Tratamiento con corticosteroides transplacentarios versus corticosteroides fetales directos para acelerar la maduración pulmonar fetal cuando hay riesgo de parto prematuro
Resumen
Antecedentes
A pesar de los importantes adelantos en la tecnología médica, la incidencia de partos prematuros aún es elevada. El uso de corticosteroides prenatales administrados por vía transplacentaria, mediante inyección intramuscular en las mujeres con riesgo de parto prematuro, ha disminuido la incidencia del síndrome de dificultad respiratoria e incrementado las tasas de supervivencia de los recién nacidos prematuros. Sin embargo, esta intervención también tiene sus propios riesgos y efectos secundarios. Los estudios en animales y los primeros estudios en embarazadas con riesgo de parto prematuro informaron sobre el uso de una vía alternativa de administración, la inyección intramuscular directa de corticosteroides en el feto con guía ecográfica, en un intento de disminuir el perfil de efectos secundarios. La administración fetal directa de corticosteroides puede tener efectos beneficiosos, en comparación con la administración materna, en cuanto a la eficacia y la seguridad.
Objetivos
Evaluar si las diferentes vías de administración de corticoesteroides (materna versus fetal directa) tienen algún efecto sobre los resultados de salud de las mujeres y sus recién nacidos.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (25 de octubre 2017), ClinicalTrials.gov, la WHO International Clinical Trials Registry Platform (ICTRP, 25 de octubre 2017) y en las listas de referencias de los estudios recuperados.
Criterios de selección
Ensayos controlados aleatorizados que comparen la vía materna y fetal directa de administración de corticosteroides prenatales en mujeres con riesgo de parto prematuro.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente evaluaron la elegibilidad de los estudios. En futuras actualizaciones de esta revisión, al menos dos autores de la revisión extraerán los datos y evaluarán el riesgo de sesgo de los estudios incluidos. También se evaluará la calidad de la evidencia mediante los criterios GRADE.
Resultados principales
No se identificaron ensayos controlados aleatorizados elegibles para esta revisión.
Conclusiones de los autores
Los estudios clínicos disponibles realizados hasta el momento en animales y en seres humanos han mostrado que la inyección intramuscular directa de corticosteroides en el feto mediante guía ecográfica es factible, pero faltan datos sobre los resultados de salud. Por lo tanto, persiste la incertidumbre en cuanto a qué método podría proporcionar el mejor perfil de eficacia y seguridad. Se requieren ensayos controlados aleatorizados que se centren en los efectos beneficiosos y perjudiciales del tratamiento con corticosteroides fetales directos versus transplacentarios. Hasta que se hayan respondido las dudas, es aconsejable seguir utilizando el estándar actual de tratamiento prenatal con corticosteroides administrados por vía materna transplacentaria.
PICO
Resumen en términos sencillos
Inyección directa de corticoesteroides al feto en comparación con la inyección a la madre para mejorar los resultados fetales cuando la madre tiene riesgo de parto prematuro
¿Cuál es el problema?
Los recién nacidos prematuros (menos de 37 semanas) presentan riesgo de muerte, hemorragia cerebral y problemas respiratorios porque sus pulmones no está completamente desarrollados. El tratamiento con corticosteroides administrado a la madre antes del parto prematuro ha demostrado ser efectivo para prevenir estos problemas y se ha convertido en la atención estándar en muchos países. El método habitual para administrar corticosteroides es mediante una inyección intramuscular a la madre. El tratamiento con corticosteroides se transfiere luego a través de la placenta (conocida como transferencia transplacentaria) al feto. Este tratamiento tiene sus propios riesgos, como la disminución del crecimiento fetal y el desarrollo cerebral, así como el aumento de los riesgos del recién nacido de sufrir enfermedades como diabetes e hipertensión arterial. Es factible inyectar corticosteroides directamente al feto mediante guía ecográfica.
¿Por qué es esto importante?
La inyección de corticoesteroides directamente en el feto, en lugar de inyectarlos en los músculos de la madre, puede prevenir el riesgo de aumento de la presión sanguínea, el incremento de los niveles de glucosa en la sangre y la susceptibilidad a la sepsis en la madre. También puede reducir la cantidad de corticoesteroides necesarios. Sin embargo, conlleva un riesgo de infección del útero y de lesiones fetales, y puede provocar un trabajo de parto y un parto prematuros. No se han realizado estudios que evalúen los efectos beneficiosos y los daños de la inyección directa en el feto, en comparación con la inyección en la madre.
¿Qué evidencia se encontró?
Se buscó la evidencia el 25 de octubre 2017 y no se encontraron ensayos controlados aleatorizados completados que evaluaran los efectos beneficiosos y los daños de la inyección directa de corticosteroides en el feto en comparación con la inyección en la madre, para las mujeres con riesgo de parto prematuro. Se encontraron dos estudios, pero uno no fue un ensayo controlado aleatorizado, y en el otro estudio los métodos no fueron claros, por lo que se estableció contacto con los autores del estudio para obtener más información.
¿Qué significa esto?
Se necesitan más estudios para evaluar los efectos de la inyección de corticoesteroides directamente en el feto en comparación con la inyección en los músculos de la madre. Los recién nacidos en estos ensayos se deben seguir durante un período largo para poder vigilar los efectos de los corticosteroides sobre el desarrollo de la niñez, incluidas las deficiencias o discapacidades como la parálisis cerebral. Para establecer si un método es mejor que el otro, se necesitan ensayos aleatorizados de buena calidad.
Authors' conclusions
Background
Description of the condition
The World Health Organization (WHO) defines preterm birth as birth before 37 weeks of gestation (WHO 2015) and this condition is associated with high neonatal morbidity and mortality. Prematurity is the main cause of perinatal mortality and morbidity in high‐income countries (Evans 1993; Goldenberg 2007). In 2013, preterm birth accounted for 16% of all perinatal mortality in Australia (AIHW 2015). Despite advances in medical technology, the primary cause of early neonatal death in preterm infants remains respiratory distress syndrome (RDS) as a consequence of immature lung development and surfactant insufficiency. With increasing gestational age, organ systems are more mature and this increases the survival rate (Doyle 2001; Saigal 2007). Preterm infants who survive the neonatal period are at increased risk of chronic disability, including but not limited to neurological disability and chronic pulmonary disease (Doyle 2001; Evans 1993). As preterm birth is associated with high mortality and long‐term adverse health impact compared with birth at term, this clearly signifies a prominent clinical as well as economic burden on healthcare resources (Goldenberg 2007; WHO 2015).
Description of the intervention
Single (Roberts 2017) or multiple (Crowther 2015) courses of antenatal corticosteroid in women at risk of preterm birth have been shown to reduce the incidence of RDS, as first described by Liggins 1972. Corticosteroid is a steroid hormone that acts by increasing protein and phospholipid synthesis, increasing levels of surfactant in the fetal lung and accelerating maturation of the fetal lung (Ballard 1995; Evans 1993). In addition to reducing the incidence of RDS, antenatal corticosteroid treatment prior to preterm birth has been shown to reduce the risk of perinatal death, neonatal death, intraventricular haemorrhage, infections, intensive care unit admission and developmental delay in childhood (Roberts 2017). These outcomes also depend on the gestational age of the pregnancy. Antenatal corticosteroids have become the standard of care for women at risk of preterm birth in many countries (Antenatal Corticosteroid Guidelines Panel 2015; Haram 2003; Jobe 2004; NIH 1995; WHO 2015).
However, the short‐ and long‐term safety profile of antenatal corticosteroid treatment is still debatable, especially in multiple corticosteroid administrations. Corticosteroids are known to inhibit cell growth and DNA replication. Animal studies have demonstrated that maternal corticosteroid administration at the minimal effective dose inhibits fetal growth, increases fetal blood pressure and perhaps modifies neurodevelopment (Fowden 1996; Jobe 1998; Moss 2003). The severity increases as the dose and number of administrations increase (Moss 2003). The known short‐term effects of antenatal corticosteroids in the fetus are decreased fetal breathing and movements, and a reduction in the amniotic fluid volume (Babovic 2009; Jackson 2003). Exposure to high levels of cortisol in a normally low fetal cortisol environment, in addition to other stress hormones produced with growth restriction, may have lifelong effects, leading to fetal programming for adult diseases, such as hypertension, insulin resistance, diabetes mellitus and metabolic syndrome (Benediktsson 1993; Dalziel 2005; Newnham 1999). Maternal administration of corticosteroid (orally, intramuscularly or intravascularly) not only affects the fetus but has the potential for negative maternal side effects, such as elevation of maternal blood pressure (Babovic 2009) and blood glucose concentrations, increasing susceptibility to sepsis (Evans 1993).
There remains variation in clinical practice on issues regarding the use of antenatal corticosteroids, including the type of corticosteroid to use, the dose and the optimal route of administration (Brownfoot 2013; Jobe 2004). The common route of administration is intramuscularly to the mother, with transplacental transfer to the fetus (Roberts 2017). Animal studies and early studies in pregnant women at risk of preterm birth have reported the use of an alternative route of administration, by direct intramuscular injection of corticosteroid into the fetus under ultrasound guidance, in an attempt to minimise the side‐effect profile. These early studies indicate that direct fetal administration may be feasible in humans but further research still needs to be conducted (Moss 2003).
How the intervention might work
Direct fetal ultrasound‐guided injection of corticosteroid is believed to induce lung maturation of the preterm fetus, with different side‐effect profiles compared to indirect administration. The most important advantages of direct administration could include the avoidance of maternal toxicity and the metabolic side effects, as well as increasing the efficacy of bypassing the placenta and therefore inducing a more rapid fetal lung maturation (Evans 1993; Jobe 1993).
One of the first few animal studies of direct fetal corticosteroid treatment used ultrasound to deliver direct fetal injection of corticosteroid or saline control to 36 pregnant ewes. The preterm lambs were delivered at 128 days gestational age, to assess the postnatal lung function (Jobe 1993). The authors found that relative to the saline control group, corticosteroid given as a single injection 48 hours before birth resulted in a significant improvement in postnatal lung function (Jobe 1993).
Another animal study comparing direct fetal against maternal administration of corticosteroid showed that administration of repeated doses of corticosteroid directly to the fetus did not produce the growth restriction induced by maternal administration (Newnham 1999). There was no reduction in the fetal birthweight or weights of major organs, with the exception of the liver (Newnham 1999).
The first human study (a single‐arm trial) administered direct intramuscular fetal therapy to six women at risk of preterm birth (Ljubic 1999). In five cases, there was an uneventful outcome of fetuses, indicating that direct fetal corticosteroid treatment improved postnatal lung function in preterm fetuses. Another single‐arm trial involving 41 women at risk of preterm birth found that direct intramuscular fetal corticosteroid administration led to an increase in fetal breathing but no change in fetal movement and growth parameters (Babovic 2009).
In an animal study involving direct corticosteroid administration, higher corticosteroid peak concentrations were found in the fetal circulation compared with maternal intramuscular administration of the same dose (Moss 2003). However, the duration of maternal and fetal exposure to corticosteroid has been shown to be shorter after direct fetal injection (Babovic 2009). These differences in corticosteroid concentration and length of exposure are thought to lead to a reduced risk of growth retardation and other maternal and fetal complications (Moss 2003). Neurodevelopmental outcomes and long‐term effects on the risk of hypertension, diabetes and metabolic syndrome in relation to direct corticosteroid treatment are as yet unknown.
As an invasive procedure, direct fetal administration of corticosteroids carries additional risks which are not present with maternal administration. These risks are likely to be similar to those of amniocentesis, which carries a 1:1000 risk of intrauterine infection and 1:100 risk of pregnancy loss (RCOG 2010). In addition, there are potential risks of fetal injury, initiation of preterm labour (Newnham 1999), preterm prelabour rupture of membranes, placental abruption and maternal‐fetal haemorrhage (Gordon 2002).
Why it is important to do this review
Despite the major advances in medical technology, the incidence of preterm birth remains high. This could be due multiple factors such as increasing rates of multiple pregnancies, greater use of reproductive technologies, increases in maternal age and changes in clinical practice (Beck 2010). Preterm birth has significant impacts on maternal health, neonatal and childhood health, families and the economy (Goldenberg 2007; Saigal 2007). The introduction of the use of antenatal corticosteroid administered by intramuscular injection to women at risk of preterm birth has reduced the incidence of RDS and increased the survival rates of preterm infants. However, this intervention also comes with its own risks and side effects. There remains uncertainty about the type of corticosteroid to use and the optimal method of administration (Brownfoot 2013). Direct fetal corticosteroid administration may have benefits over maternal administration in terms of safety and efficacy. This review assesses the effects of direct corticosteroid administration to the preterm fetus, compared with maternal administration, using the best available evidence.
Objectives
To assess if different routes of corticosteroid administration (maternal versus direct fetal) have effects on health outcomes for women and their babies.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials comparing maternal with direct fetal routes of antenatal corticosteroid administration in women at risk of preterm birth. We planned not to include quasi‐randomised trials, cluster‐randomised trials or cross‐over trials. We intended to consider studies presented only as abstracts.
Types of participants
All women at risk of preterm birth (less than 37 weeks) receiving any antenatal corticosteroid treatment prior to birth, by any maternal transplacental or direct fetal route of administration.
Types of interventions
Transplacental (oral, intramuscular or intravascular) versus direct fetal corticosteroid treatment given to women at risk of preterm birth (less than 37 weeks). We planned to consider any type, dose and regimen of corticosteroid treatment.
Types of outcome measures
Primary outcomes
Maternal
-
Maternal sepsis (as defined by authors)
Infant
-
Death (stillborn or death of a live‐born infant prior to primary hospital discharge)
-
Respiratory distress syndrome
Child
-
Survival free of any disability (as defined by authors)
-
Neurodevelopmental impairment (as defined by authors)
Child as an adult
-
Survival free of cardiometabolic disease
-
Neurodevelopmental impairment (as defined by authors)
Secondary outcomes
Maternal
-
Chorioamnionitis
-
Pyrexia after trial entry requiring the use of antibiotics
-
Intrapartum pyrexia
-
Postnatal pyrexia
-
Intensive care unit admission
-
Glucose tolerance (as defined by authors)
-
Hypertension (as defined by authors)
-
Breastfeeding
Infant
-
Gestational age at birth
-
Low Apgar score (less than seven at five minutes)
-
Intraventricular haemorrhage
-
Periventricular leukomalacia
-
Body size (birthweight, head circumference, length and skinfold thickness)
-
Placental weight
-
Neonatal blood pressure
-
Bronchopulmonary dysplasia (chronic lung disease) (as defined by authors)
-
Necrotising enterocolitis
-
Admission to neonatal intensive care
-
Composite of serious infant outcomes (as defined by authors)
-
Systemic infection in the first 48 hours of life
-
Hypothalamo‐pituitary‐adrenal axis function (as defined by authors)
Child
-
Total deaths
-
Body size measurements (including z scores for weight, height, head circumference and body mass index (BMI))
-
Asthma/wheeze
-
Risk factors for cardiovascular disease
-
Emotional and behavioural problems
Child as an adult
-
Growth measurements (including weight, head circumference, height, skin fold thickness and BMI)
-
Age at puberty
-
Abnormal lung function (including z scores for forced expiratory volume in one second, forced vital capacity and forced expiratory flow at 25% to 75% of forced vital capacity)
-
Health‐related quality of life
-
Employment status
Health services
-
Length of antenatal hospitalisation for women
-
Length of postnatal hospitalisation for women
-
Length of neonatal hospitalisation
-
Cost of maternal care
-
Cost of neonatal care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (25 October 2017)
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed by the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Excluded studies; Studies awaiting classification).
We also searched the WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov (25 October 2017). See Appendix 1 for search terms used.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
There are no included studies in this review. SeeAppendix 2 for full methods of data collection and analysis to be used in future updates of this review, as more data become available.
Selection of studies
Two review authors independently assessed for inclusion the potential studies identified by the search strategy. We resolved any disagreement through discussion, or consulted a third person.
Results
Description of studies
Results of the search
In the previous version of this review, we identified only one potential abstract (Ljubic 2000). The Ljubic 2000 study compared the efficacy and safety of transplacental versus direct fetal corticosteroid treatment for accelerating fetal lung maturation. The authors examined effects of single‐dose, ultrasound‐guided fetal administration of 4 mg dexamethasone compared with standard transplacental maternal administration of 24 mg dexamethasone. We contacted the author about the study design and await further information. The study is currently listed under Studies awaiting classification.
The updated search in 2017 identified one potential study (Babovic 2015) which we have excluded from the review as it was a non‐randomised trial (see: Excluded studies).
Our search of the WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov in October 2017 did not identify any further trials.
See: Figure 1.
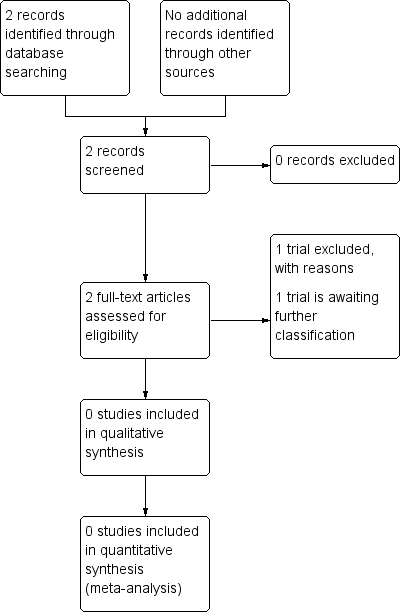
Study flow diagram.
Excluded studies
The Babovic 2015 study assessed the short‐term effects (0 to 4 hours) of transplacental versus direct fetal corticosteroid treatment on the fetal biophysical profile, baseline fetal heart rate, non‐stress test and perinatal outcomes. The authors assessed the effects of a single‐dose, ultrasound‐guided fetal administration of 4 mg dexamethasone compared with standard transplacental maternal administration of 24 mg dexamethasone (Babovic 2015). We contacted the trial author, who responded and provided clarity about the study's methodology.
Risk of bias in included studies
There are no included studies in this review.
Effects of interventions
There are no included studies in this review.
Discussion
We did not identify any randomised controlled trials for inclusion in this review comparing the benefits and harms of transplacental versus direct fetal corticosteroid treatment for accelerating fetal lung maturation where there is a risk of preterm birth.
The available studies carried out so far on animals and humans have shown that direct intramuscular injection of corticosteroid into the fetus under ultrasound guidance is feasible, but good‐quality data on health outcomes are lacking.
In the absence of sufficient data on which to base a clinical decision, uncertainty persists as to which method could provide better efficacy and safety. Randomised controlled trials are needed, focusing on the benefits and harms of maternal administration of transplacental corticosteroid treatment versus direct fetal corticosteroid treatment. Until the uncertainties have been addressed, it is advisable to stay with the current standard of transplacental maternally administered corticosteroid treatment (Roberts 2017).

Study flow diagram.

