Intervenciones para el tratamiento de la espasticidad muscular esquelética después del traumatismo craneoencefálico
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008929.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 noviembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Lesiones
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MC, KP and AS carried out study selection, data extraction and assessment of risk of bias.
OC, JW, KP, MC and AS completed analyses.
AS and KP led the writing of the manuscript, with input from MC and the coauthors.
Sources of support
Internal sources
-
From 2010 to 2012, Kate Phillips was supported by a PhD scholarship provided by Monash University, Victoria, Australia.
External sources
-
Neurotrauma Evidence Translation Program, Australia.
The Neurotrauma Evidence Translation program is funded by the Transport Accident Commission, Victoria, Australia
-
National Institute for Health Research (NIHR), UK.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Kate Phillips is the director of a private multidisciplinary community rehabilitation service and is a practising neurological physiotherapist, treating people who have had a TBI. Kate is a clinical consultant on the Clinical Panel of the Transport Accident Commission in Victoria, Australia. The Transport Accident Commission is a Victorian Government owned organisation, its role is to pay for treatment and benefits for people injured in transport accidents.
All authors were supported to complete this work by a grant received from the Transport Accident Commission, Victoria.
AS, MC, VP, DO, RG, JW, OC and LP declare no conflicts of interest.
Acknowledgements
The authors would like to thank Cochrane Injuries Group Managing Editor Helen Wakeford and Co‐ordinating Editor Emma Sydenham for their helpful advice and assistance, along with Jane Dennis (Editor) for her help incorporating two studies and developing 'Summary of findings' tables, and Jo Weldon for serving as a consumer referee. We acknowledge Anne Parkhill for consultation and advice regarding the original search strategies, and would like to thank Deirdre Beecher and Sarah Dawson (Cochrane Injuries Information Specialists) for revising and rerunning subsequent searches. Finally, we thank current and former Cochrane Australia staff (Kelly Allen, Jo McKenzie, Miranda Cumpston and Simon Turner) for their valuable methodological and statistical input.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Nov 22 | Interventions for managing skeletal muscle spasticity following traumatic brain injury | Review | Anneliese Synnot, Marisa Chau, Veronica Pitt, Denise O'Connor, Russell L Gruen, Jason Wasiak, Ornella Clavisi, Loyal Pattuwage, Kate Phillips | |
| 2011 Jan 19 | Interventions for managing skeletal muscle spasticity following traumatic brain injury | Protocol | Kate Phillips, Veronica Pitt, Denise O'Connor, Russell L Gruen | |
Differences between protocol and review
The original protocol was published in 2010. Since that time the methodology of systematic reviews has evolved. To bring the protocol and subsequent review in line with the latest Cochrane methodology we made several necessary post‐hoc adjustments to the assumptions and processes. These were separately highlighted throughout the text.
During screening, we found a number of studies with clinically diverse populations. As people with TBI were poorly represented (making up of only 26% of the participants), we therefore devised a threshold of 50% (meaning that the study needed to have at least 50% of participants with TBI to be included in the review) to ensure the evidence would be applicable to the TBI population. We also made a post‐hoc decision to use the Tardieu or Modified Tardieu Scale as our preferred measure of spasticity, in preference to the Modified Ashworth Scale, in the instance that studies reported both these measures.
We added an information size calculation post‐hoc, in line with updated Cochrane Injuries Group editorial policy, and 'Summary of findings' tables, in line with updated Cochrane standards.
Due to the paucity of data, a number of planned methods outlined in the protocol could not be implemented. These included the planned meta‐analysis, investigation of statistical heterogeneity, subgroup analysis, sensitivity analysis and assessment of reporting biases. These methods will be retained for review updates pending sufficient studies.
The following authors joined the team since the publication of the protocol: AS, MC, OC, JW and LP.
Notes
Since the publication of the protocol in 2010, network meta‐analysis methods have been developed and adopted where necessary in Cochrane Reviews. We recognise that the most appropriate design for our review (which aims to assess the effectiveness of a number of interventions) would be that of a network meta‐analysis.
However, in light of a number of issues identified while carrying out this review (such as the paucity of the data, number of available interventions, presence of heterogeneity, lack of 'pure' datasets for the condition of interest, etc.), we are aware that even if we were to alter the design of this review, a network meta‐analysis would not be feasible. The current state of the available literature also indicates that in the short‐term future updates of this review will encounter similar issues. We have hence retained the original protocol design and have presented our findings as a narrative.
The network meta‐analysis design remains a viable future plan should adequate data become available.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Baclofen [therapeutic use];
- Botulinum Toxins, Type A [therapeutic use];
- Brain Injuries, Traumatic [*complications];
- Casts, Surgical;
- Electric Stimulation Therapy;
- Head‐Down Tilt;
- Muscle Relaxants, Central [therapeutic use];
- Muscle Spasticity [etiology, *therapy];
- Neuromuscular Agents [therapeutic use];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram for searches up until June 2017. TBA: traumatic brain injury.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Nine studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study (note: only Meythaler 1996 and Verplancke 2005 contributed outcome data to the review).

Comparison 1 Casting plus botulinum toxin A versus casting plus placebo, Outcome 1 Spasticity at 12 weeks.
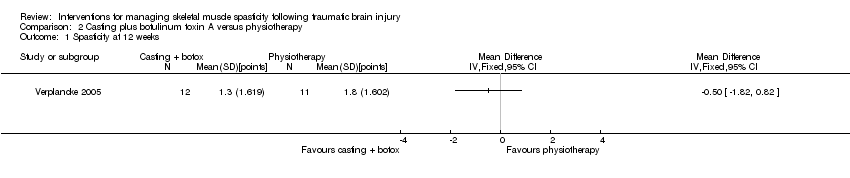
Comparison 2 Casting plus botulinum toxin A versus physiotherapy, Outcome 1 Spasticity at 12 weeks.

Comparison 3 Casting plus placebo versus physiotherapy, Outcome 1 Spasticity at 12 weeks.
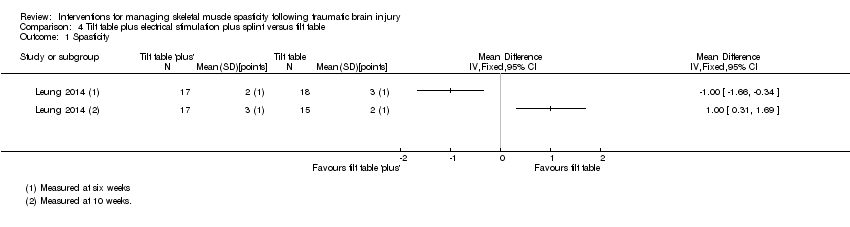
Comparison 4 Tilt table plus electrical stimulation plus splint versus tilt table, Outcome 1 Spasticity.
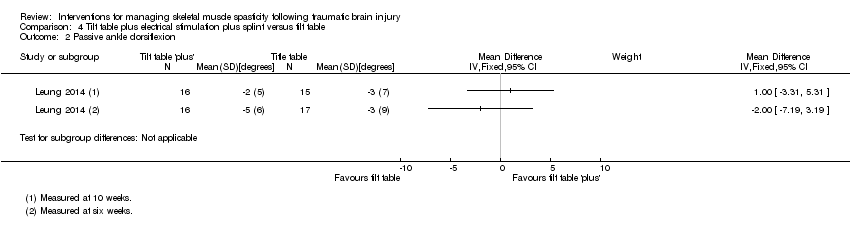
Comparison 4 Tilt table plus electrical stimulation plus splint versus tilt table, Outcome 2 Passive ankle dorsiflexion.

Comparison 4 Tilt table plus electrical stimulation plus splint versus tilt table, Outcome 3 Walking speed.
| Baclofen compared with placebo for spasticity in people with traumatic brain injury | |||
| Patient or population: adults with traumatic brain injury with spasticity in their arms and legs Settings: outpatient rehabilitation clinic (US) Intervention: intrathecal baclofen 50 μg (injected into the lumbar spine) Comparison: saline placebo | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at up to 6 hours after treatment (measured by the Ashworth Scale, 0‐, with a higher score indicating greater spasticity) | We are uncertain about the effect of baclofen on spasticity compared with placebo.1 | 11 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of baclofen on adverse events compared with placebo.4 | 11 | ⊕⊝⊝⊝ |
| Sensory functions and pain | No study measured this outcome. | ||
| Neuromusculoskeletal and movement‐related functions up to 6 hours after treatment (Measured by spasm and deep tendon reflex scores, 0‐5, with 0 being no reflexes and 5 being clonus, or repeated involuntary muscle contractions) | We are uncertain about the effect of baclofen on neuromusculoskeletal and movement‐related functions compared with placebo.6 | 11 (1)2 | ⊕⊝⊝⊝ |
| General tasks and demands | No study measured this outcome. | ||
| Mobility | No study measured this outcome. | ||
| Self‐care | No study measured this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1One study of baclofen reported an improvement in spasticity in the upper and lower limbs, compared to placebo, several hours after the injections but it was unclear how meaningful this improvement was due to reporting of P values only (Meythaler 1996). 2Three additional studies, with 35 participants, measured this outcome but had no useable results (Meythaler 1997; Meythaler 1999a; Meythaler 1999b). 3Downgraded four times due to risk of bias limitations (this study provided insufficient information about random sequence generation or allocation concealment), our concerns about indirectness of the Ashworth Score, an inability to assess imprecision relating to an absence of confidence intervals and a further downgrade for there only being one study for this outcome and the likelihood of publication bias in this area. 4No adverse events or changes in alertness level were observed in the baclofen or placebo group. 5Downgraded three times due to risk of bias limitations (no study provided sufficient information about random sequence generation or allocation concealment), the fact that there was only one study for this outcome and the likelihood of publication bias in this area. 6One study reported improvement in upper and lower limb spasm and reflexes compared to placebo several hours after treatment but it was unclear how meaningful this improvement was due to reporting of P values only (Meythaler 1996). 7Downgraded four times due to risk of bias limitations (no study provided sufficient information about random sequence generation or allocation concealment), an inability to assess imprecision relating to an absence of confidence intervals, the fact that there was only one study for this outcome and the likelihood of publication bias in this area. | |||
| Botulinum toxin A (with and without casting) compared with placebo (with and without casting) for spasticity in people with traumatic brain injury | |||
| Patient or population: adults with traumatic brain injury with spasticity in their arms (1 study) or calves (1 study) Settings: rehabilitation/neurology clinics or acute general hospital, in Europe or the UK Intervention: botulinum toxin A × 1 dose (500/1000 U) or botulinum toxin A × 1 dose of 200 U + serial casting Comparison: placebo (± casting) | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at 4‐12 weeks (measured by both Modified Ashworth Scale, 0‐5, at 12 weeks and Tardieu Scale, 0‐5, at 4 weeks) | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on spasticity.1 | 47 (2)2 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on adverse events.4 | 47 (2)2 | ⊕⊝⊝⊝ |
| Sensory functions and pain | No study measured this outcome. | ||
| Neuromusculoskeletal and movement‐related functions at 12 weeks (measured by ankle dorsiflexion) | We are uncertain about the effect of botulinum toxin A (± casting) vs placebo (± casting) on adverse events.6 | 47 (2)2 | ⊕⊝⊝⊝ |
| General tasks and demands | No study measured this outcome. | ||
| Mobility | No study measured this outcome. | ||
| Self‐care | No study measured this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1Gracies 2015 reported that "with abobotulinumtoxinA, the angle of catch (XV3 of the Tardieu Scale) improved in finger (+35 degree), elbow (+22 degree) and wrist (+12 degree) flexors" but no further outcome data were provided. For Verplancke 2005, we calculated the between‐group difference in spasticity (as measured by the Modified Ashworth Scale) as mean difference 0.30 (95% confidence interval ‐0.87 to 1.47). 2Included studies: Gracies 2015; Verplancke 2005. 3Downgraded four times due to: risk of bias concerns for both studies (downgraded twice, because either insufficient information about random sequence generation and allocation concealment, in one study, and potential selective outcome reporting in both studies), indirectness (one study included mixed traumatic brain injury and stroke populations, and measured spasticity using the Modified Ashworth Scale) and a high likelihood of publication bias in this area. 4In the main trial of Gracies 2015 (in which the traumatic brain injury population was a part (9.5%)) the most common botulinum toxin A‐related adverse event was 'mild muscle weakness' and investigators reported that all adverse events were mild or moderate only. In Verplancke 2005, botulinum toxin A was reported to be well tolerated, with only one participant with 'flu‐like' symptoms (i.e. shivering, sweating and fever). In groups who received casting (either alone, or in addition to botulinum toxin A), 41% to 50% developed 'minor' skin damage. Overall, 90.9% of those resolved spontaneously or with therapeutic dressing. 5Downgraded three times due to: risk of bias concerns for both studies (downgraded twice, because in one study there was insufficient information about random sequence generation and allocation concealment, and in both studies the adverse events data was reporting in percentages only) and a high likelihood of publication bias in this area. 6Verplancke 2005 reported between‐group differences in ankle dorsiflexion, finding no differences between groups in a one‐way ANOVA (casting + placebo versus casting + botulinum toxin A: P = 0.11). However, they did not report any summary statistics for this, or any baseline scores. 7Downgraded four times due to: risk of bias concerns for both studies (downgraded twice, because either insufficient information about random sequence generation and allocation concealment, in one study, and potential selective outcome reporting in both studies), indirectness (one study included mixed traumatic brain injury and stroke populations) and a high likelihood of publication bias in this area. | |||
| Pseudoelastic orthosis versus traditional (static) splint for spasticity in people with traumatic brain injury | |||
| Patient or population: children/young people aged 4‐18 years with traumatic brain injury and with 'mild to severe spastic tetraparesis' (weakness) in all limbs Settings: Istituro Eugenio Media (Italy) Intervention: repositioning splints equipped with participant‐specific pseudoelastic hinges Comparison: traditional splints with fixed angle braces | |||
| Outcomes | Results and conclusions | No of participants | Quality of the evidence |
| Spasticity at up to 6 hours after treatment (measured by the Modified Ashworth Scale, 0‐4, with a higher score indicating greater spasticity) | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on spasticity.1 | 25 | ⊕⊝⊝⊝ |
| Adverse events | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on adverse events.3 | 25 | ⊕⊝⊝⊝ |
| Sensory functions and pain | The included study did not report this outcome. | ||
| Neuromusculoskeletal and movement‐related functions post treatment (measured by range of movement) | We are uncertain about the effect of pseudoelastic splints compared with traditional splints on range of movement.5 | 25 (1) | ⊕⊝⊝⊝ |
| General tasks and demands | The included study did not report this outcome. | ||
| Mobility | The included study did not report this outcome. | ||
| Self‐care | The included study did not report this outcome. | ||
| GRADE Working Group grades of evidence | |||
| 1One study comparing novel pseudoelastic orthoses to traditional fixed angle splints reported no improvement in spasticity in the upper and lower limbs, over a period of one month of intervention. and that results of the two steps were not significantly different (Pittaccio 2013). 2Downgraded four times due to risk of bias limitations (study provided no information about sequence generation and allocation concealment; blinding was impossible for participants or personnel and not reported for outcome assessors; selective outcome reporting bias was high); our concerns about indirectness of the Ashworth Score and indirectness due to 36% of participants not having traumatic brain injury and one participant was of dubious eligibility; an inability to assess imprecision relating to an absence of meaningful outcome data (no numerical data were provided for spasticity; investigators reported only that there were no significant differences), and there was only one study for this comparison/outcome and that publication bias was possible in this area. 3No adverse events were reported for pseudoelastic orthoses neither did any require adjustments after fitting. Adjustments were required for 30% of traditional splints to reduce skin rash, haematomas and oedema. 4Downgraded four times due to risk of bias limitations (study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness given that 36% of participants did not have traumatic brain injury and one participant was of dubious eligibility. Furthermore, there was only one study for this comparison/outcome and publication bias was possible in this area. 5One study reported no improvement in range of movement in the upper and lower limbs, over a period of one month of intervention (Pittaccio 2013). 6Downgraded five times due to risk of bias limitations (this study provided insufficient information about sequence generation and allocation concealment, blinding was impossible for participants and personnel and not reported for outcome assessors, and selective reporting bias was high). We had concerns about indirectness due to 36% of participants not having traumatic brain injury and one participant was of dubious eligibility; our inability to assess imprecision given that means and standard deviations were only presented within a small box and whiskers plot, and a further downgrade for there only being one study for this comparison/outcome and the likelihood of publication bias in this area. | |||
| Author (year) | n total | n TBI | % TBI | Intervention | Comparator | %TBI Intervention | %TBI comparator |
| Botulinum toxin A vs placebo | |||||||
| 19 | 7 | 37 | Botulinum toxin A | Placebo | NR | NR | |
| 23 | 4 | 17 | Botulinum toxin A | Placebo | NR | NR | |
| 52 | 6 | 12 | Botulinum toxin A | Placebo | 12 | 12 | |
| 20 | 1 | 5 | Botulinum toxin A | Placebo | Cross‐over trial | ‐ | |
| 60 | 11 | 18 | Botulinum toxin A | Tizanidine or placebo | 15 | 14 and 26 placebo | |
| 21 | 2 | 10 | Botulinum toxin A | Placebo | 10 | 16 | |
| Botulinum toxin A vs therapy | |||||||
| 60 | 17 | 28 | Botulinum toxin A with rehab | Rehab only | 26 | 30 | |
| Botulinum toxin A vs botulinum toxin A (dosage) | |||||||
| 21 | 6 | 29 | High dilution botulinum toxin A with endplate target | Low dilution botulinum toxin A with end plate target | NR | NR | |
| Botulinum toxin A vs botulinum toxin A (volume) | |||||||
| 13 | 3 | 23 | High volume botulinum toxin A | Low volume botulinum toxin A | 16 | 28 | |
| 192 | 11 | 6 | High volume botulinum toxin A | Low volume botulinum toxin A | 5 | 6 | |
| Botulinum toxin A vs botulinum toxin A (location) | |||||||
| 17 | 2 | 12 | Botulinum toxin A injections towards mid belly | Botulinum toxin A injections away from mid belly | 0 | 25 | |
| 21 | 6 | 29 | High dilution botulinum toxin A with endplate target | Low dilution botulinum toxin A with end plate target | NR | NR | |
| Baclofen vs placebo | |||||||
| 19 | 2 | 11 | Intrathecal dose of baclofen | Saline | 16 | 0 | |
| 11 | 1 | 9 | Intrathecal baclofen | Placebo | 16 | 0 | |
| Cyclobenzaprine vs placebo | |||||||
| 15 | 4 | 27 | Cyclobenzaprine | Placebo | Cross‐over trial | ‐ | |
| Phenothiazine vs placebo | |||||||
| 9 | 2 | 22 | Phenothiazine | Placebo | Cross‐over trial | ‐ | |
| Tizanidine vs placebo | |||||||
| 17 | 8 | 47 | Tizanidine | Placebo | Cross‐over trial | ‐ | |
| Tizanidine vs botulinum toxin A | |||||||
| 60 | 11 | 18 | Botulinum toxin A | Tizanidine or placebo | 15 | 14 and 26 placebo | |
| Tizanidine vs diazepam | |||||||
| 105 | 16 | 15 | Tizanidine | Diazepam | 10 | 20 | |
| Casting vs control | |||||||
| 44 | 7 | 16 | Splint | No splint | 13 | 21 | |
| Casting vs therapy | |||||||
| 44 | 7 | 16 | Splint | No splint | 13 | 21 | |
| Splinting vs control | |||||||
| 10 | 2 | 20 | Individualised hand splint | No splint | 33 | 0 | |
| 28 | 2 | 7 | Stretching and hand splint | Stretching only | NR | NR | |
| 17 | 7 | 41 | Soft splints | No treatment | NR | NR | |
| Splinting vs therapy | |||||||
| 28 | 2 | 7 | Stretching and hand splint | Stretching only | NR | NR | |
| 17 | 7 | 41 | Soft splints | Stretching | NR | NR | |
| Functional electrical stimulation vs control | |||||||
| 14 | 3 | 21 | Upper limb botulinum toxin A injections for higher hand function | Upper limb botulinum toxin A injections for lower hand function | 33 | 0 | |
| Electrical stimulation + splinting vs splinting | |||||||
| 36 | 5 | 14 | Electrical stimulation to the wrist and finger extensor muscles for 1 hour a day + wrist splint for 12 hours a day, over 4 weeks | Wrist splint for 12 hours a day, over 4 weeks | 6 | 22 | |
| Repetitive peripheral magnetic stimulation vs sham | |||||||
| 66 | 3 | 5 | Repetitive peripheral magnetic stimulation | Sham stimulation | 10 | 0 | |
| Transcutaneous electrical acupoint stimulation vs another dose | |||||||
| 60 | 1 | 2 | Transcutaneous electrical acupoint stimulation (100 Hz) | Transcutaneous electrical acupoint stimulation (2 Hz) | 0 | 5 | |
| Transcutaneous electrical acupoint stimulation vs sham | |||||||
| 60 | 1 | 2 | Transcutaneous electrical acupoint stimulation (100 Hz) | Sham stimulation | 0 | 0 | |
| Ultrasound vs infrared | |||||||
| 21 | 1 | 5 | Infrared | Therapeutic ultrasound | NR | NR | |
| Robot vs bobath | |||||||
| 30 | 8 | 27 | Robot‐mediated therapy with bobath therapy | Bobath therapy | 13 | 40 | |
| n: number of participants; NR: not reported; TBI: traumatic brain injury. Some studies are listed in the table twice, given their multiple comparisons. | |||||||
| Outcome measure | Domains with score | Studies referring to this outcome | Time point analysis |
| Ashworth Scale (0‐4, lower score = better; Pandyan 1999) | 0: no increase in muscle tone. 1: slight increase in muscle tone, manifested by a catch and release or by minimum resistance through remainder of range of motion 2: more marked increase in muscle tone through most of the range of motion; limb easily moved. 3: considerable increase in muscle tone; passive movement is difficult. 4: rigid limb. | Baseline, and 1, 2, 4, 6 hours | |
| Baseline, and 4, 8 weeks | |||
| Modified Ashworth Scale (0‐5, lower score = better; Pandyan 1999) | 0: no increase in muscle tone. 1: slight increase in muscle tone, manifested by a catch and release or is moved in flexion, extension/abduction, adduction, etc. 1+: slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the range of motion. 2: More marked increase in muscle tone through most of the range of motion, but the affected part is easily moved. 3: considerable increase in muscle tone, passive movement is difficult. 4: affected part is rigid flexion or extension/abduction or adduction. | Baseline, 12 weeks | |
| Baseline, 4 weeks | |||
| Tardieu Scale (TS; 2 measurements: Quality of Muscle Reaction 0‐4, lower score better, and Angle of muscle reaction, R2 ‐ R1; Haugh 2006) | Quality of muscle reaction 0: no resistance throughout the course of the passive movement. 1: slight resistance throughout the course of the passive movement, with no clear catch at precise angle. 2: clear catch at precise angle, interrupting the passive movement, followed by release. 3: fatigable clonus (< 10 seconds when maintaining pressure) occurring at precise angle. 4: infatigable clonus (> 10 seconds when maintaining pressure) occurring at precise angle. Angle of muscle reaction (also referred to as R2 ‐ R1) Measured relative to the position of minimal stretch of the muscle (corresponding to angle) where it is relative to the resting anatomic position. R2: first measure (the maximum passive range of movement of the muscle group). R1: second measure (the angle at which the initial 'catch' or muscle resistance is felt when the muscle is moved from its shortest to longest position using a 'rapid velocity stretch'). | Baseline, 4 weeks | |
| Baseline, and 6 and 10 weeks |
| Outcome measure | Domains with score | Studies referring to this outcome | ICF classification |
| Glasgow Coma Scale (Teasdale 1974) (3‐15, higher score = better) | Eye opening (E) 4: spontaneous 3: to voice 2: to pain 1: none Verbal response (V) 5: normal conversation 4: disoriented conversation 3: words, but not coherent 2: no words, only sounds 1: none Motor response (M) 6: normal 5: localised to pain 4: withdraws to pain 3: decorticate posture (an abnormal posture that can include rigidity, clenched fists, legs held straight out, and arms bent inward towards the body with the wrists and fingers bend and held on the chest) 2: decerebrate (an abnormal posture that can include rigidity, arms and legs held straight out, toes pointed downward, head and neck arched backwards) 1: none The final GCS score or grade is the sum of these numbers. Severe: GCS 3‐8 (minimum possible score is 3) Moderate: GCS 9‐12 Mild: GCS 13‐15 | b. Body functions b1‐b8 | |
| Glasgow Outcome Scale (Jennett 1975) (1‐5, higher score = better) | To generalise and categorise the outcomes of people with TBI. 1: dead 2: vegetative state (meaning the person is unresponsive, but alive; a "vegetable" in lay language) 3: severely disabled (conscious but the person requires others for daily support due to disability) 4: moderately disabled (the person is independent but disabled) 5: good recovery (the person has resumed most normal activities but may have minor residual problems) | Body function b1‐b8 | |
| Range of movement | The joint is taken through the total arc of movement from flexion to extension | Verplancke 2005 (ankle) | Body function b7 |
| Deep tendon reflexes | 0: reflexes absent 1: hyporeflexia 2: normal 3: mild hyperreflexia 4: 3 or 4 beats clonus only 5: clonus | Body function b750 | |
| Disability Assessment Scale (DAS; Brashear 2002; 0‐3, lower score better) | People are interviewed to determine the extent of functional impairment in: hygiene, dressing, limb position and pain, according to the following scale: 0: no disability 1: mild disability (noticeable but does not interfere significantly with normal activities) 2: moderate disability (normal activities require increased effort or assistance, or both) 3: severe disability (normal activities limited) | Activities and participation | |
| ICF: International Classification of Functioning. | |||
| Adverse effect | Number of participants affected | Studies |
| Deep vein thrombosis | 1 participants withdrawn from physiotherapy group. | |
| Contracture at subtalar joint | 1 participants withdrawn from the casting + placebo group. | |
| No adverse events or changes in alertness level were observed in the baclofen group or placebo arm | Not applicable. | |
| Unspecified "treatment emergent AE [adverse effects]": "none were unexpected" | 7/23 participants, no other information given. | |
| Skin rashes/oedema/tolerability issues | No pseudoelastic device required adjustment for comfort; 30% of traditional devices did. Families reported novel treatment tolerated for 40% longer than traditional (Pittaccio 2013). 2 participants adherence to splinting was affected by 'skin problems' and 'poor tolerance' (Leung 2014). 50% of participants in casting group and 41.7% of participants in casting + botulinum toxin A group developed 'minor skin damage'. Overall, 90% of those resolved spontaneously or with therapeutic dressing (Verplancke 2005). | |
| Fainting, fatigue, storming1 | Several participants' adherence to the tilt table was affected due to fainting, fatigue and storming. | |
| 1When someone with a head injury responds to a sensation with a tonic posture or sympathetic response. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity at 12 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity at 12 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity at 12 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spasticity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Passive ankle dorsiflexion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Walking speed Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

