). Resting energy expenditure (REE). Echocardiography. Isokinetic strength measurement with Biodex® dynamometer for the dominant leg extensor at 150º/sec. Vancouver Scar Scale. The hyperglycaemia incidence was zero in both groups; therefore, these data with no events were not used in this meta‐analysis. | |
| Funding | rhGH was provided as a gift from Eli Lilly Corporation | |
| Notes | Compliance was measured with the Self‐Reported Compliance Questionnaire and with serum levels of insulin‐like growth factor‐1 (IGF‐1). Greater than 75% compliance with the daily study drug was necessary to remain in study. Scar assessment was performed by observers who were blinded to the treatment. Authors information: the data for left ventricular function are the not same as those from Mlcak 2005 Additional data were provided by the authors. No data from the scar assessment were available. Informed consent for the study was obtained from the patient or relatives. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 44 children were enrolled in the study; reconstructive procedure data were available for 32 participants. 5 participants in the treatment group and 7 participants in the control group did not complete the study. |
| Selective reporting (reporting bias) | Low risk | All presented outcome data were pre‐specified, but the study protocol was not available |
| Methods | Placebo‐controlled prospective study | |
| Participants | 16 adults (age range: 19 to 50 years) admitted to a burn centre in China between February 1996 and June 1997. Mean 81% TBSA; mean 61% third‐degree TBSA. All participants underwent eschar excision < 4 days and autografting with skin pulp. participants with associated injuries were excluded. | |
| Interventions | rhGH 0.3 IU/kg/day subcutaneously (n = 8) or 2 cc normal saline (n = 8) for 10 days, starting on the first postoperative day | |
| Outcomes | Grafted burn wound area and donor site healing time. Healing time was not defined. Wound healing rate at the 30th postoperative day. Duration of hospitalisation. Serum amino acid profile on Days 1 and 20 post‐burn. The hyperglycaemia incidence was zero in both groups; therefore, these data with no events were not used in this meta‐analysis. | |
| Funding | ||
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing data for the healing time of burn wounds or donor sites and the duration of hospital stay |
| Selective reporting (reporting bias) | High risk | Mortality and septicaemia were not pre‐specified outcomes. No study protocol was available. |
IGF‐1: insulin‐like growth factor‐1; IU: international units; RCT: randomised controlled trial; rhGH: recombinant human growth hormone; SD: standard deviation; TBSA: total body surface area
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The excluded study addressed none of the pre‐specified outcome measures | |
| The excluded study addressed none of the pre‐specified outcome measures | |
| The excluded study addressed none of the pre‐specified outcome measures | |
| The excluded study addressed none of the pre‐specified outcome measures | |
| The excluded study addressed none of the pre‐specified outcome measures | |
| This study addressed none of the pre‐specified outcome measures except hyperglycaemia. The hyperglycaemia incidence was zero in the rhGH and placebo groups; therefore, these data with no events cannot be used in this meta‐analysis. | |
| The excluded study addressed none of the pre‐specified outcome measures | |
| The study was not a RCT. Participants were not randomised. | |
| The excluded study addressed none of the pre‐specified outcome measures | |
| The excluded study addressed none of the pre‐specified outcome measures | |
| The excluded study did not address a pre‐specified outcome measure | |
| The excluded study addressed none of the pre‐specified outcome measures | |
| The excluded study addressed none of the pre‐specified outcome measures | |
| The excluded study addressed none of the pre‐specified outcome measures |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing time of burn wounds in days for adults Show forest plot | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐9.07 [‐13.76, ‐4.39] |
| Analysis 1.1  Comparison 1 Comparison of rhGH with placebo, Outcome 1 Healing time of burn wounds in days for adults. | ||||
| 2 Donor site healing time in days for adults Show forest plot | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐3.15 [‐4.75, ‐1.54] |
| Analysis 1.2  Comparison 1 Comparison of rhGH with placebo, Outcome 2 Donor site healing time in days for adults. | ||||
| 3 Donor site healing time in days for children Show forest plot | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.53, ‐0.87] |
| Analysis 1.3 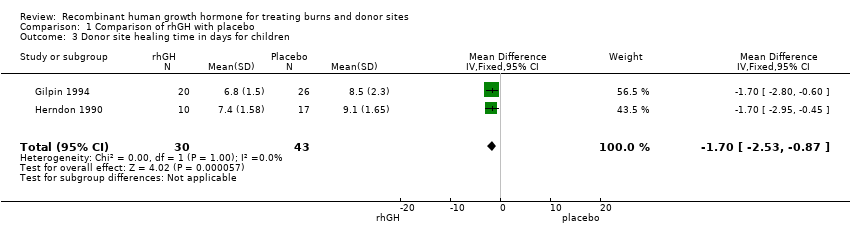 Comparison 1 Comparison of rhGH with placebo, Outcome 3 Donor site healing time in days for children. | ||||
| 4 Length of hospital stay Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Comparison of rhGH with placebo, Outcome 4 Length of hospital stay. | ||||
| 4.1 Adults | 4 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐12.55 [‐17.09, ‐8.00] |
| 4.2 Children | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐29.94, 15.94] |
| 5 Mortality Show forest plot | 5 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.22, 1.29] |
| Analysis 1.5  Comparison 1 Comparison of rhGH with placebo, Outcome 5 Mortality. | ||||
| 5.1 Adults | 4 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.19, 1.25] |
| 5.2 Children | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.08, 16.67] |
| 6 Adverse events Show forest plot | 5 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.68, 4.16] |
| Analysis 1.6 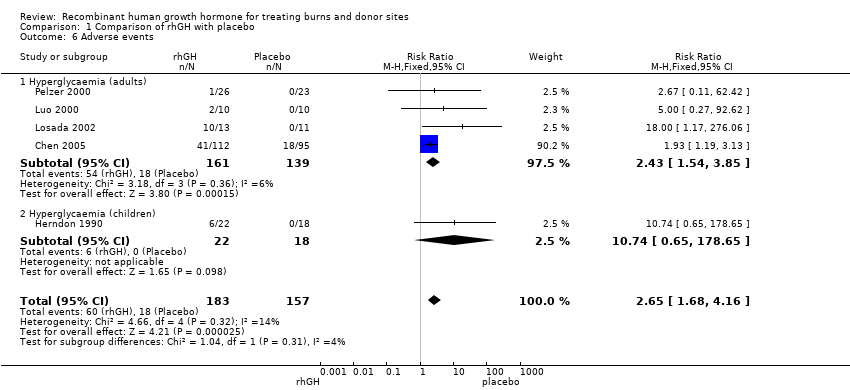 Comparison 1 Comparison of rhGH with placebo, Outcome 6 Adverse events. | ||||
| 6.1 Hyperglycaemia (adults) | 4 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.54, 3.85] |
| 6.2 Hyperglycaemia (children) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.74 [0.65, 178.65] |
| 7 Adverse event: Septicaemia in adults Show forest plot | 4 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.22] |
| Analysis 1.7 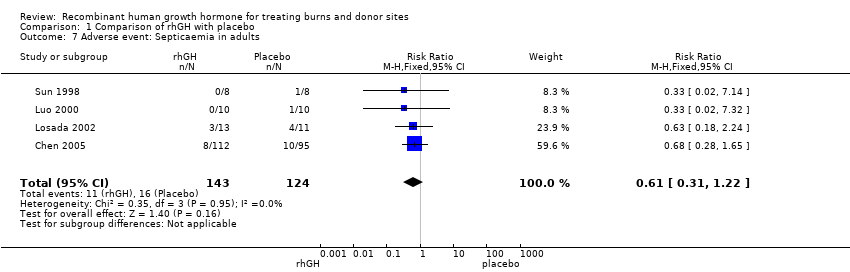 Comparison 1 Comparison of rhGH with placebo, Outcome 7 Adverse event: Septicaemia in adults. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Donor site healing in days Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Comparison of rhGH with oxandrolone, Outcome 1 Donor site healing in days. | ||||
| 2 Hyperglycaemia (blood glucose > 225 mg/dl) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Comparison of rhGH with oxandrolone, Outcome 2 Hyperglycaemia (blood glucose > 225 mg/dl). | ||||

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
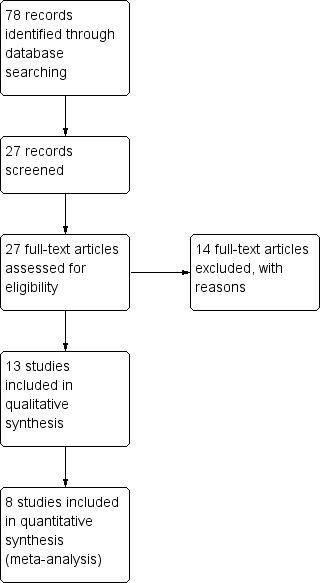
Study flow diagram.

Comparison 1 Comparison of rhGH with placebo, Outcome 1 Healing time of burn wounds in days for adults.
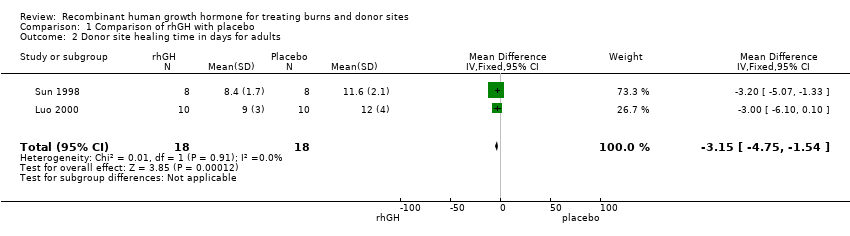
Comparison 1 Comparison of rhGH with placebo, Outcome 2 Donor site healing time in days for adults.

Comparison 1 Comparison of rhGH with placebo, Outcome 3 Donor site healing time in days for children.

Comparison 1 Comparison of rhGH with placebo, Outcome 4 Length of hospital stay.
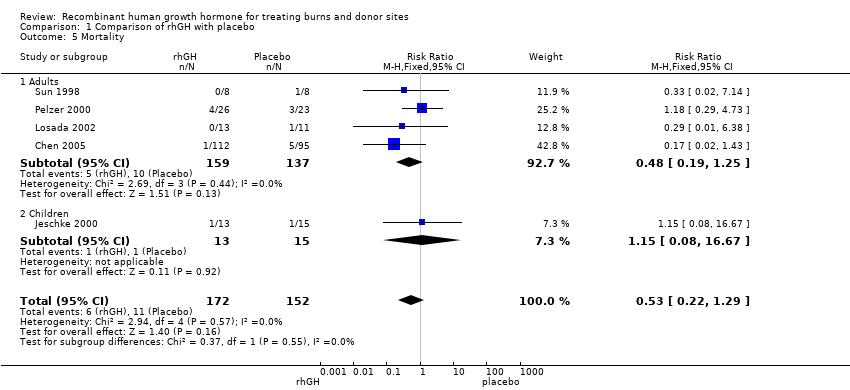
Comparison 1 Comparison of rhGH with placebo, Outcome 5 Mortality.

Comparison 1 Comparison of rhGH with placebo, Outcome 6 Adverse events.

Comparison 1 Comparison of rhGH with placebo, Outcome 7 Adverse event: Septicaemia in adults.

Comparison 2 Comparison of rhGH with oxandrolone, Outcome 1 Donor site healing in days.
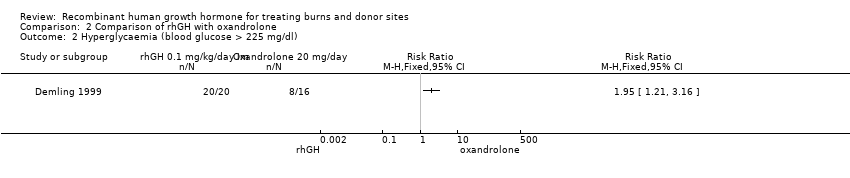
Comparison 2 Comparison of rhGH with oxandrolone, Outcome 2 Hyperglycaemia (blood glucose > 225 mg/dl).
| Recombinant human growth hormone compared with placebo for treating burns and donor sites | ||||||
| Patient or population: | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Recombinant human growth hormone | ||||||
| Healing time of burn wounds in days for adults | The mean healing time of burn wounds in days for adults in the intervention groups was 9.07 lower (4.39 to 13.76 lower) | 36 | ⊕⊕⊝⊝ | |||
| Donor site healing time in days for adults | The mean donor site healing time in days for adults in the intervention groups was | 36 | ⊕⊕⊝⊝ | |||
| Donor site healing time in days for children | The mean donor site healing time in days for children in the intervention groups was | 73 (2 studies) | ⊕⊕⊝⊝ | |||
| Mortality in adults and children | Study population5 | RR 0.53 | 324 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 4 per 100 (2 to 9) | |||||
| Low5 | ||||||
| 5 per 100 | 3 per 100 (1 to 6) | |||||
| High5 | ||||||
| 13 per 100 | 7 per 100 (3 to 17) | |||||
| Septicaemia in adults | Study population8 | RR 0.61 | 267 | ⊕⊕⊝⊝ | ||
| 13 per 100 | 8 per 100 (4 to 16) | |||||
| Low8 | ||||||
| 4 per 100 | 2 per 100 (1 to 5) | |||||
| High8 | ||||||
| 13 per 100 | 8 per 100 (4 to 16) | |||||
| Hyperglycaemia in adults and children | Study population10 | RR 2.65 | 340 | ⊕⊕⊝⊝ | ||
| 11 per 100 | 30 per 100 (19 to 48) | |||||
| Low10 | ||||||
| 0 per 100 | 0 per 100 (0 to 0) | |||||
| High10 | ||||||
| 19 per 100 | 50 per 100 (32 to 79) | |||||
| Length of hospital stay in days for adults | The mean length of hospital stay in days for adults in the intervention groups was | 99 | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Method of randomisation, allocation concealment and blinding not reported. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing time of burn wounds in days for adults Show forest plot | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐9.07 [‐13.76, ‐4.39] |
| 2 Donor site healing time in days for adults Show forest plot | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐3.15 [‐4.75, ‐1.54] |
| 3 Donor site healing time in days for children Show forest plot | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.53, ‐0.87] |
| 4 Length of hospital stay Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Adults | 4 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐12.55 [‐17.09, ‐8.00] |
| 4.2 Children | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐29.94, 15.94] |
| 5 Mortality Show forest plot | 5 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.22, 1.29] |
| 5.1 Adults | 4 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.19, 1.25] |
| 5.2 Children | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.08, 16.67] |
| 6 Adverse events Show forest plot | 5 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.68, 4.16] |
| 6.1 Hyperglycaemia (adults) | 4 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.54, 3.85] |
| 6.2 Hyperglycaemia (children) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.74 [0.65, 178.65] |
| 7 Adverse event: Septicaemia in adults Show forest plot | 4 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Donor site healing in days Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Hyperglycaemia (blood glucose > 225 mg/dl) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

