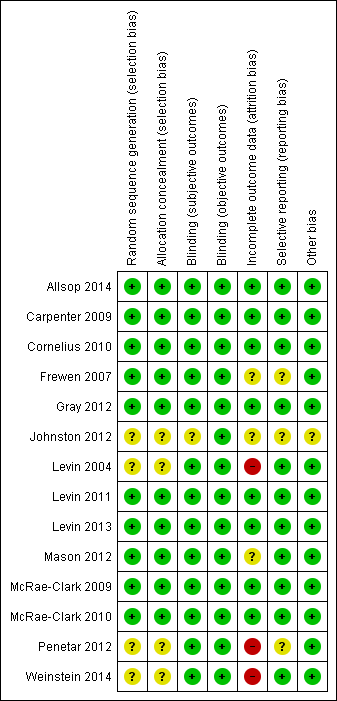
| 1 Number abstinent at end of treatment Show forest plot | 5 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 1.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.56, 2.30] |
| 1.2 Mixed action antidepressants | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.12, 5.41] |
| 1.3 SSRI antidepressants | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.68, 8.05] |
| 1.4 Anticonvulsant and mood stabiliser | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.50, 2.34] |
| 1.5 Buspirone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Atomoxetine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 N‐acetylcysteine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
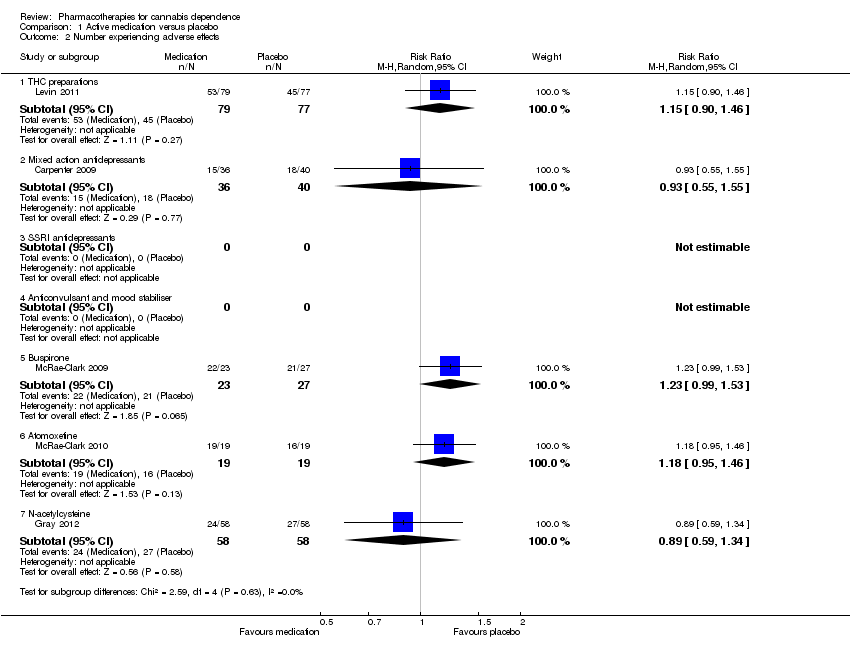
| 2 Number experiencing adverse effects Show forest plot | 5 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 2.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.90, 1.46] |
| 2.2 Mixed action antidepressants | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.55, 1.55] |
| 2.3 SSRI antidepressants | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Anticonvulsant and mood stabiliser | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.99, 1.53] |
| 2.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.95, 1.46] |
| 2.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.34] |
| 3 Number withdrawn due to adverse effects Show forest plot | 7 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 3.1 THC preparations | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.31] |
| 3.2 Mixed action antidepressants | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.11, 18.90] |
| 3.3 SSRI antidepressants | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Anticonvulsant and mood stabiliser | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.12] |
| 3.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 17.74] |
| 3.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.31] |
| 3.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.15] |
| 4 Completion of treatment Show forest plot | 12 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 4.1 THC preparations | 2 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.08, 1.55] |
| 4.2 Mixed action antidepressants | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 4.3 SSRI antidepressants | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.44, 1.53] |
| 4.4 Anticonvulsant and mood stabiliser | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.42, 1.46] |
| 4.5 Buspirone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.56, 1.77] |
| 4.6 Atomoxetine | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.60, 2.74] |
| 4.7 N‐acetylcysteine | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.83, 1.51] |
| 4.8 Bupropion | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.67] |