Farmacoterapias para dependência de maconha
Resumo
Introdução
A maconha é a droga ilícita mais prevalente no mundo. A demanda por tratamento de transtornos por uso de maconha está a aumentar. Atualmente não há farmacoterapias aprovadas para o tratamento de transtornos por uso de cannabis.
Objetivos
Para acessar a eficácia e a segurança das farmacoterapias, comparando‐se umas as outras, com placebo ou tratamento de suporte para reduzir os sintomas de abstinência de maconha e promover a cessação ou a redução do consumo de maconha.
Métodos de busca
Nós procuramos o Cochrane Central Register de Ensaios Controlados (CENTRAL) (até 4 de Março de 2014), MEDLINE (até a terceira semana de fevereiro de 2014), EMBASE (até 3 de Março de 2014) e PsycINFO (até a quarta semana de fevereiro de 2014). Nós também procuramos nas listas de referências dos artigos, fontes eletrônicas de trabalhos em curso e anais de congressos, e contactamos os investigadores selecionados ativos na área.
Critério de seleção
Estudos randomizados e quasi‐randomizados controlados envolvendo o uso de medicamentos para reduzir os sinais e sintomas de abstinência de cannabis ou para promover a cessação ou a redução do consumo de cannabis, ou de ambos, em comparação com outros medicamentos, placebo ou nenhuma medicação (tratamento de suporte) em participantes diagnosticados como dependentes de maconha ou que eram susceptíveis de ser dependentes.
Coleta dos dados e análises
Nós utilizamos os procedimentos metodológicos padrão da Colaboração Cochrane. Dois autores avaliaram independentemente os ensaios clínicos para inclusão e extração dos dados. Todos os autores da revisão confirmaram as decisões de inclusão e todo o processo.
Principais resultados
Nós incluímos 14 estudos randomizados controlados envolvendo 958 participantes. Para 10 estudos, a idade média foi de 33 anos; dois estudos direcionados aos jovens; e dados de idade não estavam disponíveis para dois estudos. A maioria (80%) participantes do estudo eram do sexo masculino. Os estudos tiveram um baixo risco de viés de seleção,de desempenho e de resultado. Três estudos estavam em risco de viés de atrito.
Todos os estudos envolvidos compararam uso de medicação ativa e placebo. Os medicamentos incluíram preparações que continham tetrahidrocanabinol (THC) (dois estudos), inibidor da recaptação da serotonina (ISRS) antidepressivos (dois estudos), antidepressivos ação mista (três estudos), anticonvulsivantes e estabilizadores de humor (três estudos), um antidepressivo atípico (dois estudos ), um ansiolítico (um estudo), um inibidor da recaptação de norepinefrina (um estudo) e um modulador glutamatérgico (um estudo). Um estudo examinou mais de uma medicação. Diversidade nos medicamentos e os resultados relatados limitaram a extensão em que a análise foi possível. Os dados foram insuficientes para avaliar a utilidade da maior parte dos medicamentos para promover a abstinência de maconha no final do tratamento.
Houve evidência moderada de qualidade que a conclusão do tratamento era mais provável com preparações que contenham THC em comparação com placebo (RR 1,29, 95% CI 1,08 a 1,55; 2 estudos, 207 participantes, p = 0,006). Houve alguma evidência de que o tratamento com preparações que contenham THC foi associado a redução dos sintomas de abstinência de maconha e do desejo de uso, mas este último resultado não poderia ser quantificado. Para antidepressivos ação mista em comparação com placebo (2 estudos, 179 participantes) a qualidade de evidência sobre a probabilidade de abstinência de cannabis no final do follow‐up (RR 0,82, 95% CI 0,12‐5,41), e evidência de qualidade moderada sobre a probabilidade de conclusão do tratamento (RR 0,93, IC 95% 0,71‐1,21). Por este mesmo resultado, foi muito baixa a qualidade da evidência para os efeitos dos antidepressivos ISRS (RR 0,82, IC 95% 0,44‐1,53; 2 estudos, 122 participantes), anticonvulsivantes e estabilizadores de humor (RR 0,78, IC 95% 0,42‐1,46; 2 estudos, 75 participantes), e antidepressivo atípico, bupropiona (RR 1,06, IC 95% 0,67‐1,67; 2 estudos, 92 participantes). Os dados disponíveis sobre a gabapentina (anticonvulsivante) e N‐acetilcisteína (modulador glutamatérgico) foram insuficientes para estimativas quantitativas da sua eficácia, mas estes medicamentos podem valer a pena uma investigação mais aprofundada.
Conclusão dos autores
Há evidências incompletas para todos as farmacoterapias investigadas, e para muitos dos resultados a qualidade foi rebaixado devido ao pequeno tamanho das amostras, inconsistência e risco de viés de atrito. As análises quantitativas que foram possíveis, combinadas com os resultados gerais dos estudos revisados, indicam que os antidepressivos SSRI, antidepressivos ação mista, antidepressivos atípicos (bupropiona), ansiolíticos (buspirona) e inibidores da recaptação de norepinefrina (atomoxetine) são provavelmente de pouco valor no tratamento da dependência de maconha. Preparações contendo tetra‐hidrocanabinol (THC) são de valor potencial no tratamento de dependência de maconha, mas limitações na evidência são tais que esta aplicação de preparações de THC deve ser considerada ainda em fase experimental. Novos estudos devem comparar diferentes preparações de THC, dose e duração do tratamento, medicamentos e terapias adjuntas. A base de evidências para o anticonvulsivante gabapentina e o modulador glutamatérgico N‐acetilcisteína é fraco, mas estes medicamentos também merecem uma investigação mais aprofundada.
PICO
Resumo para leigos
Medicamentos para o tratamento da dependência de maconha
Introdução
A maconha é a droga ilícita mais comum no mundo. A procura por tratamento pelos consumidores de maconha tem aumentado na maioria das regiões do mundo. Atualmente não há medicamentos específicos para o tratamento do consumo de maconha. Esta revisão procurou avaliar a eficácia e segurança dos medicamentos para o tratamento da dependência de maconha.
Data da busca
Nós buscamos na literatura científica em fevereiro e março de 2014.
Características dos estudos
Nós identificamos 14 estudos randomizados controlados(estudos clínicos onde pessoas foram alocadas randomicamente em um de dois ou mais grupos de tratamento) envolvendo 958 participantes dependentes de maconha. As principais características do uso de drogas dependentes são de uso compulsivo, perda de controle sobre o uso e os sintomas de abstinência quando dacessação do uso de drogas. Esta revisão incluiu estudos onde os participantes foram descritos como dependente ou eram susceptíveis de ser dependentse com base no consumo de maconha vários dias por semana, ou diariamente.
A idade média dos participantes foi de 33 anos, excluindo dois estudos que tiveram como alvo pessoas mais jovens. A maioria (80%) participantes do estudo eram do sexo masculino. A maioria (10) dos estudos foram realizados nos EUA, com três ocorrendo na Austrália e um em Israel. Os estudos envolveram uma ampla gama de medicamentos para reduzir os sintomas de abstinência de maconha e promover a cessação ou a redução do consumo de maconha.
Dois estudos receberam medicamentos do estudo da empresa de fabricação de produtos farmacêuticos, mas nenhum foi financiado por empresas farmacêuticas.
Resultados principais
Os efeitos de muitos dos medicamentos que avaliamos nesta revisão eram incertos. Com base nas evidências disponíveis, antidepressivos, bupropiona, buspirona e atomoxetina são, provavelmente, de pouco valor no tratamento de dependência de maconha. Preparações contendo tetra‐hidrocanabinol (THC), o principal ingrediente psicoactivo da maconha, são de valor potencial no tratamento de dependência de maconha, mas limitações na evidência são tais que esta aplicação de preparações de THC deve ser considerado ainda em fase experimental. Os dados disponíveis sobre gabapentina e N‐acetilcisteína sugerem que estes medicamentos pode valer a pena uma investigação mais aprofundada, mas neste momento não é possível avaliar a sua eficácia.
Qualidade da evidência
A qualidade da evidência para muitos dos resultados desta avaliação foi rebaixado porque cada medicamento foi investigada por apenas um ou dois estudos, cada estudo envolveu pequeno número de participantes, houve alguma inconsistência nos resultados, e um risco de viés devido ao abandono do tratamento por participantes do estudo.
Authors' conclusions
Summary of findings
| Active medication compared with placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active Medication | |||||
| Number abstinent at end of treatment ‐ mixed action antidepressants | Study population | RR 0.82 | 179 | ⊕⊝⊝⊝ | ||
| 250 per 1000 | 205 per 1000 | |||||
| Moderate | ||||||
| 233 per 1000 | 191 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Significant heterogeneity between studies | ||||||
| Active medication compared with placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active Medication | |||||
| Number withdrawn due to adverse effects ‐ mixed action antidepressants | Study population | RR 1.44 | 179 | ⊕⊝⊝⊝ | ||
| 11 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 19 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Studies differ in direction of effect without significant heterogeneity | ||||||
| Active medication compared with placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active Medication | |||||
| Completion of treatment ‐ THC preparations | Study population | RR 1.29 | 207 | ⊕⊕⊕⊝ | ||
| 614 per 1000 | 792 per 1000 | |||||
| Moderate | ||||||
| 618 per 1000 | 797 per 1000 | |||||
| Completion of treatment ‐ mixed action antidepressants | Study population | RR 0.93 | 169 | ⊕⊕⊕⊝ | ||
| 573 per 1000 | 533 per 1000 | |||||
| Moderate | ||||||
| 551 per 1000 | 512 per 1000 | |||||
| Completion of treatment ‐ SSRI antidepressants | Study population | RR 0.82 | 122 | ⊕⊝⊝⊝ | ||
| 790 per 1000 | 648 per 1000 | |||||
| Moderate | ||||||
| 766 per 1000 | 628 per 1000 | |||||
| Completion of treatment ‐ anticonvulsant and mood stabiliser | Study population | RR 0.78 | 75 | ⊕⊝⊝⊝ | ||
| 405 per 1000 | 316 per 1000 | |||||
| Moderate | ||||||
| 387 per 1000 | 302 per 1000 | |||||
| Completion of treatment ‐ atypical antidepressant (bupropion) | Study population | RR 1.06 | 92 | ⊕⊝⊝⊝ | ||
| 429 per 1000 | 454 per 1000 | |||||
| Moderate | ||||||
| 400 per 1000 | 424 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Significant heterogeneity between studies | ||||||
Background
Description of the condition
Cannabis is the world’s most widely produced, seized and consumed illicit drug (World Drug Report 2013).
The main psychoactive compound in all cannabis products is Δ9‐ tetrahydrocannabinol (THC) (EMCDDA Cannabis Drug Profile). The number of cannabis users globally is estimated to range between 2.8% and 5.8% of the world’s population (World Drug Report 2013). Prevalence rates of cannabis use vary widely between regions, with the highest prevalence rates in Oceania, the Americas and Africa (World Drug Report 2013). Cannabis use has increased globally, particularly in Asia, since 2009 (World Drug Report 2013) and cannabis is identified as the primary drug of concern for substantial proportions of people in treatment for drug use in Africa, Latin America and the Caribbean, and Oceania (World Drug Report 2013). Cannabis use within some indigenous communities in North America and Australia may be more prevalent than for their non‐indigenous counterparts (Beauvais 2004; Clough 2004).
Cannabis use causes significant adverse effects (Budney 2007a). The acute effects of short‐term cannabis use (Volkow 2014) include impaired memory (Solowij 2008); impaired motor co‐ordination with an associated increased risk of involvement in motor vehicle accidents (Hall 2009); altered judgement; and, in high doses, paranoia and psychosis. Long‐term or heavy use of cannabis has been associated with: the development of dependence (Budney 2007a), chronic bronchitis, and increased risk of chronic psychosis disorders in persons with a predisposition for development of such disorders (Volkow 2014). When use is commenced early in adolescence, long‐term or heavy cannabis use has also been associated with altered brain development, poor educational outcome, cognitive impairment (Solowij 2008), and diminished life satisfaction and achievement (Gruber 2003).
It has been estimated that some 10% of those who have used cannabis at least once will develop cannabis dependence (Wagner 2002). Based on a large epidemiological survey in the USA, it has been estimated that, among those exposed once to cannabis, 7.0% of males and 5.3% of females will develop cannabis dependence at some point in their life, while 47.4% of males and 32.5% of females will develop cannabis use disorders (abuse or dependence) at some point in their life (Lev‐Ran 2013a).
As with other drugs of dependence, the risk of developing dependency is influenced by multiple factors. However, intensive use of cannabis, that is daily or near daily use, is likely to increase the risk of cannabis dependence (EMCDDA 2004). It has been suggested that the earlier initiation of cannabis use (Copeland 2014), use of more potent forms of cannabis (for example the flowering heads of the female cannabis plant), and the greater use of water‐pipes may have led to an increased amount of THC consumption by some cannabis users and, therefore, possibly greater rates of cannabis dependence (Hall 2001).
The use of cannabis has consistently been found to be associated with psychotic symptoms (Minozzi 2010) and may be associated with the earlier onset of psychotic illness in some people (Large 2011). Cannabis use and cannabis use disorders have been associated with a range of mental health disorders, such as anxiety and mood disorders (Lev‐Ran 2013). These associations were particularly pronounced with bipolar disorder, substance use disorders and specific (antisocial, dependant and histrionic) personality disorders (Lev‐Ran 2013).
Estimates of the number of cannabis users experiencing withdrawal are variable (Agrawal 2008; Budney 2006; Chung 2008; Copersino 2006; Cornelius 2008; Hasin 2008). Evidence regarding factors influencing the severity of cannabis withdrawal remains limited, but there is evidence that the total number of cannabis cigarettes smoked is predictive of the intensity of withdrawal during abstinence from cannabis (McClure 2012). Smoking behaviour also appears to be a strong predictor for the severity of cannabis dependence (van der Pol 2014).
General acceptance of a specific cannabis withdrawal syndrome is indicated by the inclusion of diagnostic criteria for cannabis withdrawal in the Fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). In the DSM‐5 cannabis withdrawal is defined by development of three or more of the following signs and symptoms within approximately one week of cessation of heavy and prolonged cannabis use: (1) irritability, anger or aggression; (2) nervousness or anxiety; (3) sleep difficulty; (4) decreased appetite or weight loss; (5) restlessness; (6) depressed mood; (7) at least one of the following physical symptoms causing significant discomfort: stomach pain, shakiness or tremors, sweating, fever, chills or headache (DSM‐5). Onset of symptoms is usually within 24 to 48 hours of abstinence, reaching peak intensity within the first week (Budney 2007a). Symptoms may persist for up three to four weeks (Milin 2008), although there appears to be significant individual variability. The cannabis withdrawal syndrome is not life threatening, nor is it associated with significant medical or psychiatric consequences (Budney 2003).
Demand for treatment for cannabis related disorders has generally increased worldwide over the past decade, albeit with significant regional variation. The World Drug Report gives data on treatment demand in terms of the proportion of treatment services provided for the major drugs of dependence. Cannabis related disorders have dominated demand for drug treatment in Africa over the past 10 years with treatment rates consistently over 60%. Demand for cannabis treatment has grown significantly in some regions, more than doubling in Europe and South America and more than trebling in Oceania (World Drug Report 2013). North America as a whole was the only region to see a decrease in the contribution of cannabis to treatment demand (World Drug Report 2013) but, within the USA, cannabis admissions increased by 32% between 1996 and 2006 (SAMHSA 2008). Increases in the THC content of cannabis may be a factor in the increasing demand for treatment. In the USA, THC content, as detected in confiscated samples, has increased from about 3% in the 1980s to 12% in 2012 (Volkow 2014). Cannabis users adjust their smoking behaviour when smoking stronger cannabis but the adjustment does not fully compensate for the increased strength (van der Pol 2014). Hence, cannabis users would be expected to be exposed to higher doses of THC as a result of the increasing potency of cannabis preparations. Cannabis users who seek treatment typically have a long history of cannabis use disorder and multiple previous attempts to quit (Copeland 2014).
Description of the intervention
There are currently no accepted pharmacotherapies for the treatment of cannabis withdrawal or cessation (Nordstrom 2007). The identification and development of medications to fill this gap has become an increasing priority among researchers (Vandrey 2009). However, a number of pharmacotherapies have been proposed as possible experimental interventions to attenuate the symptoms and signs of cannabis withdrawal and to promote cessation.
These medications are diverse in nature, encompassing medications that affect cannabinoid receptor systems (for example preparations of THC), medications that affect dopamine pathways, medications that affect the specific symptoms of cannabis withdrawal or that have been used in managing withdrawal from other substances, and medications that affect mental health conditions, such as depression, that may be factors contributing to cannabis use.
How the intervention might work
The proposed pharmacologic interventions may potentially lessen the symptoms and signs of cannabis withdrawal, including craving. The availability of effective pharmacotherapy for cannabis withdrawal may encourage people who are cannabis dependent to enter treatment, and may increase the rates of completion of withdrawal, cessation of cannabis use and entry into relapse prevention treatment.
It has been reported that the experience of cannabis withdrawal symptoms may be a significant obstacle to the achievement of abstinence by people who are cannabis dependent (Budney 2006; Copeland 2001; Hart 2005). Therefore, the effective treatment of the cannabis withdrawal syndrome may promote cessation of cannabis use and provide a first step towards abstinence and recovery.
Why it is important to do this review
As discussed above, there is increasing recognition that cannabis use and dependence is an important public health issue.
Not all cannabis users will need pharmacotherapies to manage withdrawal or support cessation of their use. However, it is important that effective pharmacotherapies are identified for the treatment of cannabis withdrawal, especially in intensive cannabis users who describe withdrawal symptoms on cessation.
We believe that this is the first systematic review of pharmacotherapies for cannabis dependence, and the first review to focus on studies involving people seeking treatment for cannabis use. As such, this review seeks to establish current knowledge on the effectiveness of medications in the treatment of cannabis dependence.
Objectives
To assess the effectiveness and safety of pharmacotherapies as compared with each other, placebo or no pharmacotherapy (supportive care) for reducing symptoms of cannabis withdrawal and promoting cessation or reduction of cannabis use.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials that provided detailed information on the type and dose of intervention medication used and the characteristics of participants treated.
Types of participants
We included studies that involved participants diagnosed as cannabis dependent or who were likely to be dependent based on reported dose, duration and frequency of use (daily or multiple days per week).
Studies involving participants dependent on, and withdrawing from, both cannabis and nicotine were included, but studies involving participants dependent on and withdrawing from substances other than cannabis and nicotine were excluded. It was intended to use subgroup analyses to assess the impact of concurrent nicotine and cannabis withdrawal on the effectiveness of pharmacotherapies for cannabis withdrawal, but there were insufficient data for such analyses to be undertaken.
Studies undertaken in either inpatient or outpatient settings were included. Studies undertaken in purely research settings, such as residential research laboratory settings, were excluded. Some of these studies provide insight into the effect of different medications on signs and symptoms of cannabis withdrawal and are considered in the discussion section. However, such studies generally involved participants who were not seeking treatment for cannabis use and cessation of cannabis use was not the goal of the interventions provided, and the nature of outcomes assessed were generally different to those expected of treatment interventions. For these reasons such studies were excluded from this review.
Types of interventions
Experimental interventions involved the administration of medications with the aim of reducing the symptoms and signs of cannabis withdrawal or promoting cessation of cannabis use.
Comparison interventions involved the use of different pharmacotherapies, placebo or no pharmacotherapy (supportive care).
Types of outcome measures
Primary outcomes
-
Number of participants abstinent from cannabis at the end of treatment as determined by self‐report or urine drug screens, or both
-
Intensity of withdrawal as determined by scores on withdrawal scales, the need for symptomatic medications in addition to the experimental intervention or overall assessments by clinicians and participants
-
Nature, incidence and frequency of adverse effects and whether the planned medication regime was modified in response to adverse effects
-
Completion of scheduled treatment
Secondary outcomes
-
Level of cannabis use at the end of treatment as measured via participant reported level of use or urine drug screens, or both.
-
Number of participants engaged in further treatment following completion of the withdrawal intervention. As discussed in the 'Background' section, treatment of the cannabis withdrawal period may be considered as the first step in treatment, therefore engagement in further relapse prevention treatment may be considered to be a valid outcome of interest.
Search methods for identification of studies
All searches included non‐English language literature. No studies were found in languages other than English.
Electronic searches
We searched:
-
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (www.thecochranelibrary.com) to 4 March 2014;
-
MEDLINE (1946 to week 3 February 2014) via Ovid Online;
-
EMBASE (1980 to 3 March 2014) via Ovid Online;
-
PsycINFO (1806 to week 4 February 2014) via Ovid Online.
We developed a search strategy to retrieve references relating to the pharmacologic treatment of cannabis withdrawal. This strategy was adapted to each of the databases listed above.
For details see Appendix 1; Appendix 2; Appendix 3; Appendix 4.
We also searched some of the main electronic sources of ongoing trials:
-
Current Controlled Trials (www.controlled‐trials.com/);
-
ClinicalTrials.gov;
-
Osservatorio Nazionale sulla Sperimentazione Clinica dei Medicinali (https://oss‐sper‐clin.agenziafarmaco.it/);
-
Trialsjournal.com.
Searching other resources
We checked the reference lists of relevant review articles and retrieved studies to identify any further studies of interest that were not retrieved by the electronic search. We contacted selected researchers who are active in the area seeking information about unpublished study reports. We also checked conference proceedings likely to contain trials relevant to the review.
Data collection and analysis
Selection of studies
Two authors (KM and LG) independently assessed the titles and abstracts of records retrieved from the systematic search according to the identified inclusion and exclusion criteria. All authors agreed on the inclusion and exclusion decisions. No attempt was made to blind the authors to the names of the study authors, institutions, journal of publication and results when eligibility criteria were applied.
Data extraction and management
Two authors (KM and LG) independently extracted key information from the included studies using a data collection form to record information against the outcome measures (abstinence, intensity of withdrawal, adverse effects, completion of treatment, change in cannabis use, and engagement in follow‐up treatment). Data were confirmed by consultation with the other review authors. Key findings of studies were summarized descriptively in the first instance and the capacity for quantitative meta‐analysis was considered.
Sufficient information was extracted from reports of included studies to enable assessment of the risk of bias.
Assessment of risk of bias in included studies
The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) recommends the use of a two‐part tool to assess the risk of bias in studies included in Cochrane reviews. This tool addresses the specific domains of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and ‘other issues’. The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, in terms of low, high or unclear risk. Each included study was analysed and described according to these domains. To make these judgements, we used the criteria indicated by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and addressed their applicability to the addiction field.
We considered blinding separately for subjective and objective outcomes. Lack of blinding is a source of serious risk of bias for subjective outcomes but is less significant with objective outcomes, such as completion of treatment and duration of treatment. We only considered incomplete outcome data for the intensity of withdrawal, change in cannabis use, and nature and incidence of adverse effects. Retention in treatment (duration of treatment) and completion of treatment are frequently primary outcome measures in addiction research. See Appendix 5 for the detailed description of the criteria used.
Details of the assessments of risk of bias are included in the Characteristics of included studies.
Measures of treatment effect
Where possible, for dichotomous outcomes (for example number completing treatment) we calculated risk ratios (RR) with 95% confidence intervals (CI). No continuous data were obtained but the intention was to express continuous outcomes as a mean difference where there was a a comparable outcome measure (for example time in treatment) or as a standardized mean difference where there was variability in the outcome measure (for example withdrawal assessment scales).
Unit of analysis issues
One study included in the review involved three treatment arms (two different active medications and placebo). The active medications, compared to placebo, were included in separate subgroups and the calculation of overall totals was suppressed thereby avoiding the unit of analysis error of double‐counting participants. Where urine drug screens were reported in studies, the unit of analysis was the number of study participants and not the number of tests performed.
Dealing with missing data
It was intended to attempt to contact original investigators to request missing data. However, this was not undertaken given the limited capacity for meta‐analysis. It was also intended to use sensitivity analysis to assess the impact of different approaches to handling missing data but there were insufficient data for this.
Assessment of heterogeneity
Clinical and methodological heterogeneity was assessed by reviewing the variations between studies in terms of the characteristics of participants included, the interventions and the reported outcomes. Studies were grouped for analyses by the nature of the medication used (experimental intervention). As there was considerable heterogeneity in the types of medications, subgroup but not overall totals were calculated.
We assessed statistical heterogeneity using the Chi2 test and its P value, by visual inspection of the forest plots. and the I2 statistic. A P value of the Chi2 test lower than 0.10 or an I2 statistic of at least 50% indicated a significant statistical heterogeneity.
Data synthesis
We used Review Manager 5.2 for statistical analyses. In all analyses we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
This review aimed to consider the following potential sources of heterogeneity through subgroup analyses:
-
patterns of cannabis use and the estimated level of THC intake (as indicated by duration and level of use, number of days of use, number of uses per day (frequency), modality of use or route of administration, age at initiation of use);
-
concurrent tobacco smoking;
-
concurrent psychiatric illness and current treatment for a psychiatric illness;
-
the nature of the treatment setting;
-
the nature of adjunct treatment.
None of these analyses were possible due to limitations of the studies that met the inclusion criteria.
Sensitivity analysis
We did not use methodological quality as a criterion for inclusion in this review. We intended to assess the impact of methodological quality through sensitivity analysis. This would have involved considering the overall estimate of effect with studies with a high risk of bias included or excluded. Limitations of data reported by the studies that met the inclusion criteria meant that sensitivity analysis was not possible. However, the risk of bias was discussed in presenting the results.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies
Results of the search
Our search strategy identified 947 unique records from which 52 reports, relating to 45 different studies, were identifited as potentially relevant to this review (see Figure 1).

Study flow diagram.
Included studies
Fourteen randomised controlled trials (16 reports) involving 958 participants met the inclusion criteria for this review (see Characteristics of included studies). In total, 500 were treated with active medication and 458 received placebo. In all studies participants were offered some form of psychological therapy in addition to medication (or placebo).
All studies involved a comparison between an active medication and placebo, but the medications investigated by the studies included in this review were diverse. This limited the extent of analysis that was possible.The medications investigated, grouped according to type and mechanism of action, were:
-
preparations containing THC, dronabinol (Levin 2011) and nabiximols (Allsop 2014);
-
selective serotonin reuptake inhibitor (SSRI) antidepressants fluoxetine (Cornelius 2010), escitalopram (Weinstein 2014);
-
mixed action antidepressants (noradrenergic and serotonergic effects), nefazodone (Carpenter 2009), mirtazapine (Frewen 2007), venlafaxine (Levin 2013);
-
anticonvulsant and mood stabilisers divalproex sodium (Levin 2004), gabapentin (Mason 2012), lithium (Johnston 2012);
-
atypical antidepressant (dopamine reuptake inhibitor and weak norepinephrine reuptake inhibitor) bupropion (Carpenter 2009; Penetar 2012);
-
anxiolytic (serotonin 5‐HT1A partial agonist) buspirone (McRae‐Clark 2009);
-
selective norepinephrine reuptake inhibitor atomoxetine (McRae‐Clark 2010);
-
a supplement promoting glutamate release and modulating N‐methyl‐D‐aspartate (NMDA) receptor, N‐acetylcysteine (Gray 2012).
All except two of the studies were undertaken in outpatient settings. Allsop 2014 and Johnston 2012 were primarily studies of cannabis withdrawal, with medication administered in an inpatient (hospital) setting over six to seven days, with follow‐up interviews post‐discharge.
The majority (10) of the studies were undertaken in the USA, with three studies (Allsop 2014; Frewen 2007; Johnston 2012) in Australia and one study (Weinstein 2014) in Israel. Twelve studies reported the source of funding as (government) research grants, and the funding source was unclear for two studies (Frewen 2007; Johnston 2012). Two studies (Allsop 2014; McRae‐Clark 2010) used medications provided by the manufacturing company. Primary researchers associated with six studies declared past associations with pharmaceutical companies. Researchers associated with four studies declared no conflict of interest; no declarations were made for the remaining four studies.
Two studies (Cornelius 2010; Penetar 2012) included participants with cannabis use disorders as well as cannabis dependence, but the majority of participants met diagnostic criteria for cannabis dependence. In the other studies all participants were cannabis dependent.
For 10 studies, the average age of participants was around 33 years; data on age were not provided for two studies (Johnston 2012; Penetar 2012). The target population for the remaining two studies (Cornelius 2010; Gray 2012) was adolescents and young adults. The average age of participants in these studies was 21.1 and 18.9 years, respectively.
Two studies (Johnston 2012; Penetar 2012) did not provide information on the gender of participants; the majority (73% to 92%) of participants in the other 12 studies were male.
Participants in two studies (Cornelius 2010; Levin 2013) had comorbid depression and cannabis use disorders, and in one study (McRae‐Clark 2010) participants met diagnostic criteria for attention deficit hyperactivity disorder as well as cannabis dependence.
Excluded studies
Thirty‐one studies (36 reports) that were considered potentially relevant to the review and assessed in detail were excluded from the review (see Figure 1 and Characteristics of excluded studies). The reasons for exclusion were: study was exploratory research with participants who were not seeking treatment or participants were not cannabis dependent (14 studies); no treatment comparison (nine studies); cannabis used in combination with other drugs or not the main focus of the treatment intervention (seven studies); no medications (one study); insufficient outcome data (one study). One study was excluded for more than one reason.
Risk of bias in included studies
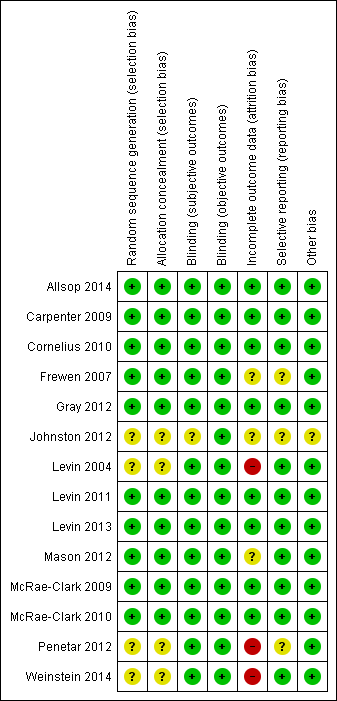
For summary results of the judged risk of bias across the included studies for each domain, see Figure 2 and Figure 3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
For four studies (Johnston 2012; Levin 2004; Penetar 2012; Weinstein 2014) the risk of bias associated with both sequence generation and concealment of allocation was unclear. All four studies were double‐blind and random allocation was stated but the methods of sequence generation and group allocation were not reported. The other studies were assessed as having a low risk of allocation bias.
Blinding
In one study (Johnston 2012) the risk of bias for subjective outcomes was unclear because the extent of blinding was unclear. Objective outcomes are unlikely to be affected by awareness of group allocation and hence we assessed Johnston 2012 as having a low risk of performance and detection bias in relation to objective outcomes.
All other studies were assessed as having a low risk of performance and detection bias for both subjective and objective outcomes.
Incomplete outcome data
This domain was considered only for the outcomes of intensity of withdrawal, adverse effects and abstinence (or use of cannabis). Completion of treatment was a primary outcome measure for the review. In three studies (Frewen 2007; Johnston 2012; Mason 2012) the risk of attrition bias due to incomplete data was unclear, and three studies (Levin 2004; Penetar 2012; Weinstein 2014) were assessed as being at high risk of attrition bias.
Selective reporting
Frewen 2007 was a secondary analysis of data from a randomised controlled trial and reported some but not all findings from the main study. The full report of the study was not available and hence the risk of reporting bias was unclear. Johnston 2012 was reported as conference abstracts only and insufficient information was available to assess the risk of reporting bias. Penetar 2012 did not discuss adverse effects making it unclear whether adverse effects were systematically assessed during the study.
Other potential sources of bias
In Johnston 2012 the risk of other sources of bias was unclear; all other studies were assessed as being at low risk of other sources of bias, such as recruitment bias, differential amounts of contact time or performance bias in the treatment groups being compared.
Effects of interventions
See: Summary of findings for the main comparison Abstinence at end of treatment; Summary of findings 2 Withdrawal due to adverse effects; Summary of findings 3 Completion of treatment
Results are presented for the outcomes identified as relevant to this review and then summarised by medication type. Where meta‐analysis was possible, only subgroup totals were calculated because of the diversity of the medications that were investigated. The summary of findings tables include results only from those analyses where more than one study provided data.
Cannabis use
The only outcome relating to cannabis use for which meta‐analysis was possible was the number of participants abstinent at the end of treatment (Analysis 1.1). These data were available for only four of the medication subgroups (THC preparations, SSRI antidepressants, mixed action antidepressants and anticonvulsants or mood stabilisers), with mixed action antidepressants being the only medication subgroup for which data were obtained from more than one study. There was no significant difference for any of these subgroups in the likelihood of abstinence from cannabis use at the end of treatment for active medication compared to placebo and, because of the small number of studies providing data and the small size of those studies, the quality of evidence in relation to this outcome was considered very low (summary of findings Table for the main comparison).
Both studies using preparations containing THC (Allsop 2014; Levin 2011) reported a reduction in cannabis use over time but with no significant group differences. Allsop 2014 reported that weekly cannabis use decreased by an average 19.02 g/day (82%) from baseline to 28‐day follow‐up, and Levin 2011 reported that the median maximum consecutive days of abstinence was six for the Dronabinol group (interquartile range (IQR) 1 to 13) compared to five for the placebo group (IQR 2 to 16).
In Weinstein 2014 there was a tendency towards participants receiving escitalopram being abstinent at the end of treatment compared to those receiving placebo. However, the high rates of dropout from treatment in this study introduced a high risk of bias for this outcome. Cornelius 2010 compared fluoxetine with placebo and reported that the count of criteria for cannabis abuse or dependence (mean ± SD) at the end of treatment was 3.88 ± 2.51 for those treated with fluoxetine (N = 34) compared to 3.61 ± 1.92 for those receiving placebo (N = 36). There were no significant group by time interactions for cannabis or depression outcomes in this study.
The two studies that reported data on the number of participants abstinent at the end of treatment for mixed action antidepressants compared to placebo had divergent findings (see Analysis 1.1). In Levin 2013 significantly fewer participants treated with venlafaxine were abstinent at the end of treatment compared to participants receiving placebo. In contrast, in Carpenter 2009 there was a tendency towards abstinence being more likely with nefazodone compared to placebo. However, there was no significant difference in the severity of dependence rating (mean ± SD) at the end of treatment for the nefazodone group (2.5 ± 1.4) compared to the placebo group (2.3 ± 1.6). A third study (Frewen 2007) using a mixed action antidepressant (mirtazapine) did not report data suitable for inclusion in the meta‐analysis but stated that mirtazapine had no effect on cannabis use, with less than 20% of participants reporting abstinence at day 56.
In addition to the data on abstinence in Analysis 1.1, Levin 2004 reported that at the end of treatment (weeks 7 and 8), participants in the divalproex group reported using cannabis on (mean ± SD) 2.75 ± 3.55 days/week, compared to 1.56 ± 2.34 days/week for the placebo group, and 4.88 ± 7.58 joints/week compared to 0.99 ± 1.18 joints/week for the placebo group. The group by time interaction was not statistically significant. For the anticonvulsant and mood stabiliser gabapentin, Mason 2012 reported a significant reduction in the grams of cannabis smoked per week, by self‐report and urinalysis, and in the days of use per week for gabapentin compared to placebo (these data were not reported in a form suitable for inclusion in meta‐analysis). Johnston 2012 did not report any data on cannabis use for lithium compared to placebo.
Carpenter 2009 reported no difference between the bupropion and placebo groups in terms of the severity of dependence rating (mean ± SD) at the completion of treatment (2.7 ± 1.5 for N = 40 receiving bupropion compared to 2.3 ± 1.6 for N = 30 receiving placebo).
In McRae‐Clark 2009, those receiving buspirone (N = 23) had 45.2% days abstinent during the trial compared to 51.4% for the placebo (N = 27) group. The amount of cannabis used per day was reduced 91% in the buspirone group and 93% in the placebo group. These differences were not statistically significant.
In McRae‐Clark 2010, 13 of 19 in the atomoxetine group compared with 9 of 19 in the placebo group had no days with heavy cannabis use during treatment. The atomoxetine group had 60.1 ± 31.5% days with cannabis use compared to 68.1 ± 31.3% for the placebo group (mean ± SD). The authors concluded that atomoxetine may improve some ADHD symptoms but does not reduce cannabis use.
Gray 2012 reported significantly greater likelihood of a negative urine cannabinoid test during treatment for the N‐acetyl cysteine group compared to the placebo group (odds ratio 2.4, 95% CI 1.1 to 5.2; P = 0.029). However, there was no significant difference in the percentage of days during treatment with cannabis use, by self‐report.
Intensity of withdrawal
Few studies reported data on the intensity of withdrawal, there was variability in the method of assessment of withdrawal, and available data were reported in different ways. As a result, meta‐analysis of data on withdrawal intensity was not possible.
The two studies that compared preparations containing THC with placebo found that withdrawal scores decreased over time for both groups but the decrease was greater with the THC preparation than with placebo. Allsop 2014 reported that on average it took 3.1 ± 3.0 days for withdrawal scores to fall below baseline with nabiximols (N = 27) compared with 4.9 ± 3.16 days for placebo (N = 24). Nabiximols reduced the withdrawal score 66% on average from baseline compared to 52% for placebo. The group receiving nabiximols had significantly lower levels of cravings, irritability, anger and aggression. Levin 2011 similarly reported a reduction in the withdrawal discomfort scores for both the dronabinol (N = 79) and placebo (N = 77) groups, but found that participants on dronabinol experienced a significantly greater drop in their withdrawal scores over time.
Frewen 2007 focused on sleep quality and did not report the full assessment of withdrawal intensity during treatment with the mixed action antidepressant mirtazapine compared to placebo. The number of participants in each group was also not reported. In this study the overall difference in sleep between the mirtazapine and placebo groups over time was not significant. Significant improvements were observed for sleep duration and sleep quality but not for sleep disturbances.
Three studies (Johnston 2012; Levin 2004; Mason 2012) compared an anticonvulsant or mood stabiliser with placebo. Levin 2004 reported a reduction in craving over time but with no significant group differences between divalproex (N = 13) and placebo (N = 12). Mason 2012 reported significant reductions in acute withdrawal symptoms with gabapentin (N = 25) compared to placebo (N = 25). Johnston 2012 reported that lithium (N = 19) did not significantly reduce the total scores on the cannabis withdrawal scale relative to placebo (N = 19), but did significantly reduce the items loss of appetite, stomach aches and nightmares or strange dreams.
In Penetar 2012, following cessation of cannabis (days 8 to 21 of the scheduled treatment protocol), withdrawal discomfort scores increased significantly for the placebo group (N = 12) but not the bupropion group (N = 10) based on change from baseline. Craving scores also increased more for the placebo group.
McRae‐Clark 2009 reported no significant difference between buspirone (N = 23) and placebo (N = 27) in terms of change in the mean withdrawal checklist score.
McRae‐Clark 2010 reported no significant difference between atomoxetine (N = 19) and placebo (N = 19) in terms of change in marijuana craving score.
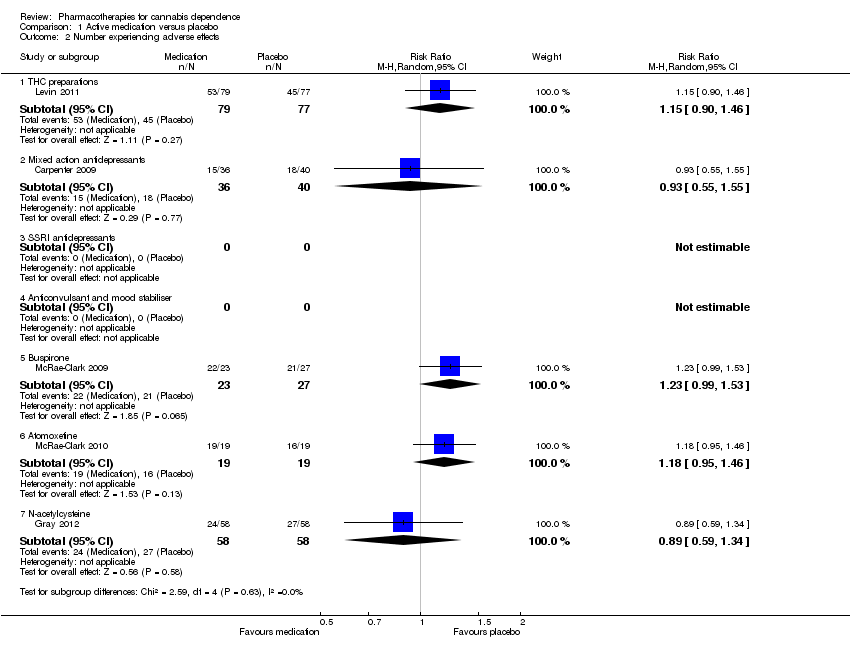
Adverse effects
Data on the number of participants experiencing any adverse effects (Analysis 1.2) suggested a tendency towards adverse effects being more likely with medication compared to placebo for preparations containing THC, buspirone and atomoxetine, but insufficient data were available to be conclusive. It appeared that the adverse effects experienced did not result in cessation of treatment (Analysis 1.3) and the number of participants withdrawing due to adverse effects was very small. The small number of events and differences between studies resulted in the evidence for this outcome being assessed as very low quality (summary of findings Table 2).
Allsop 2014 reported that study participants receiving nabiximols (N = 27) on average experienced 6.96 ± 11.02 adverse effects compared with 5.54 ± 6.70 for those receiving placebo (N = 24). This was consistent with the data shown in Analysis 1.2 from Levin 2011, indicating a somewhat higher likelihood of adverse effects with medication containing cannabinoids compared to placebo.
No data were reported on adverse effects of SSRI antidepressants in a form that was suitable for inclusion in the meta‐analysis, but Cornelius 2010 reported no moderate or severe adverse effects with fluoxetine and no participants withdrew from treatment due to adverse effects.
In Carpenter 2009, there was no significant difference in the number of participants experiencing adverse effects with nefazodone (a mixed action antidepressant) compared to placebo, but adverse effects were more likely to be moderate or severe with nefazodone. Diarrhoea was reported to be most common with nefazodone, and gastrointestinal upset with placebo.
No data suitable for inclusion in meta‐analyses were reported on the adverse effects of anticonvulsants or mood stabilisers. Levin 2004 noted that medication compliance was low for divalproex, based on blood levels, but it was not clear whether the low rate of compliance was related to adverse effects. For gabapentin compared to placebo, Mason 2012 reported no differences between the groups in the type, number and severity of adverse events reported. For lithium compared to placebo, Johnston 2012 reported no significant difference in the number or severity of adverse effects.
No data suitable for inclusion in meta‐analyses were reported on the adverse effects of bupropion, but Carpenter 2009 reported that adverse effects were more likely to be moderate or severe with bupropion compared to placebo. Headaches and nausea were most common with bupropion.
In McRae‐Clark 2009, participants receiving buspirone were more likely to experience adverse effects compared to those receiving placebo (RR 1.23, 95% CI 0.99 to 1.53; P = 0.06) (Analysis 1.2). Dizziness was reported more frequently with buspirone. Dry mouth, flushing or sweating and cold‐like symptoms were also more frequent with buspirone but the difference was not statistically significant. All adverse effects were noted as being mild to moderate in severity.
In McRae‐Clark 2010, all adverse effects were reported as mild to moderate in severity. Sexual dysfunction and gastrointestinal side effects were more common with atomoxetine than placebo.
Gray 2012 reported no significant adverse events and no significant group differences in the occurrence of adverse events for N‐acetylcysteine compared with placebo. One participant in the N‐acetylcysteine group discontinued medication due to severe heartburn.
Completion of treatment
Preparations containing THC were the only medications where completion of the scheduled treatment was more likely with active medication (N = 106) compared to placebo (N = 101) (RR 1.29, 95% CI 1.08 to 1.55; P = 0.006) (Analysis 1.4). The quality of the evidence on completion of treatment was assessed as moderate quality for preparations containing THC and mixed action antidepressants; and very low quality for SSRI antidepressants, anticonvulsants or mood stabilisers, and the atypical antidepressant bupropion (summary of findings Table 3).
Allsop 2014 also noted that participants receiving nabiximols remained in treatment for longer than those receiving placebo. Levin 2011 reported that the group receiving dronabinol attended more therapy sessions (8 ± 3.6) than those receiving placebo (6.8 ± 3.8) (mean±SD).
Weinstein 2014 compared an SSRI antidepressant with placebo and reported a high rate of dropout from the study (50%).
While not statistically significant, there was a tendency in Mason 2012 for participants receiving gabapentin to be less likely to complete treatment compared to those receiving placebo. Those receiving gabapentin remained in treatment for an average of 46.8 days compared to 48.7 days for those receiving placebo. Johnston 2012 reported no significant difference in retention rates for lithium compared to placebo.
Summary of effectiveness by medication type
(a) Preparations containing THC
The results of Allsop 2014 and Levin 2011 showed that preparations containing THC were more effective than placebo in reducing cannabis withdrawal symptoms and cravings. The THC preparations were associated with a somewhat higher likelihood of adverse effects, but these adverse effects were not sufficiently severe to cause withdrawal from treatment. Indeed, preparations containing THC were associated with significantly greater likelihood of completing treatment compared to placebo (RR 1.29, 95% CI 1.08 to 1.55; P = 0.006) (Analysis 1.4). However, THC preparations were not associated with increased likelihood of abstinence or a greater reduction in cannabis use.
(b) SSRI antidepressants
Neither of the studies comparing SSRI antidepressants with placebo (Cornelius 2010; Weinstein 2014) reported data on the intensity of withdrawal. No moderate or severe adverse effects were reported. Weinstein 2014 reported a high dropout rate with escitalopram and Cornelius 2010 found no significant difference in rates of completion of treatment for fluoxetine compared to placebo. Both studies reported no significant effect of these medications on cannabis use.
(c) Mixed action antidepressants
Three studies were included in this subgroup (Carpenter 2009; Frewen 2007; Levin 2013). Carpenter 2009 found that nefazodone had no significant effect on cannabis withdrawal symptoms. Frewen 2007 reported that mirtazapine improved sleep duration and quality but not sleep disturbances. In Carpenter 2009 there was no significant difference between nefazodone and placebo in the number of participants experiencing adverse effects, but adverse effects were more likely to be moderate or severe with nefazodone. There was no significant difference in rates of completion of treatment for this group of antidepressants compared to placebo. The effect on abstinence varied, with abstinence being significantly less likely with venlafaxine (Levin 2013) and somewhat more likely with nefazodone (Carpenter 2009), while Frewen 2007 reported that mirtazapine had no significant effect on cannabis use.
(d) Anticonvulsants and mood stabilisers
Gabapentin may have ameliorated cannabis withdrawal symptoms (Mason 2012) but it appeared that divalproex did not (Levin 2004), and lithium affected only some symptoms (Johnston 2012). Gabapentin (Mason 2012) and lithium (Johnston 2012) were not associated with adverse effects; information on adverse effects was not reported for divalproex although it was noted that compliance with medication was poor, based on blood levels. Neither medication affected retention in treatment. Gabapentin was associated with reduced cannabis use (Mason 2012) but divalproex was not.
(e) Atypical antidepressant (bupropion)
Bupropion had some capacity to reduce cannabis withdrawal and craving (Penetar 2012). Adverse effects were more likely with bupropion than with placebo. No data were available on rates of completion of treatment. Bupropion had no effect on cannabis dependence.
(f) Anxiolytic (buspirone)
A single study (McRae‐Clark 2009) found buspirone to have no effect on cannabis withdrawal symptoms or cannabis use. Adverse effects were more likely with buspirone than with placebo; there were no data on rates of completion of treatment.
(g) Norepinephrine reuptake inhibitor (atomoxetine)
A single study (McRae‐Clark 2010) found atomoxetine to have no effect on cannabis withdrawal symptoms, craving or cannabis use. Adverse effects were more likely with atomoxetine; there were no data on rates of completion of treatment.
(h) Glutamatergic modulator (N‐acetylcysteine)
A single study (Gray 2012) found that the likelihood of negative urine tests for cannabis during treatment was greater with N‐acetylcysteine than with placebo, but these data were not reported against the number of participants. Furthermore, there was no significant difference in self‐reported cannabis use. No data were reported on the intensity of withdrawal symptoms or rates of completion of treatment. There was no significant difference between N‐acetylcysteine and placebo in terms of adverse effects.
Discussion
Summary of main results
The medications considered by the studies that met the inclusion criteria for this review were diverse in nature. This and variability in the nature of data reported limited the extent of meta‐analysis that was possible. In particular, limitations in data on intensity of withdrawal prevented any meta‐analysis for this outcome. Obtaining consistent assessments of withdrawal is difficult in the context of clinical treatment, particular when undertaken in outpatient settings. For this reason, the discussion below incorporates some consideration of the findings from studies undertaken in controlled laboratory conditions that provide information on the capacity of the different medications to reduce cannabis withdrawal.
The quality of evidence available for assessment of effectiveness against the defined outcomes was generally very low (see summary of findings Table for the main comparison, summary of findings Table 2) other than for completion of treatment (preparations containing THC and mixed action antidepressants only) where the quality of the evidence was assessed as moderate (summary of findings Table 3).
This section summarises the main results and considers information from studies that were excluded from this review so as to form a more complete view of the effectiveness of medications for the treatment of cannabis dependence.
(a) Preparations containing THC
Preparations containing THC are effective in suppressing cannabis withdrawal symptoms and craving, and are associated with better retention in treatment than placebo, but are not associated with reductions in cannabis use at least in the relatively short time frames of the studies included in this review (Allsop 2014; Levin 2011). The capacity of preparations containing THC to reduce withdrawal discomfort and craving with minimal adverse effects is supported by case reports (Levin 2008; Vandrey 2013) and laboratory studies (Budney 2007; Haney 2004). The use of medications such as lofexidine (Haney 2008) and zolpidem (Haney 2013a) as adjuncts may enhance the effectiveness of THC preparations in attenuating cannabis withdrawal and improving sleep. Effectiveness may also depend on the nature of the cannabinoid preparation used. Nabilone, a synthetic analogue of THC with higher bioavailability than dronabinol, has been used by Haney and colleagues in recent laboratory studies (Haney 2013; Haney 2013a); nabiximols, used by Allsop 2014, is an extract of cannabis containing THC and cannabidiol (another cannabinoid thought to be of therapeutic importance) in a controlled ratio. While the available information indicates that preparations containing THC have considerable potential for the treatment of cannabis dependence, further research is needed to determine the relative effectiveness of different preparations, the value of adjunct medications and therapies, as well as the appropriate duration of treatment before drawing conclusions on the therapeutic value of preparations containing THC.
(b) SSRI antidepressants
In a study of fluoxetine for the treatment of alcohol dependence and comorbid depression, Cornelius 1999 identified a subgroup of study participants who were cannabis users. In this subgroup, fluoxetine treatment was associated with decreased cannabis use relative to placebo. This study provided part of the rationale for the randomised controlled trial comparing fluoxetine and placebo for the treatment of cannabis use disorder and comorbid depression in the adolescents included in this review (Cornelius 2010). In Cornelius 2010 there was no significant difference between fluoxetine and placebo in the effect on cannabis related symptoms, and depressive symptoms improved in both groups. Similarly, Weinstein 2014 found little value for the SSRI escitalopram in the treatment of cannabis dependence. However, these medications may still be of value for the treatment of depression in cannabis users (Findling 2009).
(c) Mixed action antidepressants
The studies that were included in this review found that the mixed action antidepressants nefazodone (Carpenter 2009), mirtazapine (Frewen 2007) and venlafaxine (Levin 2013) are of little value in the treatment of cannabis dependence. In a laboratory study, Haney 2003a found that nefazodone decreased some cannabis withdrawal symptoms (anxiety, muscle pain) but that participants still reported substantial discomfort (irritability, feeling miserable, sleep quality), and also concluded that nefazodone has limited potential in the treatment of cannabis dependence. Similarly, a laboratory study of mirtazapine (Haney 2010) found that mirtazapine improved sleep during abstinence and increased food intake but had no effect on withdrawal symptoms and did not decrease cannabis relapse in the laboratory model. As with SSRI antidepressants, the mixed action antidepressants may be of value in the treatment of depressive symptoms with comorbid substance use disorder but appear to have little value specifically for the treatment of cannabis dependence.
(d) Anticonvulsants and mood stabilisers
Gabapentin (Mason 2012), but not divalproex (Levin 2004), has some capacity to ameliorate cannabis withdrawal symptoms and promote reduction in cannabis use compared to placebo. In a laboratory study divalproex was found to worsen mood and cognitive performance during cannabis withdrawal (associated with the smoking of placebo rather than active cannabis cigarettes) supporting the finding that divalproex is not helpful in the management of cannabis withdrawal. Preliminary studies suggested potential therapeutic value for lithium, particularly with comorbid bipolar disorder (Geller 1998), but a subsequent randomised controlled trial that was included in this review found that lithium affected only some cannabis withdrawal symptoms and had no effect on retention in treatment (Johnston 2012).
(e) Atypical antidepressant (bupropion)
The studies that were included in this review (Carpenter 2009; Penetar 2012) indicated that bupropion may have some effect on cannabis withdrawal symptoms, but the data were inconclusive. A laboratory study (Haney 2001) found that bupropion was associated with increased ratings of irritability, restlessness, depression and trouble sleeping during the withdrawal phase when study participants were smoking placebo cannabis. The authors concluded that bupropion would not be an effective medication for the treatment of cannabis dependence.
(f) Anxiolytic (buspirone)
Buspirone showed promise in a preliminary study (McRae 2006) but the subsequent randomised controlled trial that was included in this review (McRae‐Clark 2009) found it to have little value in the treatment of cannabis dependence. However, it may be useful for the treatment of anxiety in cannabis users.
(g) Norepinephrine reuptake inhibitor (atomoxetine)
Atomoxetine is used for the treatment of attention deficit hyperactivity disorder (ADHD) and the study included in this review (McRae‐Clark 2010) investigated the effectiveness of atomoxetine in a population of cannabis users with ADHD. This study found atomoxetine to have little value in the treatment of cannabis dependence, but it may still be useful for the treatment of ADHD in cannabis users. An earlier open label pilot study of atomoxetine for the treatment of cannabis use disorders also found atomoxetine to have limited utility and to be associated with clinically significant gastrointestinal adverse effects.
(h) Glutamatergic modulator (N‐acetylcysteine)
This dietary supplement may have some effectiveness in the treatment of cannabis dependence but available data (Gray 2012) were not conclusive.
Overall completeness and applicability of evidence
Studies undertaken to date on pharmacotherapies for cannabis dependence are insufficient to guide clinical practice. There is sufficient evidence to indicate that preparations containing THC and possibly the anticonvulsant gabapentin have therapeutic potential, while further investigation of the atypical antidepressant bupropion and the glutamatergic modulator N‐acetylcysteine may be worthwhile. However, the anticonvulsants and mood stabilisers divalproex and lithium, SSRI and mixed action antidepressants, the anxiolytic buspirone and the selective norepinephrine reuptake inhibitor atomoxetine appear to be of little value in the treatment of cannabis dependence. At this point in time, psychological approaches such as motivational enhancement therapy and cognitive‐behavioural therapy remain the mainstay of treatment for cannabis use disorders (Copeland 2014; Danovitch 2012).
The studies of preparations containing THC were of relatively short duration, Allsop 2014 administered nabiximols for six days while Levin 2011 administered dronabinol for eight weeks. A minimum of three months of treatment is generally considered necessary for the achievement of sustained behavioural change in people dependent on alcohol and other drugs. Indeed, the Cochrane review of nicotine replacement therapy for smoking cessation, which is a reasonable equivalent to preparations containing THC for cannabis dependence, only includes studies with at least six months follow‐up data (Stead 2012). With a longer duration of treatment, in conjunction with psychological therapies focused on relapse prevention, it is possible that an effect on cannabis use may be seen with THC preparations.
Several other medications, including atypical antipsychotics and baclofen, have been explored for potential effects on cannabis use but no studies using these medications met the criteria for inclusion in this review.
The atypical antipsychotics olanzapine and risperidone were compared for the management of psychosis in patients with a history of cannabis use (Akerele 2007; Robinson 2006; Van Nimwegen 2008). The two medications were found to have similar efficacy on psychotic symptoms with no evidence of a differential effect on cannabis craving or use. Another atypical antipsychotic, quetiapine, was compared with placebo in a laboratory study (Cooper 2013). Relative to placebo, quetiapine improved sleep quality but was associated with increased marijuana craving and self‐administration during the 'relapse' phase of the laboratory model.
The muscle relaxant and gamma‐aminobutyric acid (GABA) derivative baclofen has been suggested to have therapeutic potential on the basis of case reports (Imbert 2014; Subodh 2011). In a laboratory study (Haney 2010), baclofen dose‐dependently decreased craving for tobacco and cannabis during a phase of active cannabis smoking but had little effect on mood during abstinence and did not decrease 'relapse' in the laboratory model. Baclofen also worsened cognitive performance regardless of cannabis smoking phase. This suggests that the case reports may not be providing a full picture of the effects of baclofen in cannabis users.
Modafinil is a vigilance promoting drug that is being considered for the treatment of cocaine and methamphetamine dependence. Sugarman 2011 compared modafinil with placebo, alone and in combination with THC, in a laboratory study for a preliminary assessment of the safety of modafinil in combination with a range of doses of THC. While it was concluded that modafinil is safe in combination with THC, there were no data to indicate potential effectiveness in the treatment of cannabis dependence.
Quality of the evidence
The studies included in this review were small, the quality of evidence was assessed as generally low (see summary of findings Table for the main comparison, summary of findings Table 2, summary of findings Table 3) and the capacity for meta‐analysis was limited. As a result, the conclusions of this review should be considered tentative at best. Nonetheless, the review provides an overview of the current status of evidence and points to future directions for research on the development of pharmacotherapies for cannabis dependence.
Potential biases in the review process
Pharmacological approaches to the management of cannabis withdrawal are still in an experimental phase with a diverse array of medications being explored many of which have shown limited effectiveness. Studies with negative or neutral findings are less likely to be published and we identified two studies for which only limited information was available (Frewen 2007; Johnston 2012). It is possible that there are further such studies that we did not locate.
Agreements and disagreements with other studies or reviews
We have identified five recently published reviews of treatments for cannabis dependence (Benyamina 2008; Copeland 2014; Danovitch 2012; Nordstrom 2007; Vandrey 2009). All are in agreement that several pharmacotherapies, in particular preparations of THC, show promise for the treatment of cannabis dependence; but there is currently insufficient evidence to support their broad therapeutic use. These reviews also identify psychotherapies, such as motivational enhancement therapy and cognitive‐behavioural therapy, as having demonstrated efficacy in decreasing cannabis use and cannabis related consequences. Hence these reviews support the conclusion that psychological approaches should continue to be the mainstay of treatment for cannabis use disorders, with pharmacotherapies continuing to be experimental.

Study flow diagram.
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Comparison 1 Active medication versus placebo, Outcome 1 Number abstinent at end of treatment.
Comparison 1 Active medication versus placebo, Outcome 2 Number experiencing adverse effects.

Comparison 1 Active medication versus placebo, Outcome 3 Number withdrawn due to adverse effects.

Comparison 1 Active medication versus placebo, Outcome 4 Completion of treatment.
| Active medication compared with placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active Medication | |||||
| Number abstinent at end of treatment ‐ mixed action antidepressants | Study population | RR 0.82 | 179 | ⊕⊝⊝⊝ | ||
| 250 per 1000 | 205 per 1000 | |||||
| Moderate | ||||||
| 233 per 1000 | 191 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Significant heterogeneity between studies | ||||||
| Active medication compared with placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active Medication | |||||
| Number withdrawn due to adverse effects ‐ mixed action antidepressants | Study population | RR 1.44 | 179 | ⊕⊝⊝⊝ | ||
| 11 per 1000 | 16 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 19 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Studies differ in direction of effect without significant heterogeneity | ||||||
| Active medication compared with placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Active Medication | |||||
| Completion of treatment ‐ THC preparations | Study population | RR 1.29 | 207 | ⊕⊕⊕⊝ | ||
| 614 per 1000 | 792 per 1000 | |||||
| Moderate | ||||||
| 618 per 1000 | 797 per 1000 | |||||
| Completion of treatment ‐ mixed action antidepressants | Study population | RR 0.93 | 169 | ⊕⊕⊕⊝ | ||
| 573 per 1000 | 533 per 1000 | |||||
| Moderate | ||||||
| 551 per 1000 | 512 per 1000 | |||||
| Completion of treatment ‐ SSRI antidepressants | Study population | RR 0.82 | 122 | ⊕⊝⊝⊝ | ||
| 790 per 1000 | 648 per 1000 | |||||
| Moderate | ||||||
| 766 per 1000 | 628 per 1000 | |||||
| Completion of treatment ‐ anticonvulsant and mood stabiliser | Study population | RR 0.78 | 75 | ⊕⊝⊝⊝ | ||
| 405 per 1000 | 316 per 1000 | |||||
| Moderate | ||||||
| 387 per 1000 | 302 per 1000 | |||||
| Completion of treatment ‐ atypical antidepressant (bupropion) | Study population | RR 1.06 | 92 | ⊕⊝⊝⊝ | ||
| 429 per 1000 | 454 per 1000 | |||||
| Moderate | ||||||
| 400 per 1000 | 424 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Significant heterogeneity between studies | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number abstinent at end of treatment Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.56, 2.30] |
| 1.2 Mixed action antidepressants | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.12, 5.41] |
| 1.3 SSRI antidepressants | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.68, 8.05] |
| 1.4 Anticonvulsant and mood stabiliser | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.50, 2.34] |
| 1.5 Buspirone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Atomoxetine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 N‐acetylcysteine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number experiencing adverse effects Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.90, 1.46] |
| 2.2 Mixed action antidepressants | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.55, 1.55] |
| 2.3 SSRI antidepressants | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Anticonvulsant and mood stabiliser | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.99, 1.53] |
| 2.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.95, 1.46] |
| 2.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.34] |
| 3 Number withdrawn due to adverse effects Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.31] |
| 3.2 Mixed action antidepressants | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.11, 18.90] |
| 3.3 SSRI antidepressants | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Anticonvulsant and mood stabiliser | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.12] |
| 3.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 17.74] |
| 3.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.31] |
| 3.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.15] |
| 4 Completion of treatment Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 THC preparations | 2 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.08, 1.55] |
| 4.2 Mixed action antidepressants | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 4.3 SSRI antidepressants | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.44, 1.53] |
| 4.4 Anticonvulsant and mood stabiliser | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.42, 1.46] |
| 4.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.56, 1.77] |
| 4.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.60, 2.74] |
| 4.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.83, 1.51] |
| 4.8 Bupropion | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.67] |

