妊娠中の女性に対するビタミンD補充
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised single‐blinded controlled trial with 2 arms: vitamin D plus calcium and placebo. | |
| Participants | 54 pregnant women at risk for pre‐eclampsia, primigravida, aged 18‐35 years old carrying singleton pregnancy at their third trimester attending maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran (latitude: 33.9889° N, 51.4772° E). Exclusion criteria: maternal severe pre‐eclampsia, IUFD, placenta abortion, preterm delivery and GDM. | |
| Interventions | Participants were randomly allocated to 1 of 2 groups: group 1 (n = 27): women received 500 mg of carbonate calcium plus 200 IU of vitamin D (cholecalciferol‐D3) daily for 9 weeks; group 2 (n = 27): women received placebo. The intervention lasted 9 weeks overall, starting at 25 weeks of pregnancy until week 34. Participants were asked not to alter their routine physical activity or usual diets and not to consume any supplement other than the one provided to them by the investigators. Health worker cadre: the trial was carried out in maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran and the investigators provided the supplements to the participants. | |
| Outcomes | Maternal: body weight and height, BMI, fasting plasma glucose levels, serum total cholesterol, triglycerol concentrations, serum HDL‐cholesterol, serum LDL‐cholesterol levels, dietary intakes, total HDL: cholesterol ratio, gestational diabetes, severe pre‐eclampsia, preterm delivery. Laboratory method used for assessment of vitamin D concentrations: serum 25‐hydroxyvitamin D [25(OH)D] concentrations were measured using a commercial ELISA kit (Immuno Diagnostic Systems). The inter‐ and intra‐assay coefficient of variation for serum 25(OH)D assays ranged from 5% to 7.5%. | |
| Notes |
Source of funding: grant from the Vice‐Chancellor for research, KUMS, and Iran. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial reported randomisation performed by the use of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Trial reported that the appearance of the placebo capsules, such as colour, shape, size, and packaging, was identical to the vitamin D3 capsules. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and investigators were blind to the interventions. |
| Blinding of outcome assessment (detection bias) | Low risk | Trial is reported as blinded, although it is not specifically described if all were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up of 3 women in the vitamin D group due to preterm delivery (n = 1), IUFD (n = 1), and placental abruption (n = 1). 3 women in the placebo group were also excluded for the following reasons: GDM (n = 1), preterm delivery (n = 1), and severe pre‐eclampsia (n = 1). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial with 2 arms: vitamin D and placebo, during March 2012 to September 2012. | |
| Participants | 48 pregnant women, primigravida, aged 18–40 years old at 25 weeks of gestation and a singleton pregnancy attending maternity clinics affiliated with Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran. Women with pre‐eclampsia, hypertension, GDM, IUFD, or those with a history of rheumatoid arthritis, hepatic or renal failure, metabolic bone disease and malabsorption, or thyroid, parathyroid, or adrenal diseases were excluded from the analysis. Also, smokers and those taking medications including nonsteroidal antiinflammatory drugs and aspirin were excluded. | |
| Interventions | Participants were randomly assigned to receive 1 of 2 groups: group 1 (n = 24) received 400 IU vitamin D (cholecalciferol‐D3) supplements daily; and group 2 (n = 24) received placebo for 9 weeks. Additionally, all participants also consumed 400 μg (0.4 mg) folic acid daily from the beginning of pregnancy and 60 mg elemental iron (as ferrous sulphate) daily from the second trimester. Health worker cadre: the trial was carried out in maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran and the investigators provided the supplements to the participants. A trained midwife at the maternity clinic performed anthropometric measurements at study baseline and at 6 weeks after the intervention. | |
| Outcomes | Maternal: weight, height, BMI, systolic blood pressure and diastolic blood pressure, serum calcium concentrations, serum 25‐hydroxyvitamin D [25(OH)D], serum hs‐C‐reactive protein, fasting plasma glucose, serum cholesterol, LDL‐cholesterol, HDL‐cholesterol concentrations, serum insulin, quantitative Insulin sensitivity check index (QUICKI) score, plasma total antioxidant capacity, plasma total glutathione, GDM, preterm delivery, IUFD, placental abruption, severe pre‐eclampsia. Laboratory method used for assessment of vitamin D concentrations: serum 25‐hydroxyvitamin D [25(OH)D] concentrations were measured using a commercial ELISA kit (Immuno Diagnostic Systems). | |
| Notes |
Source of funding: the Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, Iran. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was performed by the use of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | A trained midwife at the maternity clinic performed the randomised allocation sequence and assigned participants to the groups. Placebo pills contained microcrystalline cellulose and were packed in identical tablets and coded by the producer to guarantee blinding. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and investigators were blind to the interventions. |
| Blinding of outcome assessment (detection bias) | Low risk | Measurements of laboratory were performed in a blinded fashion, in duplicate, in pairs (before/after intervention) at the same time, in the same analytical run, and in random order to reduce systematic error and inter assay variability. |
| Incomplete outcome data (attrition bias) | Low risk | 3 in each group were lost to follow‐up but the outcomes are accounted for as they are clinical outcomes of interest for this review. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised double‐blind controlled trial; 2‐arm design with individual randomisation. | |
| Participants | 126 Asian pregnant women 28‐32 weeks of gestation attending the antenatal clinic at St George's Hospital, London, United Kingdom (latitude: 51°30'N, north of tropic of Cancer). All pregnant women were first‐generation immigrants mostly from India, Pakistan, Bangladesh, Sri Lanka, Mauritius and east Africa. Exclusion and elimination criteria: preterm deliveries, congenital malformations and maternal illnesses likely to affect fetal growth (such as diabetes) although these data are not presented. | |
| Interventions | Participants were randomly allocated to 1 of 2 groups: group 1 (n = 59 at the end of the trial): women received daily 1000 IU vitamin D (ergocalciferol‐D2) daily (estimated total dose: 56000‐84000 IU); group 2 (n = 67 at the end of the trial) received a placebo until term. Start of supplementation: weeks 28‐32 gestation. Length of the intervention/follow‐up: 8‐12 weeks from supplementation to term. Health worker cadre: St George's Hospital Medical School, London, United Kingdom. Medical doctors that were part of the team conducted the measurements and provided the supplements. | |
| Outcomes | Maternal: maternal weight gain, dietary vitamin D intake, 25‐hydroxyvitamin D (25‐OHD) concentrations in cord blood and at term. Plasma calcium (adjusted for albumin concentration), inorganic phosphate, bilirubin, albumin concentrations and total alkaline phosphatase activity, alanine transaminase and ʏ‐glutamyl transferase activities, vitamin D binding globulin concentration, compliance. Infant: weight, crown‐heel length, crown‐rump length, rump‐heel length, occipitofrontal head circumference, forearm length, lower leg length, triceps and subscapular skinfold thickness, fontanelle area, plasma cholecalciferol at day 3 and day 6. weight, length and head circumference at 3, 6, 9 and 12 months. Laboratory method used for assessment of vitamin D concentrations: Serum 25‐OHD concentration was measured by competitive protein binding assay after chromatographic purification of lipid extracts of serum. | |
| Notes |
Source of funding: The pathological research fund, St George's Hospital Medical School, and the South‐west Thames Regional Health Authority. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial reported random allocation to the groups, although the method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants received either vitamin D or placebo. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | High risk | Unclear number of randomised participants. Preterm deliveries, congenital malformations, and maternal illnesses likely to affect fetal growth (such as diabetes) were eliminated from the trial. There is not complete documentation of the exclusions. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. There were no significant baseline differences between the groups in maternal age, parity, height, vegetarian: non‐vegetarian ratio or the distribution of the various countries of origin. |
| Methods | Randomised trial; 2‐arm design with individual randomisation. | |
| Participants | 40 pregnant women attending their compulsory visit during the third month of pregnancy at the Obstetrical Unit of the Hopital Edouard Herriot, Lyon, France (latitude: 45° 45' 0" N north of tropic of Cancer). Inclusion criterion: singleton pregnancy at term and uneventful vaginal deliveries. Pre‐gestational BMI and skin pigmentation not reported. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups at the time of the compulsory visit: group 1 (n = 20): women received daily 1000 IU vitamin D (cholecalciferol‐D3) (estimated total dose: 55,000 IU); group 2 (n = 20): women received no supplement during the last trimester of pregnancy for 12 weeks from start of supplementation to term. Health worker cadre: compliance was verified by a weekly visit by a midwife. | |
| Outcomes | Maternal: serum (during last trimester of pregnancy) and cord blood immunoreactive parathyroid hormone (iPTH), 25‐hydroxyvitamin D (25‐OHD), 1‐alfa,25‐dihydroxyvitamin D (1,25(OH)2D), total calcium, ionised calcium, magnesium, inorganic phosphate. Infant: immunoreactive parathyroid hormone (iPTH), 25‐hydroxyvitamin D (25‐OHD), 1‐alfa,25‐dihydroxyvitamin D (1,25(OH)2D), total calcium, ionised calcium, magnesium, inorganic phosphate at 4 days of age. Laboratory method used for assessment of vitamin D concentrations: Serum 25‐OHD and 1,25(OH) D levels were measured by radioligand assays with slight modifications. With sample volumes of 0.75 to 1.5 mL, the inter assay variation coefficient for the 2 assays were 8% and 10%, respectively. | |
| Notes |
Source of funding: Shriners of North America, the France‐Quebec Exchange Program, and INSERM Grant 121023. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial reported as randomised but the method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | 1 group received supplements while the other received no treatment. |
| Blinding of outcome assessment (detection bias) | High risk | Compliance was verified by the midwife. As 1 group received supplements and the other received no intervention it is clear that the midwife knew which women were in each group. |
| Incomplete outcome data (attrition bias) | High risk | 1 participant from the control group (5%) and 5 (25%) from the vitamin D supplemented group. Laboratory methods reported for 25 to 30 participants (depending on the outcome) out of 40 originally randomised. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, placebo‐controlled trial; 2‐arm design with individual randomisation. | |
| Participants | 84 pregnant adolescents (13‐19 years of age) primigravidae (pregnant for the first time) with singleton pregnancies and 23‐29 weeks of gestation attending prenatal care at the Maternidade Escola, Universidade Federal do Rio de Janeiro, Brazil (latitude: 22.9083° S, 43.1964° W) from September 2009 to June 2011 and intending to exclusively or predominantly breast feed. Women with chronic health problems, pregnancy complications, smokers, users of nutritional supplements besides iron plus folate supplements provided during standard prenatal care, and mothers who decided not to breast feed were excluded from the study. | |
| Interventions | Participants were randomly assigned to: 1 of 2 groups: group 1 (n = 43) received a commercially available supplement (Rexall Sundown®) containing 600 mg calcium (as calcium carbonate) plus 200 IU vitamin D (cholecalciferol‐D3) daily; group 2 (n = 41) received placebo (capsules of microcrystalline cellulose and corn starch; Quintessencia) daily. Health worker cadre: capsules of calcium plus vitamin D or placebo were provided monthly to participants by a member of the research team during prenatal visits. Compliance was controlled by counting the remaining capsules at each visit and by telephone reminders. Calcium and vitamin D dietary intake was assessed by at least 3 24‐hour dietary recall questionnaires applied by a trained nutritionist. Standing height and body weight were measured by using a stadiometer (Seca) and a calibrated electronic scale (Filizola), respectively. The same operator performed all scanning and calibration. | |
| Outcomes | Maternal: 1 measurement at 5 and 20 weeks postpartum, serum 25(OH)D, parathyroid hormone, insulin‐like growth factor (IGF‐I), lumbar spine PA, bone mineral content, serum prolactin and estradiol. Laboratory method used for assessment of vitamin D concentrations: Serum 25(OH)D, intact parathyroid hormone (PTH), and IGF‐I were analysed by using a chemiluminescent enzyme‐labelled immunometric assay. | |
| Notes |
Source of funding: Conselho Nacional de Desenvolvimento Cientıfico e Tecnologico [grant 471872/2008‐3 (to CMD) and a doctoral fellowship (to MELD)] and the Fundacao Carlos Chagas Filho de Amparo a` Pesquisa do Estado do Rio de Janeiro (grant E‐26/102.759/2008; to CMD), Brazil. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was done by a member of the research team in a 1:1 ratio within permuted blocks of size 10. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were randomly and single‐blinded assigned to 1 of the 2 groups. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Out of 43 patients in the intervention group: decided not to breast feed (n = 1), lost to follow‐up (n = 2), pregnancy complications (n = 1), time constrains (n = 3), no reason given (n = 4), moved out of area (n = 2). Analysed at 5 weeks postpartum (n = 30). Out of 41 patients in the placebo group: decided not to breast feed (n = 1), other health issues (n = 1), lost to follow‐up (n = 2), pregnancy complications (n = 2), time constrains (n = 3), no reason given (n = 5), moved out of area (n = 1). Analysed at 5 week postpartum (n = 26). 9 mothers were lost for the 20‐week measurement, which reduced the effective sample size for the bone change over the postpartum time assessment. Nevertheless, the magnitude of significant differences between groups in bone measures at the lumbar spine at 20 weeks postpartum, after adjustment for confounding factors, probably reduced the potential bias because of uncontrolled factors, such as the unknown bone status before pregnancy. |
| Selective reporting (reporting bias) | Low risk | The trial was approved by the Ethical Committee of Maternidade Escola, Universidade Federal do Rio de Janeiro (www.clinicaltrials.gov; NCT01732328). |
| Other bias | Unclear risk | The study appears to be free of other sources of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled multi‐arm parallel study. | |
| Participants | 260 pregnant women 26‐30 weeks' gestation, with a singleton pregnancy attending community based primary care maternity clinic in Auckland, New Zealand (latitude 36°S) from April 2010 to July 2011 and then their infants, from birth to age 6 months. Women already taking vitamin D supplementation 200 IU per day, a history of renal stones or hypercalcaemia, or any serious pregnancy complication at enrolment were excluded from the study. | |
| Interventions | Participants were randomly assigned to 1 of 3 mother/infant groups: group 1 (n = 87) women received placebo from 26‐30 weeks of pregnancy until parturition and their infants also received placebo from 0‐6 months of age; group 2 (n = 87) women received 1000 IU vitamin D (cholecalciferol‐D3) from 26‐30 weeks of pregnancy until parturition and their infants received 400 IU vitamin D from 0‐6 moths of age; group 3 (n = 86) women received 2000 IU vitamin D (cholecalciferol‐D3) from 26‐30 weeks of pregnancy until parturition and their infants received 800 IU from birth to 6 months of age. Health worker cadre: the study was conducted by the research team but it is not reported who provided the supplements or measured the outcomes. | |
| Outcomes | Maternal: serum 25(OH)D concentration. Infant: serum 25(OH)D concentration. Laboratory method used for assessment of vitamin D concentrations: serum 25(OH)D concentration was measured using isotope‐dilution liquid chromatography–tandem mass spectrometry in a Vitamin D External Quality Assurance Scheme–certified laboratory. | |
| Notes |
Source of funding: Health Research Council of New Zealand, grant number 09/215R. Dr Mitchell is supported by Cure Kids. Study medicine was prepared by the Ddrops Company (Woodbridge, Ontario, Canada). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial reported computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed from research staff involved in recruitment. Trial reported randomly allocated treatment to each participant and labelled identical study medicine bottles such that study staff and participants were unaware of the treatment status. |
| Blinding of participants and personnel (performance bias) | Low risk | The study statistician randomly allocated a treatment to each participant and labelled identical study medicine bottles such that study staff and participants were unaware of the treatment status. |
| Blinding of outcome assessment (detection bias) | Low risk | The study staff and participants were unaware of the treatment status. |
| Incomplete outcome data (attrition bias) | Low risk | Reported compliance did not differ between groups. Placebo group: discontinued intervention during pregnancy (n = 3), withdrew at 30 & 32 weeks of gestation (n = 2), late fetal death at 37 weeks of gestation (n = 1). Lower dose vitamin D group: discontinued intervention during pregnancy (n = 3), withdrew @ 32 weeks of gestation (n = 2), moved from region @ 36 weeks of gestation (n = 1). Higher dose vitamin D group: discontinued intervention during pregnancy (n = 3), withdrew @ 29 & 38 weeks of gestation (n = 2), moved from region @ 36 weeks of gestation (n = 1). |
| Selective reporting (reporting bias) | Low risk | Registration was with the Australian NZ Clinical Trials Registry (ACTRN12610000483055). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Clinical controlled trial with 3 arms. | |
| Participants | 88 pregnant women with a predisposition to pregnancy‐induced hypertension, at 20‐24 weeks' gestation, a BMI index of lower than 24, and an arterial pressure of < 11.3 kPa attending an outpatient clinic and labour ward of the First Afifliated Hospital of Xi’an Medical University, Xi’an, China. | |
| Interventions | Participants were divided into 3 groups: group 1 (n = 29) received a daily dose of a tablet containing 600 mg of calcium and 200 IU of vitamin D (Caltrate‐D) daily from 20‐24 weeks until deliver; group 2 (n = 29) received 1200 mg of calcium and 400 IU vitamin D (Caltrate‐D) daily from 20‐24 weeks until deliver; group 3 (n = 30) received no intervention from 20‐24 weeks until delivery. Health worker cadre: not reported. | |
| Outcomes | Blood pressure, ionised calcium and platelet intracellular calcium, incidence rates of pregnancy‐induced hypertension. | |
| Notes |
Source of funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The assignment of the groups method is not reported. |
| Allocation concealment (selection bias) | Unclear risk | It is unclear if there was allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | 2 groups received an intervention while women from group 3 received no intervention. There is no report on blinding. |
| Blinding of outcome assessment (detection bias) | High risk | 2 groups received an intervention while women from group 3 received no intervention. There is no report on blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to make a judgement. |
| Other bias | High risk | The details of the methods are not available. The report is rather short. |
| Methods | Randomised controlled trial; 3‐arm design with individual randomisation. | |
| Participants | 77 white pregnant women 18‐36 years of age in the last trimester of pregnancy living in Northwest of France (latitude: 49° 26' 0" N north of tropic of Cancer). Pre‐gestational BMI not reported. | |
| Interventions | Participants were randomly assigned to 1 of 3 groups: group 1 (n = 21) women received daily 1000 IU of vitamin D (ergocalciferol‐D2) for the last 3 months of pregnancy (estimated total dose throughout pregnancy: 90,000 IU); group 2 (n = 27) women received a single dose of 200,000 IU (5 mg) vitamin D at the 7th month of pregnancy; group 3 (n = 29) women received no supplement and served as controls. Length of the intervention/follow‐up: 12 weeks from start of supplementation to term. Health worker cadre: the study was conducted by the research team at the maternity of Balvedere, Rouen, Frances but the roles are not described. It is unclear who provided the supplements and measured the outcomes. | |
| Outcomes | Maternal: 24‐hour urinary calcium excretion after 6 weeks supplementation, calcium, 25‐hydroxyvitamin D (25‐OHD) and1‐alfa,25‐dihydroxyvitamin D (1,25(OH)2D) metabolites of vitamin D from serum and cord during labour and delivery. Infant: serum calcium levels at days 2 and 6 of life, birthweight. Laboratory method used for assessment of vitamin D concentrations: for 25 OHD and 1,25 (OH)2 D determinations the following techniques were used: extraction with chloroform‐methanol‐water according to Preece, double step purification, first on a Sephadex LH 20 column with chloroform hexan 45‐55 vol/vol as solvent, then on a high‐pressure liquid pression system according to Shepard. Plasma metabolites were measured by competitive assay using rat protein for 25 OHD and chicken intestine cytosol for 1,25 (OH)2 D according to Jongen. Assay sensitivity for 1,25 (OH)2 D was 5 pmol/tube and for 25 OHD was 25 pmol/tube. | |
| Notes |
Source of funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | Different interventions were used: daily dose or single dose or no supplement. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | High risk | It is unclear if there was attrition, but given the uneven number of participants reported it is likely that there were losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | Groups are reported with notorious different sample size. It is unclear whether the numbers reflect the participants who finished the trial (unclear and uneven losses to follow‐up); a non randomised process; or a selection bias in which randomised participants did not receive the intervention. |
| Methods | Randomised controlled trial; 2‐arm design with randomisation at individual level. | |
| Participants | 400 pregnant women 20‐35 years of age, attending the antenatal clinic of Medical College Hospital in Rohtak, India (latitude: 76° 34' 0' north of Tropic of Cancer). Pre‐gestational BMI and skin pigmentation not reported. | |
| Interventions | Participants were allocated to 1 of 2 groups: group 1 (n = 200) received a daily supplement containing 1200 IU vitamin D and 375 mg calcium (estimated total dose from week 20‐24 of gestation to term:134,400‐168,000 IU); group 2 (n = 200) received no supplement from 20‐24 weeks of pregnancy until delivery. Length of the intervention/follow‐up: 20‐24 weeks from start of supplementation to term. Health worker cadre: not specified. | |
| Outcomes | Maternal: pre‐eclampsia (defined as blood pressure of 140 mmHg or higher systolic and/or 90 mmHg diastolic along with proteinuria higher than 300 mg/24 hours); systolic and diastolic blood pressure at 24, 28, 32 and 36 weeks of gestation. Serum calcium and creatinine. Laboratory method used for assessment of vitamin D concentrations: not assessed. | |
| Notes | Biochemical analyses were made for those who developed pre‐eclampsia (n = 12) and also in a group of women with no pre‐eclampsia (n = 25) and a control group of non pregnant women. The results of the stratified analysis are not reported in this review.
Source of funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 400 pregnant women, of these 200 were randomly selected and put on a daily supplement of calcium and vitamin D. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | There is insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | High risk | Only data on biochemical were reported for those who developed pre‐eclampsia and some of those with no pre‐eclampsia and a group of non pregnant controls. |
| Selective reporting (reporting bias) | High risk | Outcomes reported for some subgroups only. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised clinical trial; 2‐arm design with individual randomisation. | |
| Participants | 200 pregnant women, aged 22‐35 years old, attending the antenatal clinic of the Medical College Hospital, Rohtak, India (latitude: 76° 34' 0' north of Tropic of Cancer). Inclusion criterion: uncomplicated single pregnancy. Exclusion criteria: pre‐eclampsia, antepartum haemorrhage, premature delivery. Pre‐gestational BMI and skin pigmentation not reported. | |
| Interventions | Participants were allocated to 1 of the following groups: group 1 (n = 100) women received 2 doses of 600,000 IU (each dose at 7th and 8th month of pregnancy (estimated total dose: 1,200,000 IU); group 2 (n = 100): women received no intervention. Length of the intervention/follow‐up: 12 weeks from start of supplementation to term. Health worker cadre: not specified. | |
| Outcomes | Maternal: venous and cord serum calcium, serum proteins, inorganic phosphate, alkaline phosphatase, weight. Radiological examination on women with abnormal biochemistry or osteomalacia symptomatology. Side effects: back age, leg‐pains, general weakness, cramps. Infant: birthweight, low birthweight, crown‐heel length, head circumference, mid‐arm circumference within 24 hours after birth. Skinfold thickness (triceps and infrascapular). Laboratory method used for assessment of vitamin D concentrations: not assessed. | |
| Notes |
Source of funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '200 pregnant women, of these 100 were randomly selected (supplemented group) had been administered two doses of vitamin D.' Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | There is insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Unclear risk | Losses to follow‐up are not documented although exclusions included pregnancy complications. Result tables mention that each arm was comprised of 100 women, a number that corresponds to that described for the treatment allocation. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised control trial. | |
| Participants | 72 pregnant women with physiological pregnancy aged 18‐35 with low alimentary consumption of calcium (< 600 mg/day) who attended to Moscow State University of medicine and dentistry, department of obstetrics and gynaecology. (Latitude: 55.7500° N, 37.6167° E). | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 43): 1250 mg of calcium carbonate and 200 IU of vitamin D (cholecalciferol‐D3) from the second pregnancy trimester until term, in 2 takes a day; group 2 (n = 29): did not undergo the intended preventive measures. Health worker cadre: not reported. | |
| Outcomes | Maternal: resistance of uterine arteries, resistance of umbilical arteries, uterine‐placental circulation. Infant: fetal‐placental circulation, intrauterine growth retardation, assessed by dopplerometry. Laboratory method used for assessment of vitamin D concentrations: not assessed. | |
| Notes |
Source of funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "72 pregnant women with physiological pregnancy aged 18‐35 with low alimentary consumption of calcium (<600 mg/day) were randomised into two groups." Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | It is not reported whether the trial was blinded to participants, outcome assessor or care providers. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Unclear risk | There is insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Randomised placebo‐controlled trial. | |
| Participants | 160 pregnant women aged 18 < 35 years old, attending to the International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh (latitude: 23.7000° N, 90.3750° E, north of the Tropic of Cancer). Inclusion criteria: patients with residence in Dhaka, with plans to have the delivery performed at the Shimantik maternity centre, and to stay in Dhaka throughout the pregnancy and 1 month past the delivery, with gestational age of 26th to 29th (inclusive), estimated based on the first day of the last menstrual period. Exclusion criteria: Use of any dietary supplement containing more than 400 IU/day (10 mcg/day) of vitamin D within the month prior to enrolment, or refusal to stop taking supplemental vitamin D at any dose after enrolment, current use of anti‐convulsant or anti‐mycobacterial (tuberculosis) medications, severe anaemia (haemoglobin concentration < 70 g/L), complicated medical or obstetric history: cardiovascular disease, uterine haemorrhage, placenta praevia, threatened abortion, hypertension, pre‐eclampsia, preterm labour, or multiple gestation), prior history of delivery of an infant with a major congenital anomaly, birth asphyxia, or perinatal death (stillbirth or death within first week of life). | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 80): women received vitamin D (cholecalciferol‐D3) 35,000 IU per week, started at 26‐29 weeks' gestation, until delivery; group 2 (n = 80): women received placebo control administered weekly from 26‐29 weeks' gestation until delivery. Health worker cadre: supplement doses were measured in disposable plastic syringes and orally administered by study personnel. | |
| Outcomes | Maternal: serum 25‐hydroxyvitamin D concentration, serum calcium concentration, urine Ca:Cr ratio. Infant: immune function, infant growth, postnatal vitamin D status, serum calcium. Laboratory method used for assessment of vitamin D concentrations: Serum 25(OH)D was quantified by high‐performance liquid chromatography tandem mass spectroscopy (LCMS/MS) in the Department of Pathology and Laboratory Medicine at the Hospital for Sick Children. | |
| Notes |
Source of funding: The Thrasher Research Fund, Salt Lake City, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial reported computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was prepared by International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh personnel not otherwise involved in the study, and was concealed from investigators. |
| Blinding of participants and personnel (performance bias) | Low risk | Trial reported that participants and research staff (including lab personnel) were blinded to allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Trial reported that participants and research staff (including lab personnel) were blinded to allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Of the 160 participants recruited and randomly assigned to either vitamin D (35,000 IU/week) or placebo, 13 were lost to follow‐up prior to delivery (6 in the placebo group and 7 in the vitamin D group), all because of having left the Dhaka area. |
| Selective reporting (reporting bias) | Low risk | This trial was registered at ClinicalTrials.gov (NCT01126528) and all outcomes were reported as per registration. |
| Other bias | Unclear risk | The study appears to be free of other sources of bias. |
| Methods | Randomised controlled trial with 2 arms, with randomisation at the individual level from years 2010 to 2012. | |
| Participants | 180 primigravidae women with singleton pregnancy at 14–20 weeks in the Department of Obstetrics and Gynaecology in Safdarjung Hospital, New Delhi, India (28°38′08″ N, 77°13′28″ E north of Tropic of Cancer). | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 60) women did not receive any supplementation of vitamin D; group 2 (n = 120) women received vitamin D (cholecalciferol‐D3) supplementation in dosages depending upon the level of serum 25(OH)‐D levels estimated at entry into the study. Participants from this second group with sufficient levels of vitamin D (serum 25(OH)‐D levels > 50 nmol/L), received only 1 dose of 60,000 IU vitamin D (cholecalciferol‐D3) at 20 weeks; participants with insufficient levels of vitamin D (serum 25(OH)‐D levels 25–50 nmol/L) received 2 doses of 120,000 IU vitamin D (cholecalciferol‐D3) at 20 weeks and 24 weeks; and participants with deficient levels of vitamin D status (serum 25(OH)‐D levels < 25 nmol/L) received 4 doses of 120,000 IU vitamin D cholecalciferol‐D3) at 20, 24, 28 and 32 weeks. Health worker cadre: unclear what the roles of the researchers and other workers in the health worker cadre. | |
| Outcomes | Maternal: preterm labour, pre‐eclampsia, GDM, serum 25(OH)‐D concentration, serum calcium, phosphorus and serum ALP levels. Laboratory method used for assessment of vitamin D concentrations: Serum 25(OH)D was quantified by sandwich ELISA. | |
| Notes |
Source of funding: self‐funded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer‐generated random number tables. |
| Allocation concealment (selection bias) | High risk | As participants were assigned to either no intervention or intervention and the intervention dosage depended on the vitamin D status, there was a selection bias based on status of vitamin D at baseline. |
| Blinding of participants and personnel (performance bias) | High risk | 1 group received no intervention at all and the other different doses of vitamin D at different times. |
| Blinding of outcome assessment (detection bias) | High risk | 1 group received no intervention at all and the other different doses of vitamin D at different times. |
| Incomplete outcome data (attrition bias) | High risk | The level of attrition was different in groups 1 and 2: 3/60 (5%) participants in group 1 and 12/120 (10%) participants in group 2 were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other evident sources of bias. |
| Methods | Randomised controlled study with 3 arms. | |
| Participants | 990 nulliparous women attending antenatal outpatient clinics of Isfahan Health Centers (32.6333° N, 51.6500° E north of Tropic of Cancer) between April 1998 and March 2001, with singleton pregnancies, first prenatal visit before 20 weeks of gestation, systolic/diastolic blood pressure lower than 130/80 mmHg, and no proteinuria detectable by a dipstick. Women with history of cardiovascular, renal or endocrinologic problems, medical or obstetric complications and those with known hazardous condition (multifetal gestation, hydatidi‐form mole) were excluded. | |
| Interventions | Participants were randomly assigned to 1 of 3 groups: group 1 (n = 330) received 75 mg aspirin each day from 20th week of gestation until delivery; group 2 (n = 330) received a tablet containing 500 mg calcium carbonate + 200 IU vitamin D (cholecalciferol‐D3) daily from 20th week of gestation until delivery; and group 3 (n = 330) received no intervention. All cases received standard prenatal care. Health worker cadre: the women were examined by trained staff every 4 weeks through the 28 weeks of gestation, and every 2 weeks through the 36th week and weekly thereafter. Blood pressure was measured by a certified examiner. | |
| Outcomes | Maternal: blood pressure, bodyweight, BMI, maternal height, urine protein measurements, maternal weight gain, duration of gestation. Infant: neonatal weight at birth, the presence of respiratory distress syndrome, sepsis, jaundice and intrauterine growth retardation, fetal or neonatal death. | |
| Notes |
Source of funding: Research Deputy of Isfahan University of Medical Sciences grant (No: 76085). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of any allocation. It is unclear whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. |
| Blinding of participants and personnel (performance bias) | High risk | There is no mention of the study being blinded to participants of health care providers. |
| Blinding of outcome assessment (detection bias) | High risk | There is no mention of the study being blinded to participants of health care providers. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition reported. |
| Selective reporting (reporting bias) | Low risk | It appears unlikely. This study was conducted to evaluate the effect of low‐dose aspirin or calcium supplements, taken during pregnancy, on the incidence of pre‐eclampsia in nulliparous healthy women. |
| Other bias | Low risk | The study appears to be free of other evident sources of bias. |
| Methods | Randomised controlled trial; 4 x 3 block design with randomisation at individual level. | |
| Participants | 180 pregnant women (45 Indian Asians, 45 Middle Eastern, 45 Black and 45 Caucasian) women at 27 weeks' gestation attending the routine antenatal clinic at St Mary’s Hospital, London, United Kingdom (latitude: 51°30'N north of tropic of Cancer). Exclusion criteria: pre‐existing sarcoidosis, osteomalacia, renal dysfunction and tuberculosis. Pre‐gestational BMI and skin pigmentation (in addition to ethnicity) not reported. The study took place between April 2007 and November 2007. | |
| Interventions | Participants were randomised in blocks of 15 within each of the 4 ethnic groups to 3 groups; group 1 (n = 60) women received a daily dose of vitamin D (ergocalciferol D2) at 800 IU (estimated total dose 72,800 IU); group 2 (n = 60): women received a stat dose of 200,000 IU of calciferol; group 3 (n = 60): women received no treatment. Length of the intervention/follow‐up: 13 weeks from start of supplementation to term. Health worker cadre: each woman collected her tablets directly from the hospital pharmacy department or her local pharmacy. | |
| Outcomes | Maternal: maternal and cord 25‐hydroxyvitamin D levels at delivery, maternal PTH and corrected calcium levels at delivery, adverse events. Infant: small‐for‐gestational age was defined as birthweight less than the 10th percentile after adjustments for gestation at delivery, infant sex, maternal ethnicity, parity, height and weight. Laboratory method used for assessment of vitamin D concentrations: not specified. | |
| Notes | Women who did not speak English were only included if a health advocate was able to interpret and a leaflet was provided in their language.
Source of funding: Institute of Obstetrics and Gynaecology Trust, Wolfson and Weston Research Centre for Family Health, Imperial College, Du Cane Road, Hammersmith Hospital, London W12 0NN, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number lists were drawn up by an independent researcher, with randomisation in blocks of 15. |
| Allocation concealment (selection bias) | Low risk | The person seeing the pregnant women allocated the next available number on entry to the trial, and each woman collected her tablets directly from the hospital pharmacy department or her local pharmacy. |
| Blinding of participants and personnel (performance bias) | High risk | All study personnel and participants were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 loss to follow‐up on group 3. |
| Selective reporting (reporting bias) | Low risk | The study was approved by St Mary’s Hospital Ethics Committee (Ref: 06/Q0702/172) and the Medicines and Healthcare products Regulatory Agency. |
| Other bias | Unclear risk | Women were randomised within each ethnic group. It is not clear if the ethnicity can be clearly established as it was self reported. Women who did not speak English were included only if a health advocate was able to interpret and a leaflet was provided in their language (English, Arabic, Bengali and Farsi) although the ability to read was not clearly established. |
BMI: body mass index
GDM: gestational diabetes mellitus
HDL: high‐density lipoprotein
IGF‐I: insulin‐like growth factor
IU: international units
IUFD: intrauterine fetal death
LDL: low‐density lipoprotein
PA: physical activity
PTH: parathyroid hormone
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| 49 healthy, well‐nourished mothers delivering in January 1984 in the maternity wards and outpatient clinic of the Department of Paediatrics of the University Central Hospital of Tampere, Finland (latitude 61°N) and exclusively breastfeeding their infants, were divided in succession into 3 groups: group 1 (n = 17): mothers were given 2000 IU vitamin D3 a day, infants not supplemented; group 2 (n = 16): mothers were given 1000 IU vitamin D3 a day, infants not supplemented; group 3 (n = 16): mothers were not supplemented, and their breast fed infants were given 400 IU of vitamin D2 a day. During pregnancy, 33 mothers had no vitamin D supplementation, 8 mothers received 500 IU a day of vitamin D during the second trimester of pregnancy, and 8 mothers received 500 IU a day throughout the pregnancy. The mothers from these 3 groups supplemented in pregnancy were distributed in the postpartum maternal vitamin D supplementation and infant vitamin D supplementation interventions. This is not a randomised trial and the intervention includes mothers at postpartum and their infants. | |
| 54 pregnant women aged 18–40 years diagnosed with GDM by a 100‐g oral glucose‐tolerance test at 24–28 weeks' gestation attending maternity clinics affiliated with Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran (latitude: 33.9889° N, 51.4772° E), during March 2012 to September 2012 and further analysis during January 2013 to April 2013. Participants were randomly assigned to 1 of 2 groups: group 1 (n = 27), women received capsules containing 50,000 IU vitamin D (cholecalciferol‐D3) (D‐Vitin 50000; Zahravi Pharm Co) 2 times during the study (at baseline and at day 21 of the intervention): group 2 (n = 27), women received 2 placebos (Barij Essence Co) at the same times. The duration of the study was 6 weeks; however, vitamin D was given only 2 times during the 6 weeks. Additionally, all participants also consumed 400 μg (0.4 mg) folic acid daily from the beginning of pregnancy and 60 mg elemental iron (as ferrous sulphate) daily from the second trimester. The trial was carried out in maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran and the investigators provided the supplements to the participants. A trained midwife at the maternity clinic performed anthropometric measurements at study baseline and at 6 weeks after the intervention. All pregnant women in the study had a diagnosis of gestational diabetes. The type of participant is outside the scope of this review. | |
| 56 pregnant women 18‐40 years of age with gestational diabetes and 24‐28 weeks' gestation attending prenatal care at maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Iran were randomly assigned to 1 of 2 groups: group 1 (n = 28) received 1000 mg calcium per day and a 50,000 U vitamin D (cholecalciferol‐D3) pearl twice during the study (at study baseline and on day 21 of the intervention); group 2 (n = 28) received 2 placebos at the same times. Participants with premature preterm rupture of the membrane, placenta abruption, pre‐eclampsia, eclampsia, chronic hypertension, hypothyroidism, urinary tract infection, kidney or liver diseases, stressful life conditions, smokers or using oestrogen therapy or women requiring insulin therapy during the intervention (FPG > 5.8 mmol/L and 2 hours postprandial blood sugar > 6.7mmol/L).were excluded. All participants were also consuming 400 μg (0.4 mg) folic acid daily from the beginning of pregnancy and 60 mg elemental iron (as ferrous sulphate) from the second trimester. The calcium supplement and its placebo were manufactured by Tehran Shimi Pharmaceutical Company (Tehran, Iran). Vitamin D and its placebo were manufactured by Dana Pharmaceutical Company (Tabriz, Iran) and Barij Essence Pharmaceutical Company (Kashan, Iran). The trial was carried out in maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran and the investigators provided the supplements to the participants. A trained midwife at the maternity clinic performed anthropometric measurements at study baseline and at 6 weeks after the intervention. Outcomes measured included serum 25‐hydroxyvitamin D [25(OH)D] concentrations, FPG, serum calcium, cholesterol, triacylglycerol, LDL‐cholesterol and HDL‐cholesterol, serum insulin, serum high‐sensitivity C‐reactive protein, plasma total antioxidant capacity, plasma total glutathione, plasma malondialdehyde. Participants were pregnant women with diagnosis of gestational diabetes. The type of participant is outside the scope of this review. | |
| 299 pregnant women with 12 and 24 weeks of gestation of lower‐middle and middle socio‐economic groups attending the antenatal clinic in Queen Mary Hospital, Chhatrapati Sahuji Maharaj, India, were randomly assigned to 1 of 2 groups, group 1: received 1500 mcg cholecalciferol at induction into the study, or group 2 3000 mcg cholecalciferol at induction as well as at 28 weeks of gestation. All were prescribed 1 g of elemental calcium daily as calcium carbonate without vitamin D. Patients excluded from the study if they were already on calcium or vitamin D supplementation, anticonvulsants, antitubercular treatment or had any medical condition that affected calcium and vitamin D metabolism (including renal and hepatic disease). Only 97 women were followed up. Both groups received vitamin D and calcium. This type of intervention is outside the scope of our review. | |
| 1139 pregnant women were assigned to 1 of 2 wards: group 1 (n = 506) Caucasian pregnant women assigned to 1 ward of the Simpson Memorial Maternity Pavilion, Edinburgh, United Kingdom during the 9 months from September to May, were given a daily dietary supplement of 400 IU of vitamin D2 from about the 12th week of pregnancy until delivery; group 2 women (n = 633) were assigned to another ward over the same period and were given a placebo containing no vitamin D. Outcomes included plasma concentrations of calcium, phosphorus, magnesium, total proteins, and 25‐hydroxycholecalciferol at 24th and 34th weeks of pregnancy and at delivery. Infant plasma concentrations of calcium, phosphorus, magnesium, total proteins, and 25‐hydroxycholecalciferol were measured from umbilical venous blood taken from the infants at birth and on capillary blood on the 6th day. This is not a randomised trial. | |
| 174 healthy postpartum women who had delivered babies at term in Poland, were randomised to 1 of 2 groups: group 1 (n = 70) received 1200 IU/d vitamin D (cholecalciferol‐D3 as 800 IU/d alone + 400 IU/d from a multiple micronutrient supplements; group 2 (n = 67) received 400 IU/d vitamin D (cholecalciferol‐D3 as placebo + 400 IU/d from multiple micronutrient supplements) during 6 months of lactation. Outcomes measured included serum 25‐hydroxyvitamin D (S‐25‐OHD), PTH and densitometry after delivery, at 3 and 6 months postpartum. Serum and urinary calcium were assessed at 3 and 6 months postpartum. Participants from both groups received vitamin D supplements. The participants were postpartum women. The type of participant and the type of interventions are outside the scope of this review. | |
| 150 consecutive pregnant women pregnant women during their second trimester from 6 villages of a poor socio‐economic region in district Barabanki (latitude 26.8 ºN), Uttar Pradesh, north India. The participants were initially randomised to receive either no dose or 1 dose of 60,000 IU cholecalciferol under observation in the 5th gestational month. However, the first few results showed rampant vitamin D deficiency and no improvement at delivery despite good exposure to sun and calcium supplementation. Therefore, this randomisation was abandoned subsequently and 2 comparison groups were followed up, alternate women receiving either 60,000 IU in the 5th month or 120,000 IU, each in the 5th and 7th months of pregnancy. This is not a randomised trial and the comparisons are outside the scope of this review. | |
| 192 Arab women between 12–16 weeks of gestation after their last menstrual period or by ultrasound assessment who had a singleton pregnancy; and planned to receive prenatal and delivery care in primary health care clinics affiliated with Tawam Hospital, Al Ain, United Arab Emirates. Exclusion criteria were pre‐existing calcium and parathyroid conditions, active thyroid disease, liver or kidney disease, or type 1 diabetes, which are likely to affect vitamin D and calcium status. All participants received vitamin D supplementation in different regimens. The type of intervention is outside the scope of this review. | |
| 45 pregnant women with confirmed multiple sclerosis who attended an outpatient clinic in Isfahan University of Medical Sciences, Iran aged 20‐40 years with low serum 25‐hydroxyvitamin D (25(OH)D) levels were randomly allocated to 2 groups in an open‐label randomised, controlled clinical Phase I/II pilot study. 1 group received 50,000 IU/week vitamin D3 (n = 21) or routine care (n = 22) from 12 to 16 weeks of gestation till delivery. Inclusion criteria were women with a magnetic resonance imaging, clinical or laboratory‐supported diagnosis of definite multiple sclerosis, stable neurological functioning for at least 1‐month prior to study entry, and an expanded disability status scale (EDSS) score ≤ 6, serum 25(OH)D level < 20 ng/mL and a willingness to continue current medications for the duration of the study. The main outcome measures were mean change in serum 25(OH)D levels, EDSS score, and number of relapse events during pregnancy and within 6 months after delivery. Participants had a confirmed diagnosis of multiple sclerosis. This type of participant is outside the scope of this review. | |
| 160 pregnant women (24–26 weeks of gestation) who attended an obstetric clinic in Qazvin, Iran, from December 2011 to March 2012 were randomised, and included in 2 arms. Inclusion criteria were: gestational age of 24–26 weeks, singleton pregnancy and BMI of 19–26 kg/m2. Exclusion criteria at study enrolment were: diabetes before pregnancy, chronic hypertension, history of repeated abortion, rheumatoid arthritis, parathyroid disorders, hepatic or renal diseases, and use of aspirin, anticonvulsive and immunosuppressive drugs. Women in the control group received a multivitamin containing 400 IU vitamin D3 plus 200 mg elemental calcium each day until delivery. Women in the intervention group received a weekly dose of 50,000 IU oral vitamin D3 for 8 weeks (from 26 to 28 weeks of pregnancy) as well as the drug regimen (multivitamin and elemental calcium) given to the control group. Both groups received vitamin D and calcium. This type of intervention is outside the scope of our review. | |
| 200 pregnant women who attended the Department of Obstetrics & Gynaecology unit 3, Dow University and Civil Hospital Karachi, Pakistan aged between 18 and 40 years were randomised, and included in 2 arms. Participants were allocated to 1 of 2 groups: group 1 (n = 100) received along with ferrous sulphate, 4000 IU of vitamin D3; group 2 (n = 100) received routine antenatal care (ferrous sulphate and calcium). Both groups received above medications from 20 weeks of pregnancy until delivery. Maternal serum levels of 25(OH)D levels were done at the time of recruitment, and at the time of delivery. Neonatal levels were done within 48 hours of delivery. This type of intervention is outside the scope of our review. | |
| 48 pregnant women with GDM (diagnosed by performing oral glucose tolerance test at 24‐28th week of gestation) in Yazd, Iran were randomly assigned to 1 of 2 groups group 1 (n = 24) were assigned to received an intramuscular dose 300,000 IU of vitamin D and group 2 (n = 24) were assigned to receive no intervention. The participants were asked to refer to Yazd Diabetes Research Center 3‐10 days after delivery for outcome assessments that included plasma glycosylated haemoglobin A1C (HbA1C), serum 25(OH) vitamin D3, PTH, serum calcium and phosphorus. Vitamin D was provided by intramuscular injection and not orally. The type of intervention is outside the scope of this review. | |
| 876 singleton pregnant women with blood pressure lower than 140/90 mmHg at 20 weeks’ gestation, and no evidence of proteinuria, who were attending the obstetric clinic of Kumamoto University Hospital, Japan were divided into 2 groups: group 1 (n = 666) women received conventional antenatal care; group 2 (n = 210 women) were managed under a protocol for the prediction of pre‐eclampsia with an angiotensin sensitivity test and prevention of the condition by calcium supplementation. Participants from group 2 were further assigned to 1 of 4 groups according to their risk of developing pre‐eclampsia, based on the angiotensin sensitivity test and the effective pressor dose: group A received 156 mg/day of oral elemental calcium (as calcium L‐aspartate, Aspara‐Ca from 22 weeks’ gestation, followed by 312 mg/day oral elemental calcium and vitamin D3 (0.5 μg for 3 days) from 30 weeks’ gestation to term. Participants in group B received 156 mg/day oral elemental calcium from 22 weeks’ gestation and 312 mg/day oral elemental calcium from 30 weeks’ gestation to term; group C received 312 mg/day oral elemental calcium from 30 weeks’ gestation to term and group D received no supplementation. This is not a randomised trial and the type of intervention is outside the scope of this review. | |
| 881 pregnant women with either a personal history of asthma or allergies or a similar history in the spouse or partner, between 18 and 40 years of age and at an estimated gestational age between 10 and 18 weeks, were recruited at a scheduled obstetrical prenatal visit at 3 clinical centres: Boston Medical Center (Boston, MA), Washington University at Saint Louis (St. Louis, MO), and Kaiser Permanente Southern California Region (San Diego, CA). Participants were randomised to either vitamin D (cholecalciferol, 4000 IU/ day; equivalent to 100 μg/day) or placebo. All pregnant mother participants received prenatal vitamins containing 400 IU (10 μg/day) of cholecalciferol; thus, the vitamin D arm received a total of 4400 IU/day (110 μg/day) and the placebo arm received 400 IU/day (10 μg/day). Both groups received vitamin D supplements. This type of intervention is outside the scope of our review. | |
| This trial was registered in 1986 on the Oxford Database of Perinatal Trials and reports the recruitment and follow‐up completed in 1979. The registration form reports a randomised controlled trial to assess the efficacy of calcium and vitamin D supplementation versus placebo in the prevention of maternal and fetal hypocalcaemia. The reports indicates that the sample size was 55 Asian women with morbidity and laboratory results as primary outcomes but no further information is available. | |
| 45 Hindu pregnant women were randomly assigned to 1 of 2 groups: group 1 (n = 25) received tablets containing 1200 IU vitamin D and 375 mg calcium daily throughout the 3rd trimester; group 2 (n = 20) received oral single dose of 600,000 IU vitamin D2 once during 7th month and 8th month (total 2 doses). This group was compared with group 3 (n = 75) who had not received vitamin D supplements during pregnancy. The results were also compared with data from 25 non pregnant, non‐lactating healthy women. Patients with complications such as pre‐eclampsia, antepartum haemorrhage or twin pregnancies were excluded. The randomised study compares 2 doses of vitamin D supplementation. The type of study, type of participants and types of interventions are outside the scope of this review. | |
| 91 pregnant women aged 16‐42 years were admitted to Kocaeli Maternity and Children Hospital between April 2011 and April 2012. The participants were randomly divided into 3 groups: 600 IU/d (control group; n = 31); 1,200 IU/d (n = 31), and 2,000 IU/d (n = 32) of vitamin D. All groups received vitamin D supplements. This type of comparison is outside the scope of our review. | |
| 28 pregnant women were enrolled at a maternal health clinic in inner‐city Dhaka, Bangladesh Aged 18 to 34 years; at 27 to 30 weeks of pregnancy with no pre‐existing medical conditions; current vitamin D supplement use; anti‐convulsant or anti‐mycobacterial medications; severe anaemia (haemoglobin concentration less than 70 g/L); hypertension at enrolment (systolic blood pressure 140 mmHg or higher or diastolic blood pressure 90 mmHg or higher on at least 2 measurements); major risk factors for preterm delivery or pregnancy complications; or previous delivery of an infant with a congenital anomaly or perinatal death. Participants were randomly assigned to 1 of 2 groups: group 1 (N = 14) were assigned to receive a single dose of vitamin D3 70,000 IU (1.75 mg, where 1 mg = 40,000 IU) on day 0 followed by vitamin D3 35,000 IU (0.875 mg) per week starting on day 7 and continuing until delivery); Group 2 (N = 14), were assigned to receive vitamin D3 14,000 IU (0.350 mg) per week starting on day 0 and continuing until delivery. A cohort of a non pregnant participants (N = 16) received the a single dose of vitamin D3 70,000 IU on day 0 followed by vitamin D3 35,000 IU per week starting on day 7 and continuing until the last dose on day 63 (total of 10 doses). This group was used as a comparison group. All participants received vitamin D supplementation in different regimens. The type of intervention is outside the scope of this review. | |
| 51 healthy pregnant women from the beginning of their second trimester of pregnancy during the autumn and winter of 2009 in recruited from 2 primary care clinics in Yazd (31°53’50”N/54°22’04”E), Iran. Participants were distributed in 3 groups according to their serum 25(OH)D at the beginning of the second trimester of pregnancy. Participants with low concentrations (25(OH)D levels < 20 ng/mL) (n = 17) were treated with 200,000 IU (50,000 IU/week for 4 weeks) of vitamin D (as (cholecalciferol‐D3), followed by supplementation with 50,000 IU/month vitamin D (cholecalciferol‐D3). The other 34 participants were randomly assigned to 1 of 2 groups: group 1 received 50,000 IU/month vitamin D (cholecalciferol‐D3); group 2 received 100,000 IU/month vitamin D (50,000 IU every 2 weeks) of vitamin D (cholecalciferol‐D3) supplementation. All participants received vitamin D supplements. Only the participants with higher vitamin D status were randomised to different doses and regimens. The type of study design and the type of intervention are outside the scope of this review. | |
| 120 pregnant women were recruited from 2 prenatal clinics (Mojibian Hospital and Shahid Sadoughi Hospital) in Yazd, Iran, from 2009 to 2011. Exclusion criteria consisted of women with diabetes or gestational diabetes treated with insulin, women with thyroid or parathyroid disorders, polycystic ovary disease before pregnancy, BMI before pregnancy of more than 30 kg/m2 and women who received vitamin D supplementation during the prior 6 months. Participants were randomly assigned to 1 of 3 groups: group 1 received the DRI of 200 IU vitamin D (calciferol) daily, group 2 received 50,000 IU monthly (2000 IU daily) and group 3 received 50,000 IU every 2 weeks (4000 IU daily). Supplementation started in the 12th week of pregnancy and continued until delivery. All groups received vitamin D supplements. This type of comparison is outside the scope of our review. | |
| Pregnant women less than 20 weeks' gestation and over 18 years of age with no use of medications known to affect vitamin D metabolism, diagnosis of type 1 diabetes, history of thyroid, renal, or liver disease, problems with digestion or absorption participated in the study at USDA Western Human Nutrition Research Center and clinicians at UC Davis Medical Center. They were distributed into 2 groups, receiving: either 400 IU or 2000 IU of vitamin D per day for the duration of their pregnancy. Both groups received vitamin D supplements. This type of intervention is outside the scope of our review. | |
| 229 women 18‐35 years old, who were confirmed to be vitamin D deficient (vitamin D < 75 nmol/L), were randomised into the intervention, and control groups and after 15 weeks consumption of the supplement (2000 IU/day oral vitamin D) and placebo. The study was conducted among reproductive women in a high‐risk population for vitamin D deficiency. The participants of the study were not pregnant women. The type of participant is outside the scope of this review. | |
| 235 South Asian women, aged 23‐68 years, living in Auckland, New Zealand were recruited for the study and those who were insulin resistant ‐ homeostasis model assessment 1 (HOMA1) > 1.93 and had serum 25‐hydroxyvitamin D concentration < 50 nmol/L were randomly assigned to 1 of 2 groups: group 1 (n = 42) received 100 μg (4000 IU) vitamin D(3); group 2 (n = 39) received a placebo daily for 6 months. The study participants were non‐pregnant women. The type of participant is outside the scope of this review. | |
| 257 pregnant women 12‐16 weeks’ gestation were enrolled at Eau Claire Cooperative Health Center (ECCHC) in Columbia, SC, and Northwoods Community Health Center (NCHC) in North Charleston, SC, USA and were randomly assigned to receive either 2000 IU/d vitamin D versus 4000 IU/d vitamin D (cholecalciferol‐D3), followed 1‐month run‐in at 2000 IU vitamin D daily. Participants were monitored for hypercalciuria, hypercalcaemia, and 25(OH)D status. Both groups received vitamin D at different doses. The type of intervention is outside the scope of this review. | |
| 494 apparently healthy pregnant women (16‐45 years of age) with 12‐16 weeks' gestation of singletons attending prenatal care in Medical University of South Carolina, Charleston, South Carolina in South Carolina, United States were randomised into 1 of 3 groups stratified by race: group 1 received 400 IU vitamin D (cholecalciferol‐D3)/day; group 2 received 2000 IU vitamin D (cholecalciferol‐D3)/day; and group 3 received 4000 IU vitamin D (cholecalciferol‐D3)/day until delivery. All women received daily multiple micronutrients supplements. 350 women continued until delivery. Outcomes included monthly 25‐hydroxyvitamin D; 1,25(OH)2D; intact PTH, serum calcium, creatinine, phosphorus, and urinary calcium/creatinine levels, gestational age at delivery, birthweight, mode of delivery, co‐morbidities of pregnancy, pre‐eclampsia, gestational diabetes, any infection, preterm labour and premature birth. All women received vitamin D supplementation at different doses. The type of intervention is outside the scope of this review. | |
| This is an analysis of data from 2 randomised controlled trials by the same research group (Wagner 2010b; Wagner 2010a). In Wagner 2010b, women were randomised to 400, 2000, or 4000 IU vitamin D (cholecalciferol‐D3)/day, stratified by race. In Wagner 2010a, participants were randomised to 2000 or 4000 IU vitamin D (cholecalciferol‐D3)/day. | |
| 179 pregnant women 18 years of age or older, with singleton pregnancy, with plasma 25‐hydroxivitamin D (25OHD) concentrations lower than 32 ng/mL, less than 20 weeks of gestation were randomly assigned to 1 of 2 groups: group 1 (n = 89) received 5000 IU/d of vitamin D (cholecalciferol‐D3) until delivery; group 2 (n = 90) received 400 IU/d of vitamin D (cholecalciferol‐D3) until delivery. Outcomes included glycaemia and glucose tolerance, gestational diabetes at 26‐28 weeks of gestation; neonatal 25OHD, maternal hypertension, mode of delivery, prematurity, birthweight, crown‐heel length, occipitofrontal head circumference. All participants received vitamin D supplements at different doses. The type of intervention is outside the scope of this review. |
BMI: body mass index
DRI: dietary references intakes
FPG: fasting plasma glucose
GDM: gestational diabetes mellitus
IU: international units
mcg: microgram
PTH: parathyroid hormone
25OHD: 25‐hydroxycholecalciferol
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomised controlled trial on the effects of antenatal vitamin D supplementation to improve vitamin D levels in the maternal and cord blood at birth in vitamin D deficient pregnant women. |
| Methods | Randomised controlled trial. |
| Participants | Pregnant women between 14‐18 weeks' gestation at risk, defined as: dark skinned, veiled; with vitamin D deficiency that has not commenced treatment prior to recruitment. Exclusion criteria: women taking barbiturates or anticonvulsants (decreased vitamin D absorption) and severe renal failure. |
| Interventions | Participants will be individually randomised to 1 of 2 groups: group 1: 2000 international units (IU) of cholecalciferol orally daily commencing between 14 and 18 weeks' gestation. If still deficient at 28 weeks the dose will be doubled to 4000 IU orally daily until birth; group 2: No treatment during pregnancy. The mother will receive 300,000 IU cholecalciferol orally immediately and the baby 150,000 IU cholecalciferol orally immediately after birth. |
| Outcomes | Maternal: vitamin D level. Infant: vitamin D level. |
| Starting date | 1/04/2008. |
| Contact information | Name: Jodie Benson |
| Notes | Sponsor: Royal Australian and New Zealand College of Obstetrics and Gynaecology, Australia. ACTRN12609000142235. |
| Trial name or title | Vitamin D supplementation in pregnancy: regimens and long term effects on offspring. |
| Methods | Randomised, parallel group, placebo‐controlled trial. |
| Participants | Pregnant women attending antenatal clinic in Chhatrapati Shahuji Medical University (CSMMU), Uttar Pradesh, India, and in 14 to 20 weeks of pregnancy. Exclusion criteria: chronic liver disease, renal disease or treatment with antitubercular or antiepileptic drugs or vitamin D in the previous 3 months. |
| Interventions | Participants will be individually randomised to 1 of 3 groups: group 1: cholecalciferol: 400 units per day orally from recruitment till the end of pregnancy; group 2: cholecalciferol: 60,000 units orally every 4 weeks; group 3: cholecalciferol: 60,000 units orally every 8 weeks from recruitment till the end of pregnancy. |
| Outcomes | Maternal: serum 25OHD. Infant: birthweight, length, head circumference and anterior fontanelle diameter, cord serum 25OHD, neonatal serum calcium. |
| Starting date | 04‐11‐2011. |
| Contact information | Vijayalakshmi Bhatia |
| Notes | Sponsor: Department of Biotechnology, Goverment of India. CTRI/2012/02/002395. |
| Trial name or title | Study of vitamin D supplementation on improvement of gums health (vitamin D). |
| Methods | Randomised, parallel assignment, double blind. |
| Participants | Pregnant females from 12‐20 weeks of gestation who agree to participate in the study with presence of at least 20 natural teeth in mouth excluding third molars. For controls: non pregnant, healthy females matched with pregnant women with respect to age and education. Exclusion criteria: pregnant females with high vitamin D levels, women with metabolic diseases such as diabetes (type 1 or 2), presence of acute dental or periodontal disease, presence of systemic disease and/or medication affecting the periodontium; receipt of systemic antibiotic treatment or dental prophylaxis in the previous 3 months and those who do not provide informed consent. |
| Interventions | Participants will be individually randomised to 1 of 2 groups: group 1: vitamin D3 4000 mg per day, 1 tablespoon syrup per day; group 2: placebo, 1 table spoon syrup per day. |
| Outcomes | Maternal: Periodontal Probing Depth, Interleukin 6 (IL‐6), IL‐2, IL‐4, IL‐10, TNF, IFN‐ɣ and IL‐17 levels. |
| Starting date | June 2010. |
| Contact information | Farhan Raza Khan, Consultant, Dentistry, Aga Khan University. |
| Notes | Sponsor: Aga Khan University, Pakistan. (www.clinicaltrials.gov; NCT01422122 |
| Trial name or title | Vitamin D supplementation during pregnancy for prevention of asthma in childhood: an interventional trial in the ABC (Asthma Begins in Childhood) cohort. |
| Methods | Randomised double‐blind, placebo‐controlled trial with 2 arms. |
| Participants | Danish‐fluent pregnant women 18 years of age or older, with 22‐26 week of gestation living in Sealand, Denmark participating in the ABC‐cohort. The mothers in ABC also participate in an interventional trial with fish oil supplementation, and the vitamin D randomisation is stratified by fish oil treatment group. Women with intake of more than 400 IU of vitamin D during the previous 6 months, endocrinological disease such as calcium metabolic disorder, parathyroid disorder, thyroid disorder or diabetes type 1, tuberculosis, sarcoidosis or in need of diuretics or heart medication including calcium channel blockers are excluded. |
| Interventions | Participants will be individually randomised to 1 of 2 groups: group 1: receives a daily supplement with 2400 IU of vitamin D3 from week 24 of gestation to 1 week after delivery; group 2: receives placebo from week 24 of gestation to 1 week after delivery. |
| Outcomes | Maternal: 25‐OH‐vitamin D, PTH, calcium, alkaline phosphatase concentrations 1 week postpartum. Infant: upper and lower respiratory infections, allergy, eczema from 0‐3 years of age. |
| Starting date | Date of start: 03/2009. Status: recruiting participants. |
| Contact information | Hans Bisgaard, MD, DMSc Copenhagen Studies on Asthma in Childhood Copenhagen University Hospital of Copenhagen Gentofte, Denmark, 2820 Tel: +45 39777360 E‐mail: [email protected] |
| Notes | Sponsor: Copenhagen Studies on Asthma in Childhood. |
| Trial name or title | Comparison of effectiveness of vitamin D supplementation in decreasing the development of the gestational diabetes mellitus in pregnant women. |
| Methods | Single‐arm study, not blinded. |
| Participants | 75 pregnant women referring to obstetric clinic of Shahid Beheshti and Alzahra hospital in Esfahan city in 2012 (overall 225 persons). Inclusion criteria: patient satisfaction; normal BMI; gestational age below 16 weeks; no history of diabetes mellitus type 2 or GDM; no family history of diabetes mellitus type 1 in first degree relatives. Exclusion criteria: patient dissatisfaction; incorrect consumption of vitamin D supplementation; Follow‐up discontinuation. |
| Interventions | Participants will be individually randomised to 1 of 2 groups: group 1: vitamin D supplementation with dose of 50,000 unit every 2 weeks for 10 weeks; group 2: are not treated with vitamin D supplementation. Persons with level of above 25 nmol/L, were selected as normal healthy control group. |
| Outcomes | Maternal: gestational blood sugar level, serum vitamin D level. |
| Starting date | 2012‐03‐20. |
| Contact information | Name: Dr. Hatav Ghasemi Tehrani |
| Notes | Sponsor: Isfahan University Of Medical Sciences. |
| Trial name or title | Effects of prenatal vitamin D supplementation on respiratory and allergic phenotypes and bone density in the first 3 years of life. |
| Methods | Randomised interventional prevention trial. |
| Participants | 180 mothers attending antenatal clinic at St Marys Hospital, London, United Kingdom. This is a follow‐up trial of the infants of these trial participants. All of the offspring of the 180 mothers recruited in this trial are eligible and are invited to participate in this follow‐up study when their children are 3 years of age. |
| Interventions | Participants will be individually randomised to 1 of 2 groups: group 1 (n = 60): received no vitamin D; group 2 (n = 60): received 800 IU of vitamin D daily for the remainder of pregnancy; group 3 (n = 60) received a single oral dose of 200,000 IU vitamin D at 27 weeks' gestation. |
| Outcomes | Infant: wheezing episode in the first 3 years of life, measured at 36‐48 months, use of inhaled bronchodilators in the last 12 months, doctor‐diagnosed rhinitis, any wheezing episode in the preceding 12 months, doctor‐diagnosed asthma, doctor‐diagnosed eczema, doctor‐diagnosed food allergy, positive skin prick test responses, 25‐hydroxyvitamin D levels, bronchodilator responsiveness, exhaled nitric oxide level (in parts per billion), nasal secretions for inflammatory mediators, pulmonary airflow resistance and reactance at a range of frequencies using impulse oscillometry, total number of all wheezing episodes since birth and total number of upper and lower respiratory tract infections since birth, at 36‐48 months. |
| Starting date | Date of start: 01/03/2010. Status: ongoing. Anticipated end date: 31/05/2011. |
| Contact information | Dr Stephen Goldring Department of Paediatrics Wright‐Fleming Institute, Norfolk Place, London W2 1PG , United Kingdom E‐mail: [email protected] |
| Notes | Sponsor: Imperial College London (UK). |
| Trial name or title | Evaluation of the effectiveness of vitamin D supplementation to pregnant women and their infants in Pakistan. |
| Methods | Randomised controlled trial. |
| Participants | 550 apparently healthy pregnant women 15‐49 years of age from 20‐22 weeks of gestation and their infants in Pakistan. Pregnant women with pre‐existing type 1 or type II diabetes, multiple fetuses, babies (twins, triplets), with high levels of vitamin D will be excluded. Infants with multiple congenital anomalies, serious birth injury, birth asphyxia, serious infections, very low birthweight, will be excluded. |
| Interventions | Participants will be individually randomised to 1 of 2 groups: group 1 will receive a daily dose of 4000 IU of vitamin D from 20‐22 weeks of pregnancy till the time of delivery; group 2 will receive placebo. The infants will be stratified in 2 groups: group 1 will receive 400 IU of vitamin D for 6 months as intervention (if mothers are from group 1); group 2 will receive placebo (if mothers are from group 2). |
| Outcomes | Maternal: pre‐eclampsia, gestational hypertension, poor weight gain during pregnancy, stillbirth rates, prevalence and risk factors for maternal vitamin D deficiency. Infant: low birthweight, prematurity, neonatal seizures, infants with growth failure, signs and symptoms of vitamin D deficiency, infections: pneumonia, diarrhoea and receptor polymorphism, prevalence and risk factors for neonatal vitamin D deficiency. |
| Starting date | Date of start: February 2010. Status: recruiting participants. Estimated study completion date: June 2011. |
| Contact information | Muhammad Atif Habib, MBBS, MPH Project Office Aga Khan University Tel: +92 21 3 4864798 Email: [email protected] Principal investigator: Zulfiqar A Bhutta, FRCPCH, PhD Aga Khan University, Pakistan. |
| Notes | Sponsors: Aga Khan University and John Snow, Inc. |
| Trial name or title | Testing the calcium DRI during pregnancy: a study of bone health in black and white women. |
| Methods | Randomised controlled trial. |
| Participants | 120 African American or Caucasian primigravidae women 19‐40 years of age in their first trimester of pregnancy in Children's Hospital & Research Center Oakland, California, USA. Women who are smokers, have a pre‐pregnancy BMI higher than 30, have a medical condition that affects bone or taking a medication that affects bone will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 3 groups: group 1: will receive 1000 mg of calcium; group 2: will receive 2000 IU vitamin D; group 3: will receive a placebo. The intervention will be provided from week 16 of pregnancy until delivery. |
| Outcomes | Primary: Maternal: change in peripheral cortical and trabecular bone loss and gain during a reproductive cycle in black and white women. Infant: none. Secondary: change in bone markers of bone formation and resorption during pregnancy and postpartum, differences in calcium absorption in late pregnancy among black and white women, differences in adaptive immune function tests and markers of inflammation during pregnancy. Infant: none. |
| Starting date | Date of start: 05/2010. Status: currently recruiting participants. Expected study completion date: May 2013. |
| Contact information | Andrea N Hacker, MS, RD Children's Hospital & ResearchCenter Oakland, CA, USA Tel: +1 510 428‐3885 Email: [email protected] Principal Investigators: Ellen Fung, PhD, RD and Janet King, PhD, RD |
| Notes | Sponsors: Children's Hospital & Research Center Oakland and USDA, Western Human Nutrition Research Center, USA. |
| Trial name or title | MAVIDOS Maternal vitamin D osteoporosis study: study protocol for a randomised controlled trial. The MAVIDOS Study Group. |
| Methods | Randomised, double‐blind, placebo‐controlled trial. |
| Participants | Pregnant women over 18 years old, with a singleton pregnancy with less than 17 weeks' gestation at first assessment (based on last menstrual period (LMP) and dating scan), aiming to give birth at local maternity hospital, and with serum 25(OH)‐vitamin D concentration is 25‐100 nmol/L at nuchal fold/dating scan (10 to 17 weeks' gestation) |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1: will receive 1000 IU cholecalciferol orally daily; group 2: will receive placebo. Starting from 14 weeks' gestation until delivery. |
| Outcomes | Infant: whole body bone mineral content of the neonate adjusted for gestational age and age at neonatal DXA scan, whole body bone area, bone mineral density, and size corrected bone mineral density (BMC adjusted for BA, length and weight), body composition adjusted for gestational age and age at DXA scan. |
| Starting date | 2008 Apr 11. |
| Contact information | Nicholas C Harvey MRC Lifecourse Epidemiology Unit, (University of Southampton), Southampton General Hospital, Southampton, United Kingdom. Email: [email protected] |
| Notes | Sponsor: Southampton University Hospitals NHS Trust (UK). ISRCTN82927713 |
| Trial name or title | The effect of 50,000 IU vitamin D supplement administered 2 weekly on neonatal and pregnant women outcome ‐ GDM. |
| Methods | Randomised controlled trial. |
| Participants | Pregnant women, aged between 15 and 48, who came to prenatal clinic of Shahid Sadoughi and Mojibian Hospitals during first trimester with no history of corticosteroids or anticonvulsants use during the last 3 months were included. Exclusion criteria: history of thyroid or parathyroid diseases; receiving vitamin D more than maintenance dose during the last 6 months; treatment with corticosteroids or anticonvulsants and history of diabetes mellitus before pregnancy. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups:group 1: vitamin D supplement, 50,000 IU tablet, every 2 weeks; group 2: vitamin D supplement, calcium‐D tablet, 200 IU daily. |
| Outcomes | Maternal: vitamin D values and GDM. |
| Starting date | July 2011. |
| Contact information | Dr Maryam Jannati Moghadam Tel: +983516240061 Email: [email protected] Address: Ayatollah Kashani ST 8915635843 Yazd, Islamic Republic of Iran. |
| Notes | Sponsor: Shahid Sadughi Yazd University of Medical Sciences. |
| Trial name or title | DALI: vitamin D And lifestyle intervention for gestational diabetes mellitus (GDM) prevention. |
| Methods | Randomised controlled trial with a factorial design. |
| Participants | Pregnant women with gestational age at recruitment < 12 weeks, and more than 18 years of age. Inclusion criteria: pre‐pregnancy BMI (self‐reported weight, measured height) is >= 29 kg/m2), sufficiently fluent in major language of the country of recruitment, being able to be moderately physically active, giving written informed consent, agree to give birth in 1 of the participating hospitals. Exclusion criteria: pre‐existing diabetes, diagnosed with (gestational) diabetes mellitus before randomisation, defined as fasting glucose ≥ 5.1 mmol/L and/or 1 hour glucose ≥ 10 mmol/L and/or 2 hour glucose ≥ 8.5 mmol/L at baseline, not able to walk at least 100 metres safely, requirement for complex diets, advanced chronic conditions (e.g. valvular heart disease), significant psychiatric disease, unable to speak major language of the country of recruitment fluently, known abnormal calcium metabolism (hypo/hyperparathyroidism, nephrolithiasis, hypercalciuria) or hypercalciuria detected at screening (0.6 mmol/mmol creatinine in spot morning urine) and twin pregnancy. |
| Interventions | The design is that of 2 trials with a factorial design: PA, diet, PA & diet, control, vitamin D, PA & diet and placebo, vitamin D & PA & diet, placebo; to compare the impact of increased PA, enhanced nutrition and vitamin D supplementation either alone or in combination on maternal glucose tolerance, maternal weight gain and insulin sensitivity. The doses of vitamin D that will be tested in the dosing study are 500, 1000 and 1500 IU/day. 1 of these doses will be used in the trial. |
| Outcomes | Maternal: weight gain during pregnancy, fasting plasma glucose, HbA1c, fasting C peptide, leptin, triglycerides, free fatty acids, high density lipoprotein cholesterol (HDL‐C) and low density lipoprotein cholesterol (LDL‐C), adiponectin 2. 3 beta‐hydroxybutyrate, blood pressure, C‐reactive protein. Infant: neonatal growth, adiposity, adipo‐insular axis, glucose‐insulin axis, electrolyte concentrations, clinical outcomes and hypoxia exposure at birth, biparietal diameter, head circumference, abdominal circumference, femur length] and determinants of foetal body composition variables (lean body mass and fat body mass, C‐peptide, glucose, leptin, triglycerides, 3‐ beta‐hydroxybutyric acid, pH and erythropoietin, jaundice, hypocalcaemia, hypomagnesaemia. |
| Starting date | 21/11/2011. |
| Contact information | Dr David Simmons Addenbrooke's Treatment Centre United Kingdom |
| Notes | Sponsor: European Union (EU) (Belgium) ‐ Seventh Framework Programme (FP7). |
| Trial name or title | A randomised double‐blinded interventional trial to determine the effect of 50,000 IU vitamin D supplementation monthly or twice monthly from 20 weeks' gestation. |
| Methods | Randomised double‐masked clinical trial with randomisation at the individual level. Method of sequence generation: serial tossing of a coin. Allocation will be not concealed. |
| Participants | Pregnant women seeking maternity care with midwifery services involved in the study. Exclusion criteria: antenatal vitamin D level is > 75 nmol/L when enrolling in study. |
| Interventions | Participants will be assigned to 1 of 2 groups: group 1: will receive 50,000 IU tablets twice monthly, 2 weeks apart; group 2: will receive 50,000 IU monthly and a placebo monthly, 2 weeks apart from 20 weeks' gestation until delivery of baby. The placebo tablet contains lactose monohydrate, acacia, calcium carbonate, castor oil, maize starch, povidone, sucrose, purified talc, hydrated silica, powdered cellulose, magnesium stearate, shellac, gelatin, beeswax white, titanium dioxide and prepared theobroma. |
| Outcomes | Infant: vitamin D levels taken from the cord blood samples at delivery. If emergencies at delivery prevent a cord blood sample being taken then a maternal venous blood sample will be taken for analysis. |
| Starting date | Status: not yet recruiting participants. |
| Contact information | Dr Annie Judkins Newtown Union Health Service Email: [email protected] |
| Notes | Sponsors: Royal New Zealand College of GP's, New Zealand and Wellington Medical Research Foundation, New Zealand. ACTR Number: ACTRN12610001044011. |
| Trial name or title | A randomised controlled trial to investigate the effects of vitamin D supplementation on maternal and new‐born babies vitamin D status in Asian‐Indian Subjects ‐ VIDIMAN. |
| Methods | Randomised, parallel group, placebo‐controlled trial. |
| Participants | Pregnant women between 12‐16 weeks of gestation, aged between 18 to 35 years. Participants agree for delivery conducted at the AIIMS. Exclusion criteria: participants will be excluded from the study if they have more than 1 gestation in current pregnancy, cardiovascular disease, more than 3 abortions, hypertension, pre‐eclampsia or Rh isoimmunisation, prior history of delivery of an infant with chromosomal abnormalities or IUGR in previous pregnancy presence of any diagnosed systemic disease known to affect vitamin D status such as malabsorption states, liver and renal disorders, primary and tertiary hyperparathyroidism, have features suggestive of osteomalacia or severe VDD, pre‐existing hypertension, diabetes (type 1 or 2), are taking or had taken vitamin D supplementation in last 2 months in doses exceeding 600 IU/day, are using medications known to interact with vitamin D metabolism (steroids,thiazide diuretics, phenytoin, phenobarbitone and antitubercular drugs), have known hypersensitivity to vitamin D preparations, have participated in any other investigational drug study in previous 3 months, have past history of bariatric surgery, are using Ultra‐Violet (UV) radiations as a part of medical therapy, are diagnosed with albinism or have other condition associated with decreased skin pigmentation, have any medical condition that in the judgment of the investigator would jeopardise the participants' safety or evaluation of study drug for efficacy or safety. Additional exclusion criteria will be applied after biochemical screening: Having gestational diabetes between 12‐16 weeks of pregnancy, have serum calcium more than 1 mg/dL above the upper limit of normal for age, have serum S.25(OH)D level more than 100 ng/mL. |
| Interventions | Participants will be randomly assigned to 1 of 4 groups:group 1: participants will receive 1000 IU of vitamin D per day. Although dose of supplementation has been calculated from daily dose basis, but it will be given, orally as once a month dose (30,000 IU once a month orally,supervised in the hospital), supervised in the hospital till delivery; group 2: participants will receive 2000 IU of vitamin D per day. Although dose of supplementation has been calculated from daily dose basis, but it will be given, orally as once a month dose (60,000 IU once a month orally, supervised in the hospital) till delivery; group 3: participants will receive 4000 IU of vitamin D per day. Although dose of supplementation has been calculated from daily dose basis, but it will be given, orally as once a month dose (120,000 IU once a month orally, supervised in the hospital) till delivery; group 4: control group ‐ participants will receive 600 IU of vitamin D per day. Although dose of supplementation has been calculated from daily dose basis, but it will be given, orally as once a month dose (18,000 IU once a month orally,supervised in the hospital) until delivery. |
| Outcomes | Maternal: serum 25 hydroxyvitamin D, weight gain, pre‐eclampsia, preterm labour, insulin resistance in mother. Infant: fetal growth, newborn anthropometry and insulin resistance in newborn. |
| Starting date | 01‐02‐2014. |
| Contact information | Garima Kachhawa All India Institute of Medical Sciences |
| Notes | Sponsor: Indian Council of Medical Research ICMR. |
| Trial name or title | The effect of vitamin D supplementation during pregnancy on newborn's anthropometric index. |
| Methods | An interventional, randomised, not blinded, parallel trial. |
| Participants | Pregnant women with a singleton pregnancy and gestational age between 24‐28 week; BMI 19‐26; vitamin D < 30 ng/mL. Exclusion criteria: the known history of liver, renal, parathyroid, bone, metabolic diseases or epilepsy or malabsorption, medications that influence the metabolism of vitamin D and calcium, recurrent abortion, diabetes or gestational diabetes, HTN or pre‐eclampsia; fetus with anomalies, polyhydramnios, oligohydramnios or IUGR. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1: vitamin D capsule 50,000 U weekly for 8 weeks from 28 gestational age and multivitamin tablet include 400 IU vitamin D daily till termination; group 2: multivitamin tab include 400 IU vitamin D daily till termination. |
| Outcomes | Infant: height, weight, and head circumference. |
| Starting date | 1991‐11‐22. |
| Contact information | Dr Fatemeh Lalooha |
| Notes | Sponsor: Qhazvin University of Medical Sciences, Iran. Irct registration number : IRCT201205119491N2. |
| Trial name or title | Vitamin D supplementation for prevention of placenta mediated pregnancy complications. |
| Methods | Randomised, controlled trial. |
| Participants | Pregnant women > 18 years of age, from 3 maternal health care units who agree to participate in the study. Exclusion criteria: < 18 years of age, hyperparathyroidism and sarcoidosis. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1: will receive vitamin D, oral drops; group 2: will receive placebo. |
| Outcomes | Maternal: pre‐eclampsia, blood loss at delivery. Infant: blood flow in umbilical artery, growth restriction, and prematurity. |
| Starting date | 06/04/2011. |
| Contact information | N/A |
| Notes | Sponsor: Karolinska University Hospital. EudraCT Number: 2010‐019483‐37. |
| Trial name or title | Effect of high‐dose versus low‐dose vitamin D supplementation in pregnancy on maternal glucose metabolism and the risk of gestational diabetes. |
| Methods | Randomised controlled parallel trial. |
| Participants | Pregnant women, aged 18 or more, with less than 20 weeks' gestation at recruitment. Exclusion criteria: known diabetes, calcium metabolic disorder, multiple pregnancy |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1: will receive high‐dose vitamin D supplementation (5000 IU/day); group 2: standard dose pregnancy vitamin supplementation (400 IU vitamin D daily), administered as an oral capsule, from the time of the first antenatal clinic visit (around 12 weeks' gestation) until delivery. |
| Outcomes | Maternal: diagnosis of gestational diabetes, determined by an oral glucose tolerance test (blood analysis after ingestion of 75 g oral glucose) performed at 26‐28 weeks' gestation, or other evidence of hyperglycaemia recorded throughout pregnancy. Maternal glucose metabolism in late second trimester (26‐28 weeks' gestation) assessed by glucose tolerance test (blood analysis after ingestion of 75 g oral glucose). Infant: weight at birth, infant length (assessed with a newborn stadiometer) and head circumference. |
| Starting date | 10/02/2010 |
| Contact information | Mark McLean University of Western Sydney‐ Blacktown Clinical School |
| Notes | Sponsor: Hospital Westmead, Australia and University of Western Sydney, Australia. ACTRN 12612001145897. |
| Trial name or title | The effect of vitamin D and calcium plus vitamin D for leg cramps in pregnant women: a randomised controlled trial. |
| Methods | Randomised controlled trial. |
| Participants | Pregnant women with gestational age of 25‐30 weeks aged 18 to 35 years old; having leg cramps at least twice a week; being literate. Exclusion criteria: having known thyroid, cardio‐vascular, diabetes or renal diseases; intake of calcium and vitamin D supplementation during pregnancy; having allergy history to studied drugs. |
| Interventions | Eligible women will be selected with convenience sampling and will be randomly assigned into 3 groups of 42 participants with block sizes of 3 and 6: group 1: will receive Vitamin D tablets (1000 units); group 2: will receive calcium‐vitamin D tablets (300 mg calcium carbonate plus 1000 units vitamin D); group 3: the control group will receive placebo. |
| Outcomes | Maternal: the number, severity and duration of leg cramps. |
| Starting date | 2013‐04‐30. |
| Contact information | Dr. Mozhgan Mirghafourvand |
| Notes | Sponsor: Tabriz University of Medical Sciences (Project number: 388). IRCT 2013040810324N12. |
| Trial name or title | Effect of vitamin D supplementation on glucose status, lipid profiles and inflammatory factors in mothers with a history of gestational diabetes. |
| Methods | Randomised parallel trial. |
| Participants | Women between 20‐45 years of age with gestational diabetes at their recent pregnancy from the list of GDM Diabetes Research Center of Yazd University, and without thyroid disease, kidney disease, bone disease, PCO, liver disease and not using anti‐epilepsy drugs, glucocorticoids, and statins. Exclusion criteria: risk of any illness that requires medication and lack of any willingness to cooperate. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups:group 1: intramuscular injection of vitamin D with 300,000 IU dose; group 2: control: not receive any intervention. |
| Outcomes | Maternal: glucose status, lipid profiles and inflammatory factors. |
| Starting date | 2010‐01‐25. |
| Contact information | Dr Hassan Mozaffari |
| Notes | Sponsor: Shahid Sadughi University of Medical Sciences and Health Services. IRCT138902113840N1. |
| Trial name or title | Assessment of dose effectiveness of vitamin D supplementation during pregnancy: a dose‐comparison trial. |
| Methods | Blind, randomised controlled trial with 3 arms. |
| Participants | 315 pregnant women aged 15‐45 years with less than 16 weeks of gestation in a hospital in Pakistan. Pregnant women with pre‐existing type 1 or type II diabetes, pre‐existing hypertension, multiple fetuses, babies (twins, triplets) or with a diagnosis of pregnancy with a fetal anomaly in scan will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 3 groups: groups 1 will receive a dose of 400 IU/day till the time of delivery; group 2 will receive 2000 IU/ day till the time of delivery; group 3 will receive 4000 IU/ day till the time of delivery. |
| Outcomes | Vitamin D deficiency, pre‐eclampsia, preterm labour, preterm birth, low birthweight, stillbirths. |
| Starting date | June 2013. |
| Contact information | Dr Sidrah Nausheen, Division of Women & Child Health, Aga Khan University, Karachi, Pakistan. |
| Notes | Sponsor: Aga Khan University, Pakistan. |
| Trial name or title | Effects of vitamin D supplement before and during and after pregnancy on complications, birthweight and bone mineral density during lactation. |
| Methods | Double‐blind, randomised, placebo‐controlled trial. |
| Participants | 400 apparently healthy women 30‐35 years of age, all with concentrations of P‐25‐hydroxyvitamin D (25OHD)‐ lower than 50 nmol/L. All women included attempts to get pregnant. Visits take place at Clinic of Osteoporosis, Department of Endocrinology, at Aarhus University Hospital, Aarhus, Denmark. Women with infertility, an intake of 400 IU or more vitamin D/day, cancer, history of alcohol or drug abuse, calcium metabolic disturbances or spontaneous abortion within last 6 months will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 3 groups: group 1: will receive 35 µg per day cholecalciferol; group 2: will receive 70 µg per day cholecalciferol; group 3: will receive placebo. All women will receive 2 tablets daily from baseline until 16 weeks after delivery. Intervention with cholecalciferol or placebo starts before pregnancy is achieved and continues until 4 months after the women has given birth. |
| Outcomes | Primary: Infant: birthweight. Maternal: none. Secondary: Infant: weight, crown‐heel length and head circumference, and infections within 16 weeks after birth. Concentration of 25OHD in umbilical cord and venous sample 16 weeks after birth. Maternal: postpartum effects of vitamin D supplement on maternal bone mineral density, concentration of 25OHD in mothers milk, incidence of pre‐eclampsia and abortions. |
| Starting date | Date of start: 12/2009. Status: recruiting participants. Estimated study completion date: December 2011. |
| Contact information | Gitte Bloch Rasmussen, MD Department of Endocrinology, Aarhus University Hospital University of Aarhus Tel: +45 89 4976 81 Email: [email protected] |
| Notes | Sponsor: University of Aarhus, Denmark. |
| Trial name or title | Randomised placebo‐controlled trial of maternal vitamin D supplementation during pregnancy and lactation to improve infant linear growth in Dhaka, Bangladesh (MDIG). |
| Methods | Randomised placebo‐controlled trial. |
| Participants | Women aged 18 years and above with a gestational age of 17 to 24 completed weeks estimated based on recalled last menstrual period and/or ultrasound, who Intend to permanently reside in the trial catchment area for at least 18 months. Exclusion criteria: history of medical conditions that may predispose the participant to vitamin D sensitivity, altered vitamin D metabolism and/or hypercalcaemia, or history of renal calculi, high‐risk pregnancy based on 1 or more of the following findings by point‐of‐care testing: Severe anaemia: haemoglobin < 70 g/L assessed by Hemocue, Moderate‐severe proteinuria: ≥ 300 mg/dL (3+ or 4+) based on urine dipstick, Hypertension: systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg, multiple gestation, major congenital anomaly, or severe oligohydramnios based on maternal history and/or ultrasound, unwillingness to stop taking non‐study vitamin D or calcium supplements or a multivitamin with calcium and/or vitamin D, currently prescribed vitamin D supplements as part of a physician's treatment plan for vitamin D deficiency and previous participation in the same study. |
| Interventions | Participants will be randomly assigned to one of 5 groups: group 1: prenatal period 0 IU; postpartum period 0 IU (placebo); group 2: prenatal period 4200 IU/week of vitamin D3 (= 600 IU/d); postpartum period 0 IU/week (placebo); group 3: prenatal period 16,800 IU/week of vitamin D3 (= 2400 IU/d); postpartum period 0 IU/week (placebo). Group 4: prenatal period 28,000 IU/week of vitamin D3 (= 4000 IU/d); postpartum period 0 IU/week (placebo); group 5: prenatal period 28,000 IU/week of vitamin D3 (= 4000 IU/d); postpartum period 28,000 IU/week (= 4000 IU/d). Overall: the prenatal period will start at enrolment (17‐24 weeks' gestation) and last until delivery. The postpartum period will last from delivery until 6 months postpartum. |
| Outcomes | Infant: infant length‐for‐age Z‐Scores with prenatal supplementation, infant length‐for‐age Z‐Scores with postpartum supplementation, serum calcium, stunting (LAZ < ‐2 SD below the median) at 1 and 2 years of age, attained length and LAZ at 2 years of age, birthweight, low birthweight %, small‐for‐gestational age %, preterm birth %, stillbirth %, acute respiratory infections and diarrhoea, biomarker concentrations, perinatal, neonatal and infant severe morbidity and mortality, epigenetic patterns of genes involved in vitamin D metabolism. Maternal: severe morbidity and mortality. |
| Starting date | March 2014. |
| Contact information | Daniel Roth, MD Tel: +1 416 8135795 Email: [email protected] |
| Notes | Sponsors: The Hospital for Sick Children, Canada; International Centre for Diarrhoeal Disease Research, Bangladesh; Shimantik; Bill & Melinda Gates Foundation, USA. ClinicalTrials.gov Identifier: NCT01924013. |
| Trial name or title | Vitamin D supplementation in gestational diabetes mellitus. |
| Methods | Randomised clinical trial. |
| Participants | Women with gestational diabetes, defined by the WHO criteria: fasting glucose ≥ 7.0 mmol/L or; oral glucose tolerance test: 75 g glucose, 2‐hour glucose ≥ 7.8 mmol/L recognised during pregnancy with a written informed consent, aged between 18‐42. Exclusion criteria: impaired renal function: creatinine * 150 *mol/L or a creatinine clearance < 50 ml/min, to the discretion of the investigator, cardiac problems: decompensated heart failure (NYHA III and IV); diagnosis of unstable angina pectoris; myocardial infarction within the last 12 months. Mental retardation, or psychiatric treatment for schizophrenia, organic mental disorder or bipolar disorder currently or in the past; Iinsufficient knowledge of the Dutch language; vitamin D plasma level ≥ 100 nmol/L or < 15 nmol/L; hypercalcaemia of any reason; granulomatous diseases influencing vitamin D levels; urolithiasis; pre‐existent diabetes mellitus of any type; substance abuse, other than nicotine; participation in any other trials, involving investigational products within 30 days prior to trial entry; any condition that the Investigator and/or co‐ordinating Investigator feels would interfere with trial participation or evaluation of results. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1: cholecalciferol 15,000 IU once a week during pregnancy; group 2: placebo 15,000 IU once a week during pregnancy. |
| Outcomes | Primary: Maternal: insulin sensitivity (HOMA‐index and β‐cell function) measured through fasting insulin and blood glucose levels. Secondary: Maternal: serum 25OHD, HbA1c values, blood pressure, thyroid function, lipid profile and BMI, pregnancy characteristics, maternal outcomes, and adverse effects. Infant: neonatal outcome. |
| Starting date | 1 Jul 2012. |
| Contact information | Drs. Y.H.M. Poel Medisch Centrum Alkmaar Wilhelminalaan 12 Tel: +31 (0)72 5484444 Email: [email protected] |
| Notes | Sponsor: Medisch Centrum Alkmaar. NTR Number: NTR3158. |
| Trial name or title | Preventing health disparities during pregnancy through vitamin D supplementation. |
| Methods | Randomised control trial with 2 arms. |
| Participants | 450 pregnant women (18‐45 years of age) who presents to her obstetrician or midwife at the Medical University of SC (MUSC), Charleston, United States of America obstetrical facilities within the first 14 weeks after her last menstrual period with confirmation of a singleton pregnancy will be eligible for enrolment in the study. Women with diverse ethnic backgrounds (African‐American, Asian, Caucasian and Hispanic) will be actively recruited. Women with pre‐existing calcium, uncontrolled thyroid disease, parathyroid conditions, or who require chronic diuretic or cardiac medication therapy including calcium channel blockers will not be eligible for enrolment into the study. Mothers with pre‐existing sickle cell disease (not trait only), sarcoidosis, Crohn's disease, or ulcerative colitis may not participate in the study. In addition, because of the potentially confounding effect of multiple fetuses, mothers with multiple gestations will not be eligible for participation in the study. A subgroup of approximately 100 participants with known diabetes, hypertension, HIV, or morbid obesity (BMI > 49) will participate in the study. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1 will receive vitamin D3 4000 IU in gummy form (participants will be asked to consume 4 gummies/day beginning at 10‐14 weeks of your pregnancy) plus the daily multiple micronutrient supplement; group 2 will receive placebo gummy plus the standard multiple micronutrient supplement (containing also 400 IU vitamin D3. |
| Outcomes | Maternal and neonatal health status as a function of maternal and infant vitamin D status. |
| Starting date | January 2013. |
| Contact information | Dr Carol Wagner Medical University of South Carolina Charleston, South Carolina, United States, 29425 Tel: +1 843‐792‐2401 Email: [email protected] |
| Notes | Sponsor: Medical University of South Carolina, USA and W.K. Kellogg Foundation, USA. |
BMI: body mass index
DRI: dietary references intakes
GDM: gestational diabetes mellitus
HTN: hypertension
IU: international units
IUGR intrauterine growth restriction
LMP: last menstrual period
PA: physical activity
PCO: polycystic ovary
PTH: parathyroid hormone
Rh: Rhesus
SD: standard deviation
VDD: Vitamin D deficiency
25OHD: 25‐hydroxycholecalciferol
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pre‐eclampsia (ALL) Show forest plot | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.25, 1.05] |
| Analysis 1.1  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 1 Pre‐eclampsia (ALL). | ||||
| 2 Gestational diabetes (ALL) Show forest plot | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.45] |
| Analysis 1.2  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 2 Gestational diabetes (ALL). | ||||
| 3 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 54.73 [36.60, 72.86] |
| Analysis 1.3  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 3 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL). | ||||
| 4 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by start of supplementation) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| Analysis 1.4 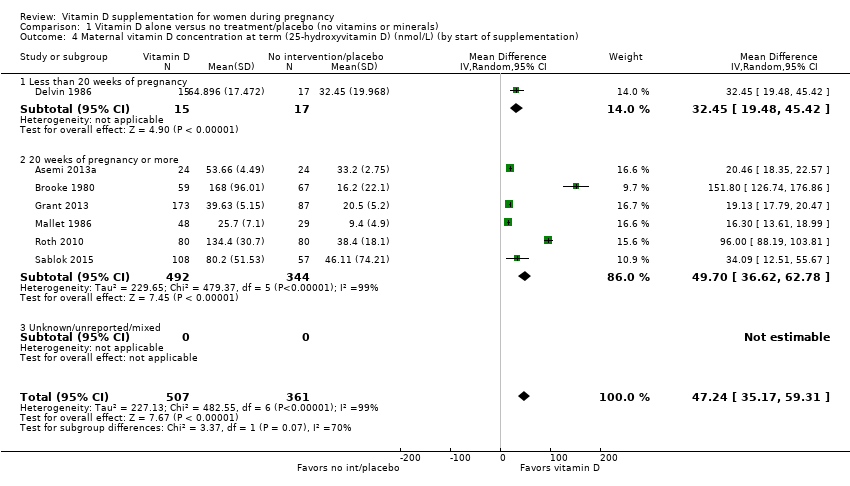 Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 4 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by start of supplementation). | ||||
| 4.1 Less than 20 weeks of pregnancy | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 32.45 [19.48, 45.42] |
| 4.2 20 weeks of pregnancy or more | 6 | 836 | Mean Difference (IV, Random, 95% CI) | 49.70 [36.62, 62.78] |
| 4.3 Unknown/unreported/mixed | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by pre‐gestational body mass index (kg/m2)) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| Analysis 1.5  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 5 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by pre‐gestational body mass index (kg/m2)). | ||||
| 5.1 Underweight (lower than 18.5) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Normal weight (18.5 to 24.9) | 1 | 165 | Mean Difference (IV, Random, 95% CI) | 34.09 [12.51, 55.67] |
| 5.3 Overweight (25 or higher) | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 19.54 [18.34, 20.74] |
| 5.4 Unknown/unreported/mixed | 4 | 395 | Mean Difference (IV, Random, 95% CI) | 73.18 [21.00, 125.36] |
| 6 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by supplementation scheme/regimen) Show forest plot | 8 | 1043 | Mean Difference (IV, Random, 95% CI) | 44.12 [30.24, 58.00] |
| Analysis 1.6  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 6 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by supplementation scheme/regimen). | ||||
| 6.1 Single dose | 3 | 340 | Mean Difference (IV, Random, 95% CI) | 15.16 [5.68, 24.63] |
| 6.2 Daily | 6 | 703 | Mean Difference (IV, Random, 95% CI) | 57.80 [38.37, 77.23] |
| 6.3 Weekly | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by skin pigmentation based on Fitzpatrick skin tone chart) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| Analysis 1.7 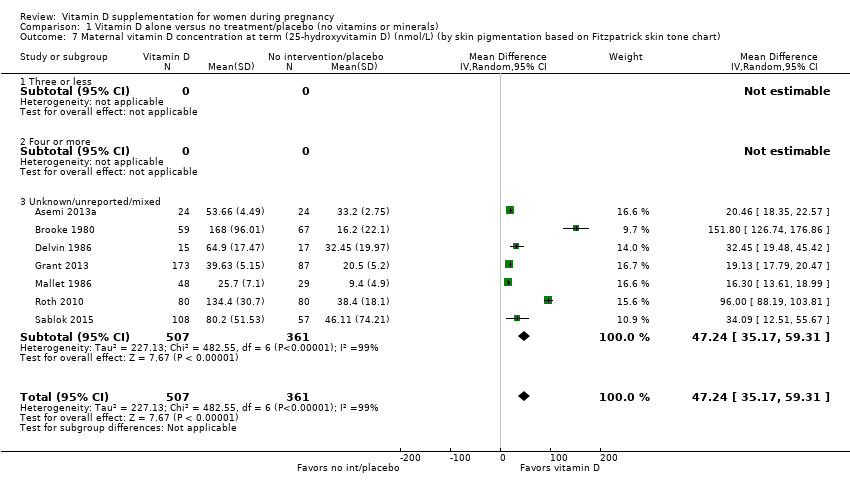 Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 7 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by skin pigmentation based on Fitzpatrick skin tone chart). | ||||
| 7.1 Three or less | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Four or more | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Unknown/unreported/mixed | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| 8 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by latitude) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| Analysis 1.8  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 8 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by latitude). | ||||
| 8.1 Between Tropics of Cancer and Capricorn | 1 | 260 | Mean Difference (IV, Random, 95% CI) | 19.13 [17.79, 20.47] |
| 8.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 6 | 608 | Mean Difference (IV, Random, 95% CI) | 55.73 [35.67, 75.80] |
| 8.3 Unknown/unreported | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by season at the start of pregnancy) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| Analysis 1.9  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 9 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by season at the start of pregnancy). | ||||
| 9.1 Summer | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 96.0 [88.19, 103.81] |
| 9.2 Winter | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 16.30 [13.61, 18.99] |
| 9.3 Mixed seasons | 5 | 631 | Mean Difference (IV, Random, 95% CI) | 37.24 [27.46, 47.02] |
| 10 Preterm birth (less than 37 weeks' gestation) (ALL) Show forest plot | 3 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.93] |
| Analysis 1.10  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 10 Preterm birth (less than 37 weeks' gestation) (ALL). | ||||
| 11 Low birthweight (less than 2500 g) (ALL) Show forest plot | 3 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.24, 0.67] |
| Analysis 1.11  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 11 Low birthweight (less than 2500 g) (ALL). | ||||
| 12 Impaired glucose tolerance | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Caesarean section Show forest plot | 2 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.69, 1.31] |
| Analysis 1.13  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 13 Caesarean section. | ||||
| 14 Gestational hypertension | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Adverse effects (nephritic syndrome) (ALL) Show forest plot | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.06] |
| Analysis 1.15 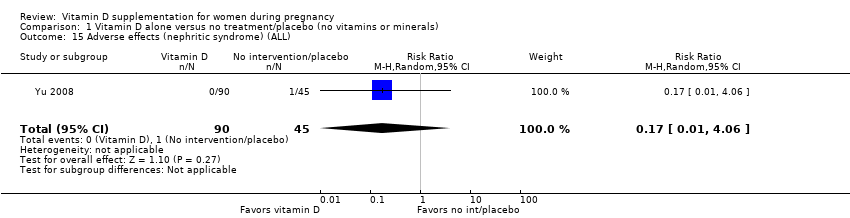 Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 15 Adverse effects (nephritic syndrome) (ALL). | ||||
| 16 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) Show forest plot | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.16  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 16 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL). | ||||
| 17 Birth length (cm) (ALL) Show forest plot | 4 | 638 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.02, 1.43] |
| Analysis 1.17  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 17 Birth length (cm) (ALL). | ||||
| 18 Head circumference at birth (cm) (ALL) Show forest plot | 4 | 638 | Mean Difference (IV, Random, 95% CI) | 0.43 [0.03, 0.83] |
| Analysis 1.18 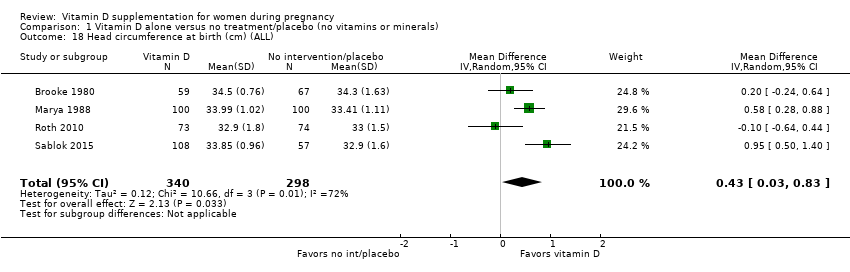 Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 18 Head circumference at birth (cm) (ALL). | ||||
| 19 Birthweight (g) (ALL) Show forest plot | 5 | 715 | Mean Difference (IV, Random, 95% CI) | 66.60 [‐137.22, 270.41] |
| Analysis 1.19  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 19 Birthweight (g) (ALL). | ||||
| 20 Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Stillbirth (ALL) Show forest plot | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.06, 1.99] |
| Analysis 1.21  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 21 Stillbirth (ALL). | ||||
| 22 Neonatal death (ALL) Show forest plot | 2 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.04, 1.67] |
| Analysis 1.22  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 22 Neonatal death (ALL). | ||||
| 23 Apgar score less than seven at five minutes Show forest plot | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.53] |
| Analysis 1.23  Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 23 Apgar score less than seven at five minutes. | ||||
| 24 Neonatal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Very preterm birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pre‐eclampsia (ALL) Show forest plot | 3 | 1114 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.80] |
| Analysis 2.1 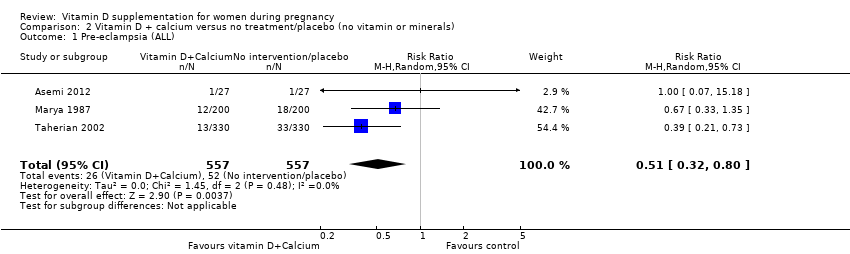 Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 1 Pre‐eclampsia (ALL). | ||||
| 2 Gestational diabetes (ALL) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| Analysis 2.2  Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 2 Gestational diabetes (ALL). | ||||
| 3 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Preterm birth (less than 37 weeks' gestation) (ALL) Show forest plot | 3 | 798 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.02, 2.43] |
| Analysis 2.4  Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 4 Preterm birth (less than 37 weeks' gestation) (ALL). | ||||
| 5 Low birthweight (less than 2500 g) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Impaired glucose tolerance | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Caesarean section | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Gestational hypertension Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.12] |
| Analysis 2.8  Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 8 Gestational hypertension. | ||||
| 9 Adverse effects (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal death (death while pregnant or within 42 days of termination of pregnancy) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Birth length (cm) (ALL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Head circumference at birth (cm) (ALL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Birthweight (g) (ALL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Stillbirth (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Neonatal death (ALL) Show forest plot | 1 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.15] |
| Analysis 2.16 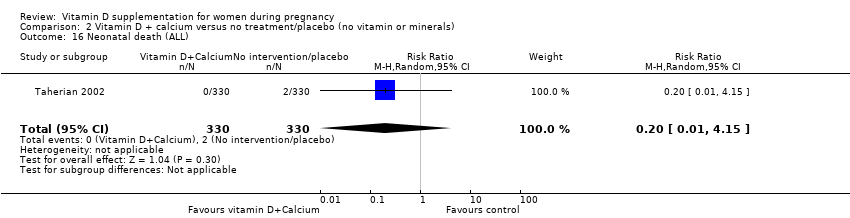 Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 16 Neonatal death (ALL). | ||||
| 17 Apgar score less than seven at five minutes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Neonatal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Very preterm birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

Study flow diagram for this update

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
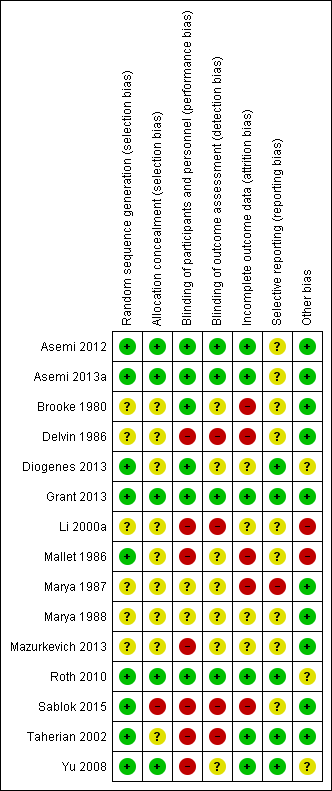
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 1 Pre‐eclampsia (ALL).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 2 Gestational diabetes (ALL).
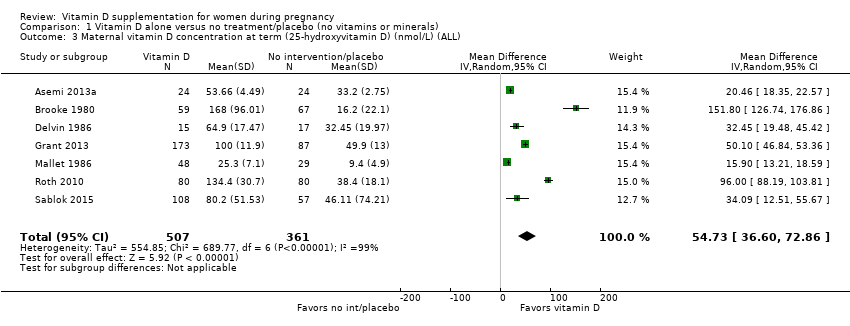
Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 3 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 4 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by start of supplementation).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 5 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by pre‐gestational body mass index (kg/m2)).
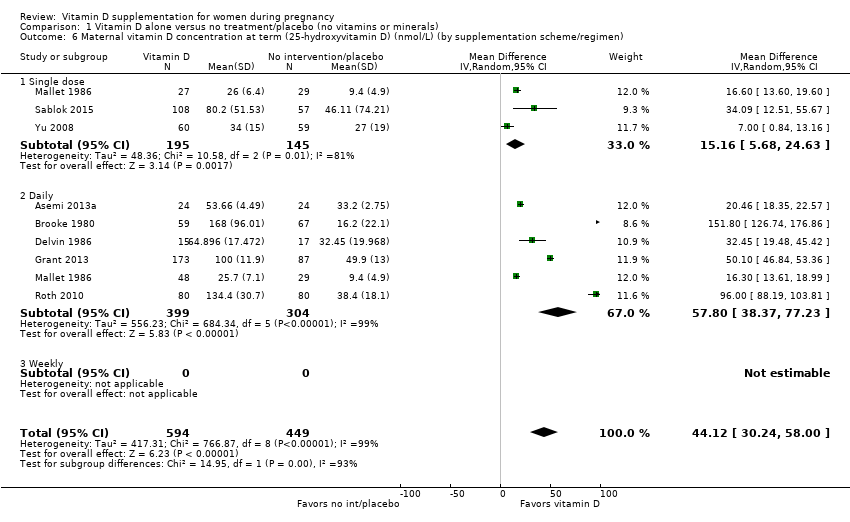
Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 6 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by supplementation scheme/regimen).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 7 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by skin pigmentation based on Fitzpatrick skin tone chart).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 8 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by latitude).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 9 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by season at the start of pregnancy).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 10 Preterm birth (less than 37 weeks' gestation) (ALL).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 11 Low birthweight (less than 2500 g) (ALL).
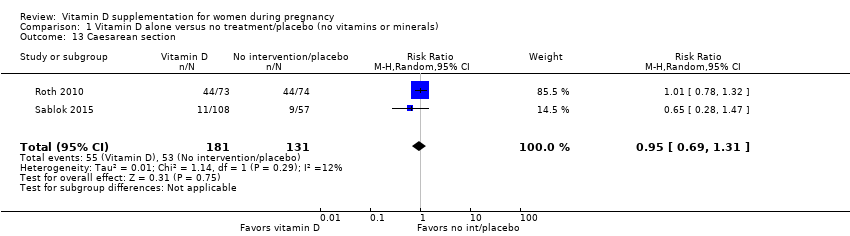
Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 13 Caesarean section.

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 15 Adverse effects (nephritic syndrome) (ALL).
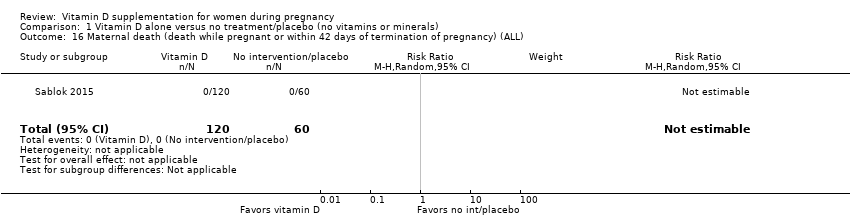
Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 16 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 17 Birth length (cm) (ALL).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 18 Head circumference at birth (cm) (ALL).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 19 Birthweight (g) (ALL).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 21 Stillbirth (ALL).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 22 Neonatal death (ALL).

Comparison 1 Vitamin D alone versus no treatment/placebo (no vitamins or minerals), Outcome 23 Apgar score less than seven at five minutes.

Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 1 Pre‐eclampsia (ALL).
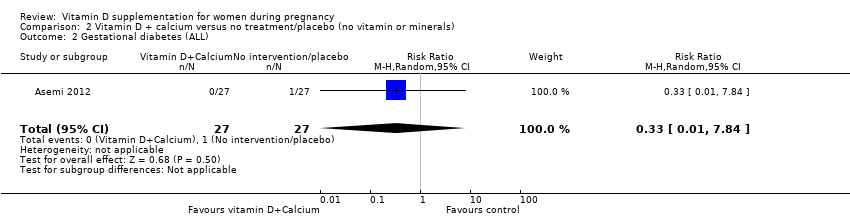
Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 2 Gestational diabetes (ALL).

Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 4 Preterm birth (less than 37 weeks' gestation) (ALL).

Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 8 Gestational hypertension.

Comparison 2 Vitamin D + calcium versus no treatment/placebo (no vitamin or minerals), Outcome 16 Neonatal death (ALL).
| Population: women during pregnancy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no treatment/placebo (no vitamins or minerals) | Risk with vitamin D alone | |||||
| Pre‐eclampsia (ALL) | Study population | RR 0.52 | 219 | ⊕⊕⊝⊝ | ||
| 155 per 1000 | 80 per 1000 | |||||
| Moderate | ||||||
| 124 per 1000 | 64 per 1000 | |||||
| Gestational diabetes (ALL) | Study population | RR 0.43 | 219 | ⊕⊝⊝⊝ | ||
| 24 per 1000 | 10 per 1000 | |||||
| Moderate | ||||||
| 27 per 1000 | 12 per 1000 | |||||
| Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) | The mean maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) in the intervention group was 47.24 higher (35.17 to 59.31 higher) | 868 | ⊕⊕⊝⊝ | |||
| Adverse effects | Study population | RR 0.17 (0.01 to 4.06) | 135 (1 RCT) | ⊕⊕⊝⊝ | ||
| 22 per 1000 | 4 per 1000 (0 to 90) | |||||
| Preterm birth (less than 37 weeks' gestation) (ALL) | Study population | RR 0.36 | 477 | ⊕⊕⊕⊝ | ||
| 99 per 1000 | 36 per 1000 | |||||
| Moderate | ||||||
| 46 per 1000 | 17 per 1000 | |||||
| Low birthweight (less than 2500 g) (ALL) | Study population | RR 0.40 | 493 | ⊕⊕⊕⊝ | ||
| 199 per 1000 | 80 per 1000 | |||||
| Moderate | ||||||
| 193 per 1000 | 77 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies contributing data had design limitations (high risk for allocation concealment and attrition bias). 2 Wide confidence interval crossing the line of no effect. 3 Wide confidence interval crossing the line of no effect & few events. 4 Statistical heterogeneity (I² > 60%). Considerable variation in size of effect. | ||||||
| Population: women during pregnancy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no treatment/placebo (no vitamin or minerals) | Risk with vitamin D + calcium | |||||
| Pre‐eclampsia (ALL) | Study population | RR 0.51 | 1114 | ⊕⊕⊕⊝ | ||
| 93 per 1000 | 48 per 1000 | |||||
| Moderate | ||||||
| 90 per 1000 | 46 per 1000 | |||||
| Gestational diabetes (ALL) | Study population | RR 0.33 | 54 | ⊕⊕⊝⊝ | ||
| 37 per 1000 | 12 per 1000 | |||||
| Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) | (0 studies) | No trial assessed this outcome. | ||||
| Adverse effects | (0 studies) | No trial assessed this outcome. | ||||
| Preterm birth (less than 37 weeks' gestation) (ALL) | Study population | RR 1.57 | 798 | ⊕⊕⊕⊝ | **Because there were zero events in some of the groups in two out of three of the included trials, GRADEpro GDT did not produce corresponding risks for a moderate risk population. | |
| 73 per 1000 | 114 per 1000 | |||||
| Moderate | ||||||
| ** | ** | |||||
| Low birthweight (less than 2500 g) (ALL) | Study population | Not estimable | (0 studies) | No trial assessed this outcome. | ||
| Not pooled | Not pooled | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies contributing data had design limitations (selection bias was unclear and high risk of attrition bias) 2 Wide confidence interval crossing the line of no effect, few events & small sample size. | ||||||
| Author | Location and setting | Participants (age, number, gestational week) | Randomisation process | Vitamin D supplement dose(s) | Type of vitamin D supplement | Results |
| India (antenatal clinics) | 299 pregnant women; 12‐24 weeks of gestation (lower middle and middle socio‐economic) | Random number tables | 60,000 IU; 120,000 IU; Usual care | Cholecalciferol | 25OHD at term was higher in those on the higher dose compared to the lower dose. Birthweight, length and HC were greater in the supplemented groups versus usual care. | |
| United Arab Emirates (primary and tertiary perinatal care centres) | 192 pregnant women; 12‐16 weeks of gestation | Stratified block design (seasonally balanced) using computer‐generated lists | 400 IU/d; 2,000 IU/d; 4000 IU/d | Cholecalciferol | 25OHD at term was higher with 4000 IU/d versus 2000 IUd versus 400 IU/d (P < 0.001). | |
| Iran (obstetric clinic) | 160 pregnant women; 24–26 weeks of gestation; singleton pregnancy and BMI:19–26 kg/m2 | Computer‐generated random numbers (open‐label randomised) | 400 IU/d (+200 mg calcium; 50,000 IU/week (+ calcium) | Cholecalciferol | Length, HC and weight were significantly higher in the intervention group compared with the control group. | |
| US (prenatal clinical centres in Boston, Saint Louis, San Diego) | 881 pregnant women, history of asthma/allergies; aged 18‐40 years; 10‐18 weeks' gestation | Stratified permuted blocks with randomly varied block sizes of 4 and 6, and 1 block allocation list per stratum | 400 IU/d; 4400 IU/d | Cholecalciferol | Not published yet. | |
| India | 45 Hindu pregnant women | Not reported | No vitamin D; 1200 IU/d (+375 mg calcium); 600,000 IU (at 7th and 8th month). | Ergocalciferol | Supplementation with 1200 U/d led to significantly higher birthweight and this was even higher in those taking the 2 doses of 600,000. | |
| Turkey (Hospital) | 91 pregnant women; aged 16‐42 years; singleton | Reported: simple randomisation method | 600 IU/d; 1,200 IU/d; 2,000 IU/d | Cholecalciferol | 25OHD was significantly higher in the 2,000 IU/d group versus the other groups | |
| Bangladesh (maternal health clinic) | 28 pregnant women; aged 18‐34 years; 27 to 30 weeks | Not specified | 70,000 IU (single dose on day 0) + 35,000 IU/week (from day 7); 14,000 IU/week (from day 0; control group) | Cholecalciferol | A dose‐response effect was observed in 25OHD with the higher dose versus control | |
| Iran (2 primary care clinics) | 51 healthy pregnant women; second trimester of pregnancy; autumn and winter | Not specified | 50,000 IU/month; 100,000 IU/month | Cholecalciferol | 76% of neonates in group with 50,000 IU/d month and 100% of neonates in group with 100,000 IU/month had 25OHD > 20 ng/mL. | |
| Iran (2 prenatal clinics) | 120 healthy non‐obese pregnant women; < 12th week of pregnancy | Not specified | 200 IU/d; 50,000 IU/month; 50,000 IU every 2 weeks | Cholecalciferol | 25OHD was higher in those on the higher dose versus middle versus lower doses. Supplementation with 50,000 IU every 2 weeks significantly improved insulin resistance | |
| USA (Research Center and university care centres) | 57 healthy pregnant women; <20 weeks' gestation;>18 years | Not specified | 400 IU/d; 2,000 IU/d | Cholecalciferol | Greater increase in 25OHD and higher infant birthweight among those on the 2000 IU/d group. | |
| USA (University prenatal care) | 494 healthy pregnant women; aged 16‐45 years; 12‐16 weeks of gestation; singleton | Stratified blocked randomisation to balance by ethnicity. | 400 IU/d; 2000 IU/d; 4000 IU/d. All women received daily multiple micronutrients supplements | Cholecalciferol | Greater increase in 25OHD among those on the 2000 IU/d and 4000 IU/d groups. | |
| USA (University prenatal care) | 257 healthy pregnant women; aged 16‐45 years; 12‐16 weeks of gestation; singleton | Randomisation stratified based on initial 25OHD, using lists generated by computer prior to the start of the study. | 2000 IU/d; 4000 IU/d. All women received daily multiple micronutrients supplements | Cholecalciferol | 25OHD significantly increase in both groups from baseline. Preterm birth was inversely associated with 25OHD at delivery | |
| Australia | 179 pregnant women; 18 years of age or older; singleton pregnancy; with low baseline 25OHD; < 20 weeks of gestation | Randomisation was in a 1:1 ratio, with a permuted block size of 6 and sequential assignment. | 400 IU/d; 5000 IU/d | Cholecalciferol | No difference in maternal OGTT between groups. | |
| Abbreviations used 25OHD: 25‐hydroxycholecalciferol | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pre‐eclampsia (ALL) Show forest plot | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.25, 1.05] |
| 2 Gestational diabetes (ALL) Show forest plot | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.45] |
| 3 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 54.73 [36.60, 72.86] |
| 4 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by start of supplementation) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| 4.1 Less than 20 weeks of pregnancy | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 32.45 [19.48, 45.42] |
| 4.2 20 weeks of pregnancy or more | 6 | 836 | Mean Difference (IV, Random, 95% CI) | 49.70 [36.62, 62.78] |
| 4.3 Unknown/unreported/mixed | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by pre‐gestational body mass index (kg/m2)) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| 5.1 Underweight (lower than 18.5) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Normal weight (18.5 to 24.9) | 1 | 165 | Mean Difference (IV, Random, 95% CI) | 34.09 [12.51, 55.67] |
| 5.3 Overweight (25 or higher) | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 19.54 [18.34, 20.74] |
| 5.4 Unknown/unreported/mixed | 4 | 395 | Mean Difference (IV, Random, 95% CI) | 73.18 [21.00, 125.36] |
| 6 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by supplementation scheme/regimen) Show forest plot | 8 | 1043 | Mean Difference (IV, Random, 95% CI) | 44.12 [30.24, 58.00] |
| 6.1 Single dose | 3 | 340 | Mean Difference (IV, Random, 95% CI) | 15.16 [5.68, 24.63] |
| 6.2 Daily | 6 | 703 | Mean Difference (IV, Random, 95% CI) | 57.80 [38.37, 77.23] |
| 6.3 Weekly | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by skin pigmentation based on Fitzpatrick skin tone chart) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| 7.1 Three or less | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Four or more | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Unknown/unreported/mixed | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| 8 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by latitude) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| 8.1 Between Tropics of Cancer and Capricorn | 1 | 260 | Mean Difference (IV, Random, 95% CI) | 19.13 [17.79, 20.47] |
| 8.2 North of the Tropic of Cancer or South of the Tropic of Capricorn | 6 | 608 | Mean Difference (IV, Random, 95% CI) | 55.73 [35.67, 75.80] |
| 8.3 Unknown/unreported | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (by season at the start of pregnancy) Show forest plot | 7 | 868 | Mean Difference (IV, Random, 95% CI) | 47.24 [35.17, 59.31] |
| 9.1 Summer | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 96.0 [88.19, 103.81] |
| 9.2 Winter | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 16.30 [13.61, 18.99] |
| 9.3 Mixed seasons | 5 | 631 | Mean Difference (IV, Random, 95% CI) | 37.24 [27.46, 47.02] |
| 10 Preterm birth (less than 37 weeks' gestation) (ALL) Show forest plot | 3 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.93] |
| 11 Low birthweight (less than 2500 g) (ALL) Show forest plot | 3 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.24, 0.67] |
| 12 Impaired glucose tolerance | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Caesarean section Show forest plot | 2 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.69, 1.31] |
| 14 Gestational hypertension | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Adverse effects (nephritic syndrome) (ALL) Show forest plot | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.06] |
| 16 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) Show forest plot | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Birth length (cm) (ALL) Show forest plot | 4 | 638 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.02, 1.43] |
| 18 Head circumference at birth (cm) (ALL) Show forest plot | 4 | 638 | Mean Difference (IV, Random, 95% CI) | 0.43 [0.03, 0.83] |
| 19 Birthweight (g) (ALL) Show forest plot | 5 | 715 | Mean Difference (IV, Random, 95% CI) | 66.60 [‐137.22, 270.41] |
| 20 Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Stillbirth (ALL) Show forest plot | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.06, 1.99] |
| 22 Neonatal death (ALL) Show forest plot | 2 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.04, 1.67] |
| 23 Apgar score less than seven at five minutes Show forest plot | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.53] |
| 24 Neonatal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Very preterm birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pre‐eclampsia (ALL) Show forest plot | 3 | 1114 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.32, 0.80] |
| 2 Gestational diabetes (ALL) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.84] |
| 3 Maternal vitamin D concentration at term (25‐hydroxyvitamin D) (nmol/L) (ALL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Preterm birth (less than 37 weeks' gestation) (ALL) Show forest plot | 3 | 798 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.02, 2.43] |
| 5 Low birthweight (less than 2500 g) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Impaired glucose tolerance | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Caesarean section | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Gestational hypertension Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.12] |
| 9 Adverse effects (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal death (death while pregnant or within 42 days of termination of pregnancy) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Birth length (cm) (ALL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Head circumference at birth (cm) (ALL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Birthweight (g) (ALL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Stillbirth (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Neonatal death (ALL) Show forest plot | 1 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.15] |
| 17 Apgar score less than seven at five minutes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Neonatal infection | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Very preterm birth | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

