Pharmacothérapies pour la dépendance au cannabis
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double‐blind, randomised, placebo‐controlled trial. Setting: inpatient (two hospitals), New South Wales, Australia. Funding: research grant (Australian National Health and Medical Research Council), with study drugs provided by manufacturer (GW Pharmaceucticals, UK). Declaration of conflict of interest not published | |
| Participants | N = 51 adults seeking treatment for cannabis use, dependent by DSM‐IV‐TR. Average age 35; 76% male; 53% unemployed; 25% married or in de facto relationship; on average using 23 g cannabis per day, average duration of use 20 years; 71% also nicotine dependent. Dependence on alcohol or other drugs except nicotine or caffeine and unstable medical or psychiatric conditions were exclusion criteria. Groups well matched apart from differences in baseline withdrawal score and disability scale scores | |
| Interventions | (1) N = 27, nabiximols (cannabis extract, Sativex®), maximum dose 86.4 mg THC, 80 mg cannabidiol; 6 days medication, 3 days washout, or (2) N = 24, placebo. Cognitive‐behavioural therapy tailored to inpatient cannabis withdrawal as adjunct intervention. Total 9 days inpatient admission. Follow‐up interview after 28 days. Participants compensated AUD 40 for follow‐up interviews | |
| Outcomes | Overall withdrawal score, irritability, craving, and depression reported as graphs and results of statistical analyses with imputation for missing data. Number retained in treatment at all time points, median days inpatient stay. Change in amount of cannabis use from baseline to 28‐day follow‐up | |
| Notes | Withdrawal and craving assessed with Cannabis Withdrawal Scale (19 items on 11‐point Likert scale for the previous 24 hours). Drug use by modified timeline follow‐back | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician generated a randomization list for each site using random block sizes in Stata, version 11.1 ..." |
| Allocation concealment (selection bias) | Low risk | Comment: Method of allocation concealment not reported, but generation of lists by independent statistician and use of matching placebos would be expected to provide good control of bias |
| Blinding (subjective outcomes) | Low risk | Quote: "Patients, investigators, and outcome assessors were blind to treatment allocation until all research procedures were complete. Blinding was maintained by the use of a matched placebo ... The success of patient blinding was formally assessed before hospital discharge." |
| Blinding (objective outcomes) | Low risk | Quote: "Patients, investigators, and outcome assessors were blind to treatment allocation until all research procedures were complete. Blinding was maintained by the use of a matched placebo ... The success of patient blinding was formally assessed before hospital discharge." |
| Incomplete outcome data (attrition bias) | Low risk | Statistical methods used to impute missing data and assess data as missing at random |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Double‐blind, randomised, placebo‐controlled trial. Setting: outpatient clinic, New York, USA. Funding from research grant (NIDA). One author declared past associations with pharmaceutical companies | |
| Participants | N = 106 participants seeking treatment for problems related to cannabis use, cannabis dependent by DSM‐IV and smoking at least 5 cannabis joints per week. Average age 32; 76% male (63% in bupropion group); 34% Caucasian, 28% Hispanic, 27% African‐American; 91% employed. Exclusion criteria for the trial included "significant and unstable psychiatric condition", “chronic organic mental disorder” and "DSM‐IV dependence criteria for another substance" | |
| Interventions | Placebo for 1 week then (1) N = 36, oral nefazodone, 150 mg/day to maximum 600 mg/day (2) N = 40, oral bupropion‐SR 150 mg to maximum of 300 mg/day, or (3) N = 30, oral placebo for 10 weeks. Riboflavin added to medication to monitor adherence. All participants received placebo for 2 weeks after medication phase. Participants attended treatment clinic twice weekly (paid USD 5 for transport costs at each visit); medications dispensed weekly. Weekly individual psychosocial intervention based on coping skills as adjunct therapy. Scheduled duration 13 weeks | |
| Outcomes | Number completing 13 weeks of study, number abstinent at week 10, dependence severity at baseline and week 10 (and improvement), withdrawal symptoms, sleep, HAM‐A at baseline and week 10. Total side effects during study | |
| Notes | Cannabis use assessed by self‐report and urine toxicology of observed samples provided at each clinic visit, with a cut‐off of 100 ng/ml (rather than usual 50 ng/ml) to minimise false positives. Severity of dependence symptoms assessed by Clinical Global Impression (scores from 1 = no pathology, to 7 = extreme pathology). Sleep quality self‐reported once a week using the St Mary's Hospital Sleep Questionnaire. Irritability self‐reported every other week with the Snaith Irritability Scale (4 items each rated 0 to 3). Hamilton anxiety scale (14 items each rated 0 to 4) administered by clinician every other week. If either urine or self‐report data were missing for a given week, it was considered a non‐abstinent week | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A research pharmacist who was independent of the research team, conducted the randomization" Comment: Method of sequence generation not reported, but the involvement of an independent pharmacist would be expected to protect against bias |
| Allocation concealment (selection bias) | Low risk | Quote: "All capsules were prepared at the research pharmacy and looked identical for all three treatment conditions" Comment: although not specifically stated, treatment allocation was likely to have been through medication provided by the research pharmacist making it unlikely that participants or investigators could foresee intervention assignment. Characteristics of participants in three groups similar, except significantly more females in bupropion group |
| Blinding (subjective outcomes) | Low risk | Study stated to have been conducted double‐blind, without specification as to whether participants, observers and treating personnel were all blinded to group allocation. However, the provision of active and placebo medications in identical capsules, and the use of urine screening to support self‐report data would be expected to be associated with a low risk of bias |
| Blinding (objective outcomes) | Low risk | Study conducted double‐blind and these outcomes less likely to be affected by knowledge of treatment allocation. The use of riboflavin to confirm medication adherence would help to reduce the risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | There was substantial dropout from all three groups, with only 52 of 106 (49%) participants randomised completing the 10‐week medication phase and 43% completing the full 13‐week trial. Quote: "Survival analysis revealed no statistically significant group differences on treatment retention... there were no differences between those participants who completed the trial and those who did not on demographic indices or baseline substance use measures." Comment: Missing data on cannabis use regarded as indicative of “non‐abstinence”; statistical methods used to allow for missing data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Double‐blind, randomised, placebo‐controlled trial. One physician remained non‐blinded to handle any potential problems. Setting: outpatient clinic, Pittsburgh, USA. Funding from research grants (NIDA, NIAAA, Veterans Affairs). All authors declared no conflict of interest | |
| Participants | N = 70 adolescents and young adults (aged 14 to 25 at baseline) with comorbid major depression and cannabis use disorder by DSM‐IV criteria. Average age 21.1; 61% male; 56% Caucasian, 37% African‐American; 94% cannabis dependent, using on average of 76% of days in prior month; 28.6% also alcohol dependent. Bipolar disorder, schizoaffective disorder, schizophrenia, substance abuse or dependence other than alcohol, nicotine or cannabis, history of IV drug use were exclusion criteria | |
| Interventions | (1) N = 34, fluoxetine, 10 mg increasing to 20 mg/day after 2 weeks (2) N = 36, placebo. Nine sessions (delivered at each clinic visit) of manual‐based cognitive‐behavioural therapy for depression and cannabis use and motivation enhancement therapy for cannabis use as adjunct intervention. Scheduled duration 12 weeks | |
| Outcomes | Severity of abuse or dependence (criteria count), days cannabis used in past week, number completing treatment | |
| Notes | Depressive symptoms rated by observer with Hamilton Rating Scale for Depression and by participants with Beck Depression Inventory. Cannabis use behaviours assessed by timeline follow‐back method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patient randomization was conducted by urn randomization stratified by gender…” |
| Allocation concealment (selection bias) | Low risk | “Active medication and matching placebo were prepared by the research pharmacy…” |
| Blinding (subjective outcomes) | Low risk | Quote: "The study was conducted in a double‐blind fashion, though [one] physician ... remained non‐blinded in order to handle any problems which may have arisen." This suggests it is likely that participants, treating personnel and observers were all blind to group allocation |
| Blinding (objective outcomes) | Low risk | Study conducted double‐blind, as indicated above |
| Incomplete outcome data (attrition bias) | Low risk | Authors note “low percentage of missing data”. Missing data handled by carrying forward last observation |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Double‐blind, randomised, placebo‐controlled trial. Setting: outpatient, Sydney, Australia. Funding: not reported. No declaration of conflict of interest made | |
| Participants | N = 81 adults, seeking treatment for cannabis use, used cannabis in 72 hours prior to assessment interview, dependent by DSM‐IV‐TR in previous 3 months. Average age 31.4; 81% male; 78% Australian‐born; 64% employed; 92% living in stable accommodation; 63% not in a relationship. Average of 12 years cannabis use; 97% daily smokers; 63% daily tobacco smokers. Psychiatric or medical instability were exclusion criteria. Characteristics of participants similar to characteristics of general population seeking treatment for cannabis use | |
| Interventions | 1) Oral mirtazapine 30 mg/day or 2) placebo Weekly individual cognitive‐behavioural therapy as adjunct intervention Reimbursement of AUD 30 for expenses at the day 56 interview Scheduled 4 weeks medication, with follow‐up 28 days later | |
| Outcomes | Withdrawal symptoms in first seven days related to subsequent cannabis use for groups combined (effect of medication not considered in this analysis). Measures of sleep quality and disruption | |
| Notes | Withdrawal symptoms measured daily for 14 days with the Marijuana Withdrawal Scale (32 items, rated from 0 = "none" to 3 = "severe"). Cannabis use assessed with the drug scale from the Opiate Treatment Index. Sleep problems recorded with the Karolinksa Sleep Questionnaire for 7 days, and the Pittsburgh Sleep Quality Index (24 items, global score 0 to 21, with higher scores indicative of poorer sleep) at baseline and days 28 and 56 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were randomized ... using permuted block randomisation.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomisation was independently assigned by pharmacy staff offsite.” " ... the placebo was identically matched in colour, shape, size and taste to the medication." Comment: As independent pharmacy staff controlled the randomization process, it is likely to have prevented investigators and participants from foreseeing allocation assignment |
| Blinding (subjective outcomes) | Low risk | Quote: "All treating physicians, psychologists and research staff were blind to the randomisation until all participants had completed the final research interview." |
| Blinding (objective outcomes) | Low risk | Quote: "All treating physicians, psychologists and research staff were blind to the randomisation until all participants had completed the final research interview." |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information available to form a view |
| Selective reporting (reporting bias) | Unclear risk | Limited study results available |
| Other bias | Low risk | None apparent |
| Methods | Double‐blind, randomised, placebo‐controlled trial. Setting: outpatient, university clinic, South Carolina, USA. Funding: research grants (NIDA, National Center for Research Resources). Authors declared "no competing interests" | |
| Participants | N = 116 adolescents (age 13 to 21), cannabis‐dependent and using cannabis regularly. Average age 18.9 years; 73% male; 83.5% Caucasian; 73.9% enrolled in school. Average 22.6 days with cannabis use in 30 days prior to baseline; 57% smoked tobacco; 13.8% had a psychiatric comorbidity. Dependence on other substances (except nicotine) and unstable psychiatric or medical illness were exclusion criteria | |
| Interventions | (1) N = 58, N‐acetylcysteine 1200 mg twice daily or (2) N = 58, placebo. Twice‐weekly contingency management and weekly brief (10 minute) individual cessation counselling as adjunct therapies. Initial contingent reward USD 5 (cash) for both adherence and abstinence with amount increased by USD 2 for each successive visit; reward reset to baseline if conditions not met. Seen in clinic weekly. Follow‐up 4 weeks after treatment conclusion. Scheduled duration 8 weeks | |
| Outcomes | Likelihood of negative urine test reported as odds ratio and 95% confidence interval. Occurrence of adverse events (number of events and number of participants). Proportion of medication doses consumed, discontinuation of medication due to adverse effects. Number completing treatment, median days in treatment, contingency rewards earned | |
| Notes | Urine cannabinoid testing at all visits. Self‐reported cannabis use by timeline follow‐back. Medication diaries and weekly pill counts used to determine adherence. Participants lost to follow‐up or absent for visits were coded as having a positive urine test | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in 1:1 parallel group allocation stratified by age and baseline cannabis use. No significant group differences at baseline suggesting appropriate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "university investigational drug service oversaw randomization, encased medication in identical‐appearing capsules, and dispensed them in weekly blister packs..." |
| Blinding (subjective outcomes) | Low risk | Quote: "Participants, investigators and clinical staff remained blind to treatment assignment throughout the study." |
| Blinding (objective outcomes) | Low risk | Quote: "Participants, investigators and clinical staff remained blind to treatment assignment throughout the study." |
| Incomplete outcome data (attrition bias) | Low risk | Missing data and non‐attendance regarded as indicating non‐abstinence |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Double‐blind, randomised, placebo‐controlled trial. Setting: inpatient withdrawal unit; Sydney, Australia. Funding source not reported. No declaration of conflict of interest made | |
| Participants | N = 38 cannabis dependent adults. No other participant characteristics reported | |
| Interventions | (1) N = 19, lithium carbonate, 500 mg bd or (2) N = 19, placebo. Scheduled 7 days inpatient treatment. Follow‐up at 14, 30 and 90‐days post‐discharge | |
| Outcomes | Withdrawal severity by Cannabis Withdrawal Scale; retention; number and severity of adverse effects | |
| Notes | Conference abstracts only ‐ limited data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated; method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Random allocation stated; method of allocation concealment not reported |
| Blinding (subjective outcomes) | Unclear risk | Double‐blind stated, but adequacy of control for assessment of subjective outcomes (withdrawal severity) unclear |
| Blinding (objective outcomes) | Low risk | Double‐blind stated and these outcomes unlikely to be affected by awareness of group allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information reported to assess risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information reported to assess risk |
| Other bias | Unclear risk | Insufficient information reported to assess risk |
| Methods | Double‐blind, randomised, placebo‐controlled trial. Study included a cross‐over phase which was not included in this review due to substantial dropout (> 30%) in the first 2 weeks. Setting: outpatient with two clinic visits per week; New York, USA. Funding: Research grants (NIDA). Declaration of conflict of interest not published | |
| Participants | N = 27 enrolled, N = 25 randomized; cannabis dependent by DSM‐IV, using daily. Average age 32; 92% male; 56% Caucasian, 20% Hispanic, 24% African American; average (± SD) joints smoked per week at baseline (1) 28.3 ± 23.2 (2) 19.4 ± 16.4. Dependence on other substances, except caffeine and nicotine, and psychiatric disorder requiring medical intervention were exclusion criteria | |
| Interventions | Two‐week single‐blind placebo lead‐in phase, then (1) N = 13, oral divalproex sodium commenced at 500 mg/day, increasing to maximum of 2 g/day, depending on response, or (2) N = 12, placebo. Medication in 2 doses per day. Weekly individual cognitive‐behavioural relapse prevention therapy as adjunct. Scheduled duration 8 weeks (plus subsequent cross‐over phase that was excluded from this review) | |
| Outcomes | Outcomes reported for N = 19 who completed 8 weeks of study: frequency and amount of cannabis use and craving score at baseline and weeks 7 and 8; number completing scheduled treatment; number with 2 or more weeks of assumed abstinence | |
| Notes | Urine samples collected and analysed at each visit. Participants reported cannabis use and completed a visual analogue scale of intensity and desire for cannabis each week. Clinician‐rated global impression assessment for cannabis use completed weekly. "Strict abstinence" defined as at least one negative urine sample and no self‐reported cannabis use for that week. "Assumed abstinence" if patient reported no cannabis use and urine samples had THC‐COOH levels at least 50% below the previous week | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty‐seven participants were enrolled and 25 were randomized." Comment: method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: [participants] "...were randomly assigned to receive either divalproex or a matching placebo." Comment: method of allocation concealment not reported |
| Blinding (subjective outcomes) | Low risk | Quote: "Following randomization, patients received...either divalproex sodium or a placebo using a double‐blind design" Comment: use of urine screening to support determination of "abstinence" would be expected to help reduce bias in these outcomes |
| Blinding (objective outcomes) | Low risk | Quote: "Following randomization, patients received...either divalproex sodium or a placebo using a double‐blind design" Comment: these outcomes considered unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | High risk | Rates of dropout were similar in the two groups, but there was no discussion of possible differences between those retained and those who dropped out of the study. Cannabis use outcomes were reported only for those who completed treatment |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | The cross‐over phase of the trial was excluded from analyses and this review due to high rates of dropout in the first two weeks |
| Methods | Randomised, double‐blind, placebo‐controlled, trial. Randomisation after 1‐week placebo lead‐in phase. Those who used cannabis less than twice a week during the placebo lead‐in phase were not randomised. Setting: outpatient with clinic attendance twice weekly, New York, USA. Funding: research grant (NIDA). One author declared prior associations with pharmaceutical companies | |
| Participants | N = 156 adults seeking outpatient treatment for problems related to cannabis use, dependent by DSM‐IV‐TR, using cannabis at least 5 days a week in prior 28 days. Average age 38; 82% male; 60% employed full‐time, 13% part‐time; 31% married. Significant psychiatric condition and dependence on other substances except nicotine were exclusion criteria. No significant group differences in demographic or clinical characteristics at baseline | |
| Interventions | Placebo for 1 week, then 1) N = 79, oral dronabinol, commenced at 10 mg/day, titrated to 20 mg twice a day or the maximum tolerated, or 2) N = 77, placebo. Medication maintained to end of week 8 then tapered over 2 weeks. Weekly individual therapy based on coping skills plus motivational enhancement therapy as adjunct intervention. Participants earned vouchers with value increased by USD 1.50 for each consecutive visit, with value reset for non‐attendance, and USD 10 for returning their pill bottle and remaining medication. Maximum possible earnings were USD 570. Cash payments of USD 5 to 20 were made at each visit for transport costs | |
| Outcomes | Number achieving 2 weeks abstinence in weeks 7 and 8 and median maximum consecutive days abstinence; number retained in study to week 8; average number of therapy sessions attended; number experiencing any adverse effects, requiring dose reduction, serious adverse events and number withdrawn due to adverse events; withdrawal scores reported as graph and results of statistical modelling; medication compliance | |
| Notes | Cannabis use assessed by timeline follow‐back. Urine samples tested at each clinic visit for confirmation of self‐report. Withdrawal symptoms assessed twice a week using the Withdrawal Discomfort Score (10 items, scores 0‐30). Craving by Marijuana Craving Questionnaire with the 47‐item version completed once a month, and the 12‐item version weekly. Side effects assessed twice a week using the Modified Systematic Assessment for Treatment and Emergent Events (SAFTEE). Hamilton Anxiety and Depression scales used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “participants were randomized ...using a fixed block size of 4, stratified by joints used per week…and whether or not they were receiving a psychotropic medication.” |
| Allocation concealment (selection bias) | Low risk | Quote:“A research pharmacist, who was independent of the research team, conducted the randomization.” |
| Blinding (subjective outcomes) | Low risk | Quote: "Donabinol...or matching placebo...was prepared by the pharmacy...packaged in matching gelatin capsules with lactose filler and an equal amount of riboflavin. All capsules looked identical..." Comment: double‐blind stated. Participants may have been able to distinguish the effects of dronabinol, but use of urine screening to support self‐report would be expected to reduce risk of bias |
| Blinding (objective outcomes) | Low risk | Double‐blind stated. Packaging of medication in identical capsules as above. Objective outcomes less likely to be influenced by awareness of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “All analyses were conducted on the intent‐to‐treat population.” “...missing data in weeks 7 and 8 were scored as indicating cannabis use...” |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind, placebo‐controlled trial. Outpatient setting with twice weekly clinic attendance, New York, USA. Funding: research grants (NIDA). Two authors declared prior associations with pharmaceutical companies | |
| Participants | N = 103 seeking treatment for problems related to cannabis use, cannabis dependence and major depressive disorder or dysthymia by DSM‐IV. Average age 35; 74% male; 40% working full‐time; 18% currently married; average 27.4 days of use in month prior to baseline. No significant group differences on demographic or clinical characteristics at baseline. Physical dependence on substances other than cannabis or nicotine was an exclusion criterion | |
| Interventions | One‐week placebo lead‐in phase ‐ those who improved as assessed by Clinical Global Impression rating were not randomised. (1) N = 51, venlafaxine‐extended release, up to 375 mg on a fixed‐flexible schedule or (2) N = 52, placebo. Medication dose titrated over 3 weeks, then maintained for 8 weeks. Weekly individual cognitive behavioural therapy that primarily targeted cannabis use as adjunct intervention. Participants received USD 5 to 20 per visit for transport costs, and USD 10 per week if they returned their pill bottles and any remaining medication. Scheduled duration 12 weeks | |
| Outcomes | Abstinence defined by 2 or more consecutive urine‐confirmed abstinent weeks. Improvement in depressive symptoms by Hamilton Depression Rating Scale | |
| Notes | Cannabis use assessed by timeline follow‐back. Urine THC levels tested at each visit, with cut‐off of 100 ng/ml to decrease the probability of false positives. Side effects assessed weekly using the Modified Systematic Assessment for Treatment and Emergent Events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized at the end of the [placebo] lead‐in phase using a computer‐generated fixed‐block size of 4, with a 1:1 allocation ratio, and stratified by joints used per week...and severity of depression" Comment: similarities of groups at baseline suggest adequate method of sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "A research pharmacist, who was independent of the research team, conducted the randomization and maintained the allocation sequence." Venlafaxine or placebo "was prepared by the pharmacy...packaged in matching gelatin capsules with lactose filler." Comment: allocation by pharmacy and identical appearance of medication and placebo would support adequate concealment of allocation |
| Blinding (subjective outcomes) | Low risk | Quote: "Participants, care providers and outcome assessors were kept blinded to the allocation." |
| Blinding (objective outcomes) | Low risk | Quote: "Participants, care providers and outcome assessors were kept blinded to the allocation." |
| Incomplete outcome data (attrition bias) | Low risk | Patients who dropped out were significantly younger and less likely to be married, but rates of dropout were similar in the two arms. Those who dropped out without achieving 2 continuous weeks of abstinence were classified as not abstinent |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind, placebo controlled trial. Setting: outpatient with weekly clinic visits, California, USA. Funding: research grants (NIDA). One author declared past associations with pharmaceutical companies | |
| Participants | N = 50 treatment‐seeking volunteers with current cannabis dependence by DSM‐IV, smoked cannabis at least once in week prior to randomisation. Average age 33.9 years, 88% male, average 11.6 years of daily cannabis use, smoking an average of 11.0 g/week; 62% employed full‐time; 40% married. Abuse or dependence on substances other than cannabis or nicotine, and significant psychiatric disorders were exclusion criteria. No significant group differences on demographic or clinical variables at baseline | |
| Interventions | 1) N = 25, oral gabapentin 300 mg, increasing to 1200 mg/day, or 2) N = 25, matched placebo. Abstinence‐oriented individual counselling weekly. Scheduled duration 12 weeks | |
| Outcomes | Change in amount of cannabis use, frequency of use and withdrawal symptoms, as graphs and results of statistical tests. Number completing treatment | |
| Notes | Cannabis use by weekly urine toxicology and self‐report by timeline follow‐back interview. Withdrawal symptoms by Marijuana Withdrawal Checklist. Marijuana Problems Scale completed at baseline and end of treatment (week 12) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “subjects were randomly assigned … in a 1:1 ratio, on the basis of a computer‐generated randomization code.” |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomization code was kept by the study pharmacist, who provided subjects with a 1‐week supply of medication in a blister card package at each weekly study visit…” Comment: allocation by pharmacy and identical appearance of medication and placebo would support adequate concealment of allocation |
| Blinding (subjective outcomes) | Low risk | Quote: "Subjects, care providers, and those assessing outcomes were blinded to the identity of drug assignment. Gabapentin was purchased and over‐encapsulated to match placebo capsules." |
| Blinding (objective outcomes) | Low risk | Quote: "Subjects, care providers, and those assessing outcomes were blinded to the identity of drug assignment. Gabapentin was purchased and over‐encapsulated to match placebo capsules." |
| Incomplete outcome data (attrition bias) | Unclear risk | High rate of dropout. Extent of missing data, and adjustments for missing data unclear |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind, placebo‐controlled trial. N = 93 randomised; N = 34 did not receive study drug (21 failed to return for second baseline visit); analysis based on those randomised who received study drug and completed at least one post‐baseline visit. Setting: outpatient with clinic visits 1 to 2 times per week, South Carolina, USA. Funding: research grant (NIDA). Two authors declared past associations with pharmaceutical companies | |
| Participants | N = 50 with current cannabis dependence by DSM‐IV. Average age 31.6; 90% male; 86% Caucasian; on average used cannabis on 89% of days prior to study entry, using average 3.8 g/day. Dependence on other substances except caffeine or nicotine, history of psychotic disorder, current major depression were exclusion criteria. Treatment groups similar on baseline characteristics | |
| Interventions | (1) N = 23, oral buspirone, initiated at 5 mg twice a day, increased 5 to 10 mg every 3 to 4 days as tolerated to maximum 60 mg per day or (2) N = 27, placebo. Motivational interviewing (3 sessions) as adjunct intervention for first four weeks. Subjects received USD 10 for time and travel associated with study visits. Scheduled duration 12 weeks | |
| Outcomes | Urinalysis data reported as per cent of screens that were negative, not participants with negative screens. Mean change in withdrawal score. Number experiencing any adverse effect. Number completing treatment. Change in reported cannabis use per using day, % days abstinent during study | |
| Notes | Cannabis use by timeline follow‐back for 90 days prior to study entry, and weekly throughout the study. Craving by Marijuana Craving Questionnaire, withdrawal, by Marijuana Withdrawal Checklist. Urine drug screens at baseline and weekly during study. Side effects evaluated weekly with open‐ended questions. Adjustment for missing data by last observation carried forward | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Urn randomization ...was used to determine treatment assignment. Urn variables used were age ... gender, and [anxiety] score..." |
| Allocation concealment (selection bias) | Low risk | Quote: [participants] "Randomized at central pharmacy..." "Buspirone and placebo tablets were packaged in identical opaque gelatin capsules with cornstarch." |
| Blinding (subjective outcomes) | Low risk | Double‐blind stated. Urinalysis to support self‐report data would be expected to reduce bias, although authors noted some inconsistencies between urine screen and self‐report data |
| Blinding (objective outcomes) | Low risk | Double‐blind stated and these outcomes considered unlikely to be affected by awareness of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | High rate of dropout but statistical methods used to adjust for missing data (GEE modelling and last observation carried forward) |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind, placebo‐controlled trial; 78 participants were randomised but only 46 received study medication and only 38 returned for at least one post‐baseline assessment. Analyses based on this group. Setting: outpatient, South Carolina, USA. Funding: research grants (NIDA), with medication and placebo provided by manufacturer (Eli Lilly and Company). Two authors declared past associations with pharmaceutical companies | |
| Participants | N = 38 adults, cannabis dependence and attention deficit hyperactivity disorder (with age of onset before 12 years of age) by DSM‐IV. Average age 29.9 years; 76% male; 92% Caucasian; used cannabis on average 87% of days prior to baseline, using average of 4.1 times per day. Dependence on other substances except caffeine or nicotine, and other psychiatric disorders were exclusion criteria. No significant group differences on baseline characteristics | |
| Interventions | (1) N = 19, oral atomoxetine started at 25 mg, increased to 40 mg in week 2, and to 80 mg in week 3 as tolerated, with further increase to 100 mg/day in week 4 if required, or (2) N = 19, matching placebo. Motivational interviewing (3 sessions) as adjunct intervention. Nominal monetary reimbursement for completion of study assessments. Scheduled duration 12 weeks | |
| Outcomes | Self‐reported cannabis use during week 12 (last observation carried forward for participants who did not complete the trial). Number completing treatment. Change in craving scores. Number experiencing adverse effects and type of adverse effects | |
| Notes | Cannabis use self‐reported by timeline follow‐back weekly and assessed by Clinical Global Impression of Severity and Improvement Scales. Urine drug screens at baseline and then weekly. Medication side effects weekly by standard checklist. Craving by Marijuana Craving Questionnaire. Compliance assessed by patient report and pill count | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomization was used to assign treatments to participants using a 1:1 allocation ratio." |
| Allocation concealment (selection bias) | Low risk | Quote: "...participants were randomized at the central pharmacy..." |
| Blinding (subjective outcomes) | Low risk | Double‐blind stated. Use of matching capsules along with urine screening to validate self‐report data would be expected to reduce the risk of bias |
| Blinding (objective outcomes) | Low risk | Double‐blind stated and these outcomes unlikely to be affected by awareness of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | High rates of dropout in both groups. Last observation carried forward and statistical techniques used to allow for missing data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind, placebo‐controlled trial. Setting: outpatient with daily clinic attendance Monday to Friday, Harvard Medical School, USA. Funding: research grant (NIDA). Disclosures of interests according to ICMJE criteria were a requirement of publication | |
| Participants | N = 22 treatment seeking, with cannabis abuse or dependence by DSM‐IV, with at least 3 years of heavy use (smoking on 5 or more days a week or more than 25 times per month) and with 2 or more negative symptoms in previous quit attempts. Demographic data were provided only for N = 9 who completed the study (5 male, average age 31.2 years, 7 met criteria for dependence). Abuse or dependence on any other drug (including nicotine) was an exclusion criterion | |
| Interventions | Participants used cannabis as usual for 7 days then commenced 1) N = 10, oral bupropion‐SR (sustained release) 150 mg/day for days 1 to 3, then 150 mg twice a day or 2) N = 12, placebo. Cannabis use stopped on day 8 (Quit Day). Tobacco and caffeine continued throughout the study. Weekly individual motivational enhancement therapy (3 sessions) as adjunct intervention. Scheduled duration 21 days | |
| Outcomes | Data reported as graphs and results of statistical tests. Relevant outcomes reported were completion of study, change in withdrawal discomfort and change in craving | |
| Notes | Withdrawal by Marijuana Withdrawal Checklist (29 items each rated 0‐3). Withdrawal discomfort score calculated from 10 items (max score 30). Drug use, sleep and withdrawal recorded by participants in daily diary. With each medication administration participants consumed identical appearing capsule that contained riboflavin to measure compliance. Urine testing to confirm drug use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation to treatment group stated, but method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (subjective outcomes) | Low risk | Double‐blind stated and "Bupropion tablets were repackaged into gelatin capsules...Placebo consisted of identical appearing gelatin capsules". Use of urine screening to verify self‐report expected to reduce risk of bias |
| Blinding (objective outcomes) | Low risk | Double‐blind stated, placebo used, and these outcomes less likely to be affected by awareness of group allocation |
| Incomplete outcome data (attrition bias) | High risk | High rate of dropout and demographics reported only for those who completed treatment. Unclear whether there were differences between the groups, or between those who did and did not complete the study. Unclear how missing data were handled |
| Selective reporting (reporting bias) | Unclear risk | Data on adverse effects not reported |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind, placebo‐controlled trial. Setting: outpatient, Tel Aviv, Israel. Funding: research grant (Israeli anti‐drug authority). Authors declared no conflict of interest | |
| Participants | N = 52, regular cannabis users, dependent by DSM‐IV. Average age 32.7, 75% male. Dependence on other drugs or alcohol and significant psychiatric disorders were exclusion criteria | |
| Interventions | One week "induction" with placebo, then (1) N = 26, escitalopram 10 mg/day, or (2) N = 26 placebo. Medication for 9 weeks, follow‐up sessions for further 14 weeks. Blinding broken after 9 weeks; participants able to continue open‐label escitalopram use. Participants instructed to stop cannabis use after 4 weeks of medication. Weekly (9 sessions) cognitive‐behaviour (relapse prevention) and motivation enhancement therapy in combination with medication. Scheduled duration 9 weeks | |
| Outcomes | Number completing treatment, number abstinent, number reporting not taking medication, results of statistical analyses of withdrawal scores | |
| Notes | Urine samples collected every second week. Questionnaires administered to assess anxiety and depression. Revised Clinical Institute Withdrawal Assessment Scale (CIWA) adapted for assessment of cannabis withdrawal (score of 10 or more indicated significant withdrawal) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants were blindly randomized..." Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "participants were blindly randomized..." Method of allocation concealment not reported |
| Blinding (subjective outcomes) | Low risk | Double‐blind stated. Subjective outcomes not reported |
| Blinding (objective outcomes) | Low risk | Double‐blind stated and these outcomes unlikely to be affected by awareness of group allocation |
| Incomplete outcome data (attrition bias) | High risk | High (50%) rate of dropout. Those who did not complete study were younger, and more likely to be daily alcohol drinkers. Non‐completers marginally more depressed, but difference not statistically significant |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Participants were diagnosed with abuse or dependence on marijuana or cocaine. Data was reported separately for cocaine and marijuana use, but it was not possible to extract data just for those dependent on marijuana. All participants were diagnosed with schizophrenia; the management of substance use in the context of schizophrenia was the main focus of the study | |
| Secondary analysis of data from a randomised controlled trial comparing two behavioural interventions. No use of medications | |
| Laboratory study involving non‐treatment seeking cannabis users. Not all users were cannabis dependent, and participants were not trying to reduce their cannabis use | |
| Laboratory study involving marijuana smokers who were not seeking treatment. Investigation of research model of withdrawal and relapse rather than treatment intervention | |
| Randomised controlled trial comparing fluoxetine and placebo for treatment of alcohol dependence with comorbid major depression. Effect on subgroup with diagnosed marijuana abuse considered as secondary analysis | |
| Reports cannabis withdrawal symptoms in participants entering two separate trials of fluoxetine. No treatment intervention for cannabis dependence | |
| No treatment comparison. Unclear if participants are cannabis dependent. Insufficient information on participants and treatment regimes | |
| Randomised controlled trial comparing fluoxetine and placebo for treatment of depressive symptoms in adolescents with comorbid substance use disorder. Cannabis use reported by 88.2% of participants (41.2% dependent). The emphasis of this study is on the amelioration of depression. Outcome data not reported separately for the subset of cannabis‐dependent participants | |
| Randomised controlled trial comparing lithium and placebo for treatment of adolescents with bipolar disorder and comorbid substance use disorder. Majority of participants were polydrug users ‐ 2 of 25 were dependent on cannabis only | |
| Reports the use of nitrous oxide for treatment of withdrawal associated with the smoking of methaqualone combined with cannabis. Unclear how many participants were cannabis dependent. All participants received placebo then analgesic nitrous oxide. Effectiveness assessed only in terms of improvement in withdrawal symptoms | |
| Open‐label single group study investigating the effectiveness of N‐Acetylcysteine in promoting cessation of cannabis use. No treatment comparison | |
| Comparison of bupropion and placebo in terms of effect on mood when administered in conjunction with active or placebo cannabis cigarettes. Laboratory study which aimed to assess the therapeutic potential of buproprion, but not a treatment intervention | |
| Investigation of mechanism of effects of cannabis through comparison of naltrexone and methadone, administered prior to oral THC, and different doses of oral THC administered in combination with naltrexone or placebo. No treatment intervention | |
| Laboratory study comparing the effect of nefazodone (450mg/day) and placebo on the acute effects of cannabis, and on cannabis withdrawal symptoms. The study aimed to assess the therapeutic potential of nefazodone in cannabis withdrawal but was not a treatment intervention | |
| Two separate laboratory‐based studies, one assessing THC and the other divalproex, compared to placebo, in terms of effects on cannabis withdrawal. Studies aimed to assess the therapeutic potential of THC and divalproex but were not treatment interventions | |
| Laboratory study investigating the effect of lofexidine and THC (separately and in combination) compared with placebo on cannabis withdrawal symptoms and a model of cannabis relapse. The study aimed to test the therapeutic potential of lofexidine in cannabis withdrawal but was not a treatment intervention | |
| Controlled laboratory study investigating the effects of baclofen or mirtazapine on cannabis smoking, craving and withdrawal. Exploratory study of the potential therapeutic value of baclofen and mirtazapine, but not a treatment intervention | |
| Laboratory study with aim of assessing effect of nabilone on marijuana withdrawal symptoms, and laboratory measure of relapse. The study aimed to test the therapeutic potential of nabilone but was not a treatment intervention | |
| Laboratory study investigating the effect of zolpidem and nabilone (separately and in combination) compared with placebo on marijuana withdrawal symptoms and a model of marijuana relapse. The study aimed to test the therapeutic potential of zolpidem in marijuana smokers but was not a treatment intervention | |
| Laboratory study assessing the effect of oral THC or placebo on smoking of marijuana. Aim of study was to explore therapeutic potential of THC, but not a treatment intervention | |
| Reports single case involving the use of baclofen to manage cannabis dependence. No treatment comparison | |
| Not a controlled study. Two case studies and a review of the use of dronabinol for cannabis dependence | |
| Open label study of buspirone for treatment of cannabis dependence. No treatment comparison | |
| Randomised controlled trial comparing olanzapine and risperidone for treatment of schizophrenia in people with a history of cannabis use disorders. Primary goal of treatment was management of schizophrenia. Comparison of substance use outcomes was secondary. Data on substance use was reported only for those who completed treatment | |
| An open label study investigating the use of baclofen for the treatment of cannabis dependence. No treatment comparison | |
| Controlled study assessing the safety of modafinil in combination with THC. While the study contributes to assessment of the therapeutic potential of modafinil, this study did not involve a treatment intervention. Participants were occasional cannabis users (people who were heavy users or dependent were excluded) | |
| An open label study investigating the use / effect of atomoxetine for the treatment of marijuana dependence. No treatment comparison | |
| Randomised controlled trial comparing olanzapine and risperidone for treatment of schizophrenia. Majority of participants were not using cannabis and cannabis dependence was not assessed | |
| Cross‐over study comparing zolpidem and placebo during short (3‐day) periods of abstinence from cannabis in terms of sleep parameters. Not a full treatment intervention for cannabis dependence | |
| Comparison of dronabinol and placebo in terms of effect on cannabis withdrawal and subjective effects of smoked cannabis, but without providing a treatment intervention for cannabis dependence | |
| An open label study investigating the use of lithium carbonate for the management of cannabis withdrawal. No treatment comparison |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number abstinent at end of treatment Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Active medication versus placebo, Outcome 1 Number abstinent at end of treatment. | ||||
| 1.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.56, 2.30] |
| 1.2 Mixed action antidepressants | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.12, 5.41] |
| 1.3 SSRI antidepressants | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.68, 8.05] |
| 1.4 Anticonvulsant and mood stabiliser | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.50, 2.34] |
| 1.5 Buspirone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Atomoxetine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 N‐acetylcysteine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number experiencing adverse effects Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Active medication versus placebo, Outcome 2 Number experiencing adverse effects. | ||||
| 2.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.90, 1.46] |
| 2.2 Mixed action antidepressants | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.55, 1.55] |
| 2.3 SSRI antidepressants | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Anticonvulsant and mood stabiliser | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.99, 1.53] |
| 2.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.95, 1.46] |
| 2.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.34] |
| 3 Number withdrawn due to adverse effects Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Active medication versus placebo, Outcome 3 Number withdrawn due to adverse effects. | ||||
| 3.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.31] |
| 3.2 Mixed action antidepressants | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.11, 18.90] |
| 3.3 SSRI antidepressants | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Anticonvulsant and mood stabiliser | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.12] |
| 3.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 17.74] |
| 3.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.31] |
| 3.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.15] |
| 4 Completion of treatment Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Active medication versus placebo, Outcome 4 Completion of treatment. | ||||
| 4.1 THC preparations | 2 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.08, 1.55] |
| 4.2 Mixed action antidepressants | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 4.3 SSRI antidepressants | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.44, 1.53] |
| 4.4 Anticonvulsant and mood stabiliser | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.42, 1.46] |
| 4.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.56, 1.77] |
| 4.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.60, 2.74] |
| 4.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.83, 1.51] |
| 4.8 Bupropion | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.67] |

Study flow diagram.
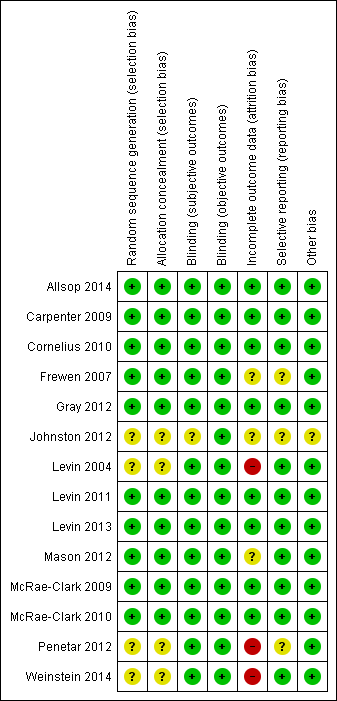
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Comparison 1 Active medication versus placebo, Outcome 1 Number abstinent at end of treatment.
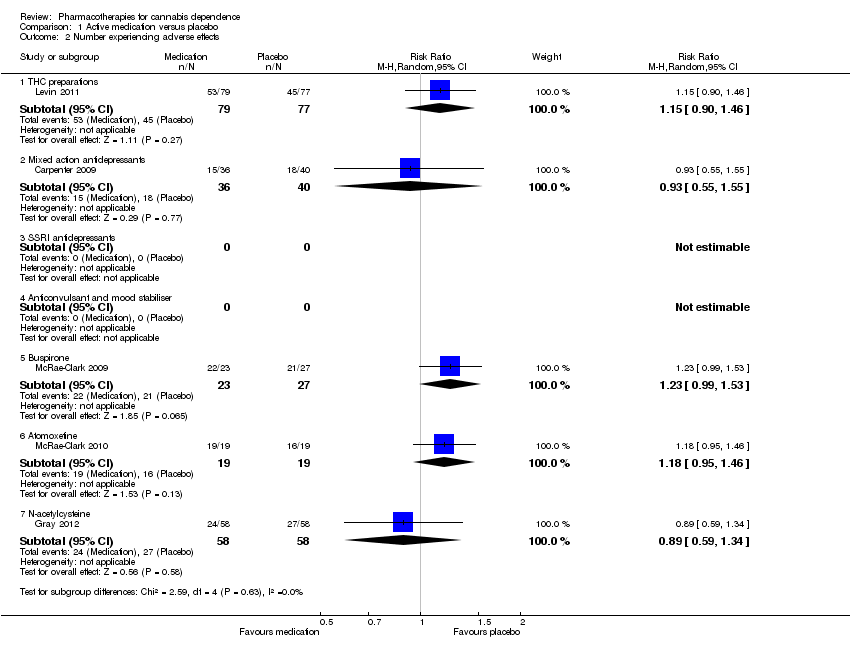
Comparison 1 Active medication versus placebo, Outcome 2 Number experiencing adverse effects.

Comparison 1 Active medication versus placebo, Outcome 3 Number withdrawn due to adverse effects.

Comparison 1 Active medication versus placebo, Outcome 4 Completion of treatment.
| Active medication compared with placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active Medication | |||||
| Number abstinent at end of treatment ‐ mixed action antidepressants | Study population | RR 0.82 | 179 | ⊕⊝⊝⊝ | ||
| 250 per 1000 | 205 per 1000 | |||||
| Moderate | ||||||
| 233 per 1000 | 191 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Significant heterogeneity between studies | ||||||
| Active medication compared with placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active Medication | |||||
| Number withdrawn due to adverse effects ‐ mixed action antidepressants | Study population | RR 1.44 | 179 | ⊕⊝⊝⊝ | ||
| 11 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 19 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Studies differ in direction of effect without significant heterogeneity | ||||||
| Active medication compared with placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active Medication | |||||
| Completion of treatment ‐ THC preparations | Study population | RR 1.29 | 207 | ⊕⊕⊕⊝ | ||
| 614 per 1000 | 792 per 1000 | |||||
| Moderate | ||||||
| 618 per 1000 | 797 per 1000 | |||||
| Completion of treatment ‐ mixed action antidepressants | Study population | RR 0.93 | 169 | ⊕⊕⊕⊝ | ||
| 573 per 1000 | 533 per 1000 | |||||
| Moderate | ||||||
| 551 per 1000 | 512 per 1000 | |||||
| Completion of treatment ‐ SSRI antidepressants | Study population | RR 0.82 | 122 | ⊕⊝⊝⊝ | ||
| 790 per 1000 | 648 per 1000 | |||||
| Moderate | ||||||
| 766 per 1000 | 628 per 1000 | |||||
| Completion of treatment ‐ anticonvulsant and mood stabiliser | Study population | RR 0.78 | 75 | ⊕⊝⊝⊝ | ||
| 405 per 1000 | 316 per 1000 | |||||
| Moderate | ||||||
| 387 per 1000 | 302 per 1000 | |||||
| Completion of treatment ‐ atypical antidepressant (bupropion) | Study population | RR 1.06 | 92 | ⊕⊝⊝⊝ | ||
| 429 per 1000 | 454 per 1000 | |||||
| Moderate | ||||||
| 400 per 1000 | 424 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Significant heterogeneity between studies | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number abstinent at end of treatment Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.56, 2.30] |
| 1.2 Mixed action antidepressants | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.12, 5.41] |
| 1.3 SSRI antidepressants | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.68, 8.05] |
| 1.4 Anticonvulsant and mood stabiliser | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.50, 2.34] |
| 1.5 Buspirone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Atomoxetine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 N‐acetylcysteine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number experiencing adverse effects Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.90, 1.46] |
| 2.2 Mixed action antidepressants | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.55, 1.55] |
| 2.3 SSRI antidepressants | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Anticonvulsant and mood stabiliser | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.99, 1.53] |
| 2.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.95, 1.46] |
| 2.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.34] |
| 3 Number withdrawn due to adverse effects Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.31] |
| 3.2 Mixed action antidepressants | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.11, 18.90] |
| 3.3 SSRI antidepressants | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Anticonvulsant and mood stabiliser | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.12] |
| 3.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 17.74] |
| 3.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.31] |
| 3.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.15] |
| 4 Completion of treatment Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 THC preparations | 2 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.08, 1.55] |
| 4.2 Mixed action antidepressants | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 4.3 SSRI antidepressants | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.44, 1.53] |
| 4.4 Anticonvulsant and mood stabiliser | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.42, 1.46] |
| 4.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.56, 1.77] |
| 4.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.60, 2.74] |
| 4.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.83, 1.51] |
| 4.8 Bupropion | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.67] |

