Hormona de crecimiento humana recombinante para el tratamiento de las quemaduras y los sitios donantes
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008990.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 septiembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Roelf S. Breederveld: conceived the review question, developed the review, completed the first draft, made an intellectual contribution to the review and approved the final version prior to submission.
Wim E. Tuinebreijer: developed the review, co‐ordinated the review development, completed the first draft of the review, made an intellectual contribution to the review and approved the final version prior to submission.
Contributions of the editorial base:
Nicky Cullum edited the review; advised on the methodology, interpretation and content; and approved the final review prior to submission.
Sally Bell‐Syer co‐ordinated the editorial process; advised on the methodology, interpretation and content; and edited the review and the updated review.
Amanda Briant ran some of the searches.
Sources of support
Internal sources
-
Red Cross Hospital, Beverwijk, Netherlands.
Use of library and internet facilities.
External sources
-
The National Institute for Health Research (NIHR) is the sole funder of the Cochrane Wounds Group, UK.
Declarations of interest
There are no known conflicts of interests.
Acknowledgements
The authors would like to acknowledge the contribution of the peer referees who commented on this review: Wounds Group editors (Marian Brady, Dirk Ubbink, Ruth Foxlee), referees (Jane Burch, Richard Kirubakaran, Heather Cleland, Arturo Marti‐Carvajal, Amy Zelmer) and copy editor Jenny Bellorini.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Sep 15 | Recombinant human growth hormone for treating burns and donor sites | Review | Roelf S Breederveld, Wim E Tuinebreijer | |
| 2012 Dec 12 | Recombinant human growth hormone for treating burns and donor sites | Review | Roelf S Breederveld, Wim E Tuinebreijer | |
| 2011 Feb 16 | Recombinant human growth hormone for treating burns and donor sites | Protocol | Roelf S Breederveld, Wim E Tuinebreijer | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Adult; Aged; Child; Child, Preschool; Humans; Infant; Middle Aged; Young Adult;
PICO

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
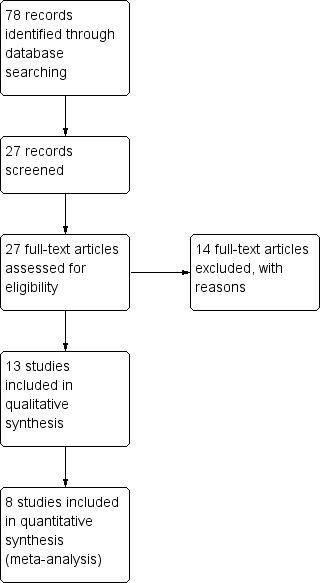
Study flow diagram.

Comparison 1 Comparison of rhGH with placebo, Outcome 1 Healing time of burn wounds in days for adults.
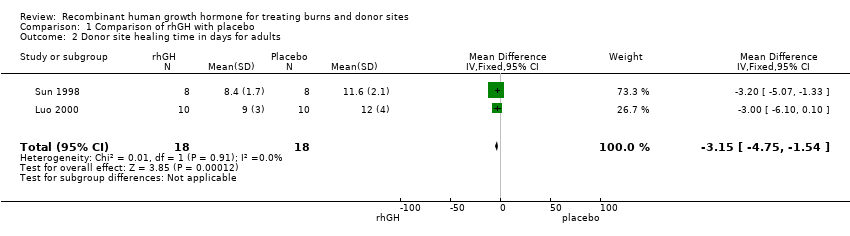
Comparison 1 Comparison of rhGH with placebo, Outcome 2 Donor site healing time in days for adults.

Comparison 1 Comparison of rhGH with placebo, Outcome 3 Donor site healing time in days for children.

Comparison 1 Comparison of rhGH with placebo, Outcome 4 Length of hospital stay.
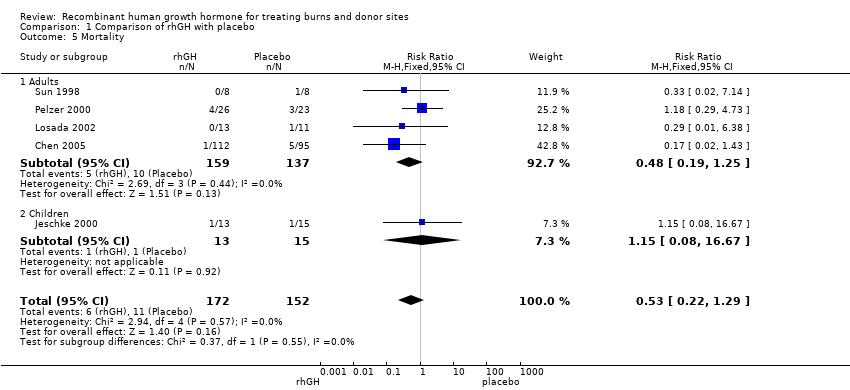
Comparison 1 Comparison of rhGH with placebo, Outcome 5 Mortality.

Comparison 1 Comparison of rhGH with placebo, Outcome 6 Adverse events.

Comparison 1 Comparison of rhGH with placebo, Outcome 7 Adverse event: Septicaemia in adults.

Comparison 2 Comparison of rhGH with oxandrolone, Outcome 1 Donor site healing in days.
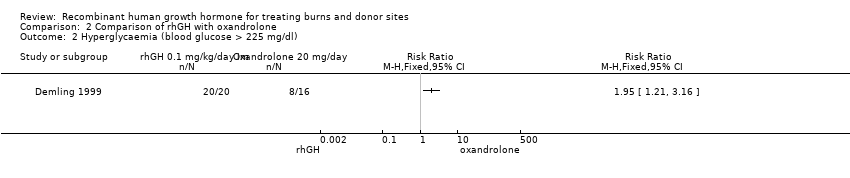
Comparison 2 Comparison of rhGH with oxandrolone, Outcome 2 Hyperglycaemia (blood glucose > 225 mg/dl).
| Recombinant human growth hormone compared with placebo for treating burns and donor sites | ||||||
| Patient or population: | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Recombinant human growth hormone | ||||||
| Healing time of burn wounds in days for adults | The mean healing time of burn wounds in days for adults in the intervention groups was 9.07 lower (4.39 to 13.76 lower) | 36 | ⊕⊕⊝⊝ | |||
| Donor site healing time in days for adults | The mean donor site healing time in days for adults in the intervention groups was | 36 | ⊕⊕⊝⊝ | |||
| Donor site healing time in days for children | The mean donor site healing time in days for children in the intervention groups was | 73 (2 studies) | ⊕⊕⊝⊝ | |||
| Mortality in adults and children | Study population5 | RR 0.53 | 324 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 4 per 100 (2 to 9) | |||||
| Low5 | ||||||
| 5 per 100 | 3 per 100 (1 to 6) | |||||
| High5 | ||||||
| 13 per 100 | 7 per 100 (3 to 17) | |||||
| Septicaemia in adults | Study population8 | RR 0.61 | 267 | ⊕⊕⊝⊝ | ||
| 13 per 100 | 8 per 100 (4 to 16) | |||||
| Low8 | ||||||
| 4 per 100 | 2 per 100 (1 to 5) | |||||
| High8 | ||||||
| 13 per 100 | 8 per 100 (4 to 16) | |||||
| Hyperglycaemia in adults and children | Study population10 | RR 2.65 | 340 | ⊕⊕⊝⊝ | ||
| 11 per 100 | 30 per 100 (19 to 48) | |||||
| Low10 | ||||||
| 0 per 100 | 0 per 100 (0 to 0) | |||||
| High10 | ||||||
| 19 per 100 | 50 per 100 (32 to 79) | |||||
| Length of hospital stay in days for adults | The mean length of hospital stay in days for adults in the intervention groups was | 99 | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Method of randomisation, allocation concealment and blinding not reported. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing time of burn wounds in days for adults Show forest plot | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐9.07 [‐13.76, ‐4.39] |
| 2 Donor site healing time in days for adults Show forest plot | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐3.15 [‐4.75, ‐1.54] |
| 3 Donor site healing time in days for children Show forest plot | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.53, ‐0.87] |
| 4 Length of hospital stay Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Adults | 4 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐12.55 [‐17.09, ‐8.00] |
| 4.2 Children | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐29.94, 15.94] |
| 5 Mortality Show forest plot | 5 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.22, 1.29] |
| 5.1 Adults | 4 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.19, 1.25] |
| 5.2 Children | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.08, 16.67] |
| 6 Adverse events Show forest plot | 5 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.68, 4.16] |
| 6.1 Hyperglycaemia (adults) | 4 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.54, 3.85] |
| 6.2 Hyperglycaemia (children) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.74 [0.65, 178.65] |
| 7 Adverse event: Septicaemia in adults Show forest plot | 4 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Donor site healing in days Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Hyperglycaemia (blood glucose > 225 mg/dl) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

