Inhaladores de dosis medida versus nebulizadores para la administración de broncodilatadores en aerosol en pacientes adultos que reciben asistencia respiratoria mecánica en unidades de cuidados intensivos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Single‐blind, randomized, cross‐over study | |
| Participants | 13 male, 5 female Age: mean 69 years All patients who were ventilator‐dependent and were to receive bronchodilator aerosols for suspected airways obstruction All patients were clinically stable, assessed through an absence of hypotension, tachycardia and/or cardiac arrhythmias 12 patients required ventilation for acute respiratory failure caused by a primary lung disease, 6 had undergone major surgical procedures 11 patients were considered to have asthma or COPD, 15 were smokers | |
| Interventions | Patients received sequentially in a random order albuterol by MDI and NEB administered by the same respiratory therapist MDI: 3 puffs (3 x 90µg albuterol)
NEB: 2.5mg albuterol in 3ml saline
| |
| Outcomes | Respiratory mechanics and vital signs (systemic blood pressure, heart rate) Outcomes were assessed before and 30 minutes after the end of each modality of administration Primary outcomes:
Secondary outcomes
| |
| Notes | 2 patients were excluded from the analysis and report as tests did not confirm a diagnosis of airways obstruction Study was funded by United States Government grant HL38107/HL/NHLBI NHHHS/United States | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The same respiratory therapist delivered each aerosol treatment in every patient and determined the sequence of delivery modes with the flip of a coin" (p68) |
| Allocation concealment (selection bias) | Unclear risk | "The same respiratory therapist delivered each aerosol treatment in every patient and determined the sequence of delivery modes with the flip of a coin" (p68) |
| Blinding (performance bias and detection bias) | Low risk | "Investigators responsible for data acquisition and post sampling analysis were blinded to the treatment sequence" (p68) |
| Incomplete outcome data (attrition bias) | Low risk | "Twenty adult ventilator‐dependent patients...consented to be studied...Two patients were excluded from this report, because our tests did not confirm a diagnosis of airway obstruction" (p66) Study findings appear to report on all 18 participants (p69) "Two patients in who VPmean was greater than 0.8L/s had been excluded from this study." (p69) Results presented for 18 patients in Figure 3 and Figure 4 |
| Selective reporting (reporting bias) | High risk | 3 hypotheses were to be examined:
Results are presented for bronchodilator responsiveness and cost comparison. Data collected for cardiovascular side effects included systemic blood pressure, but only heart rate reported |
| Other bias | Unclear risk | "When necessary, excess secretions were removed by endotracheal suctioning before baseline Pao/V curves, systemic blood pressure, and heart rate were acquired" (p68) No further information as to how the need for suctioning was assessed or decided, or how many of the patients received this prior to commencing data collection |
| Appropriate design? | Low risk | "All patients were clinically stable, as indicated by the absence of hypotension, tachycardia, and/or cardiac arrhythmias" (p66) |
| Order of treatments randomized? | Low risk | "The same respiratory therapist delivered each aerosol treatment in every patient and determined the sequence of delivery modes with the flip of a coin" (p68) |
| Free from carry‐over effects? | Low risk | "Baseline and posttreatment measurements were repeated 4 h later after crossover to the alternate delivery mode" (p68) |
| Unbiased data available? | Low risk | “using paired Student’s t‐test statistics” (p68) No dropouts or systematic differences between two study periods |
| Methods | Single blind randomized cross‐over study | |
| Participants | 13 male, 5 female Age: 67 years ± 3 All patients were orotracheally intubated and were mechanically ventilated and all patients had COPD 10 patients had acute exacerbation of COPD, 8 patients had pneumonia 6 patients had received other bronchodilator agents but these were withheld for at least 4 hours before the onset of investigation | |
| Interventions | Patients received sequentially in a random order fenoterol‐ipratroprium bromide by MDI and NEB administered by the same respiratory therapist MDI: 4 puffs (4 x 50µg fenoterol/20µg ipratropium bromide)
NEB: 1.25mg fenoterol/500µg ipratropium bromide in 5ml saline
| |
| Outcomes | Respiratory mechanics and vital signs (heart rate, oxygen saturations and systemic blood pressure) Outcomes were assessed before and 30 minutes after the end of each modality of administration Primary outcomes:
Secondary outcomes
| |
| Notes | Research funded from a grant from Baxter who manufactured the MDI and nebulizer used in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients received sequentially in a random order (random order table)” (p1037) |
| Allocation concealment (selection bias) | Unclear risk | Random order table was used (p1037), but no information provided as to how this was used/interpreted or any indication if there was blinding or concealment used at this stage |
| Blinding (performance bias and detection bias) | Low risk | “The investigators who performed the post sampling analysis of the respiratory signals were blinded to the treatment modality” (p1038) |
| Incomplete outcome data (attrition bias) | Low risk | All data presented as n=18. No missing data apparent |
| Selective reporting (reporting bias) | Unclear risk | “We aimed at studying in detail the respiratory mechanics, and specifically the flow resistive properties of the respiratory system” (p1036) No pre‐set outcomes stated |
| Other bias | Unclear risk | 6 patients received other inhaled bronchodilators (other than the fenoterol‐ipratropium bromide under study) before entry into the study. In these patients, treatment was withheld for at least 4 hours before the onset of investigation |
| Appropriate design? | Low risk | "They all had COPD which was diagnosed by clinical history, chest radiographs and pulmonary function tests." (p1037) "Acute respiratory failure had been triggered by acute exacerbation in 10 patients and pneumonia in eight patients. They were investigated 1 to 10 d after the onset of tracheal intubation and ventilation" (p1037) |
| Order of treatments randomized? | Low risk | “patients received sequentially in a random order (random order table) fenoterol‐ipratropium bromide by MDI and NEB” (p1037) |
| Free from carry‐over effects? | Low risk | “A period of at least 10 h was allowed between the administration of the bronchodilator with the two modalities” (p1037) |
| Unbiased data available? | Low risk | "The comparison of the values of respiratory mechanics and vital signs before and after inhalation were made within and between delivery modalities by using Student's paired t tests" (p1038) No dropouts or systematic differences between two study periods |
| Methods | Prospective randomized cross‐over study | |
| Participants | 6 males, 4 females Age range 44‐78 years (mean 66 years) All patients admitted to the ICU who required mechanical ventilation and had a difference of more than 15cm H2O between their peak and pause airway pressures on tidal volume inflation and who gave informed consent 3 patients had pneumonia, 2 patients had COPD, 1 patient had lung cancer | |
| Interventions | Patients were prospectively randomized to receive albuterol therapy by MDI or nebulizer; 4 hours were allowed for wash out of the first course of albuterol. The patient was then crossed over to receive albuterol by the alternative method of administration MDI: doses of 10, 20, 30 and 40 puffs at 30 minute intervals
NEB: successively increasing doses 2.5, 5, and 7.5 mg in 3 ml of saline at 30 minute intervals
| |
| Outcomes | Respiratory mechanics and dose‐response relationship including the development of toxicity. Toxicity was defined by heart rate increment of 20 per minute, more than 4 premature ventricular or atrial contractions per minute, tremulousness or nausea Primary outcomes:
Secondary outcomes
| |
| Notes | Grant from National Institute of Health (US Government Grant) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided but p1567 patients were described as: "prospectively randomized" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | "prospectively randomized 10 mechanically ventilated patients" (abstract p1567) Results presented for 10 patients (figure 1 p1568 / figure 2 p1569). No missing data apparent |
| Selective reporting (reporting bias) | Unclear risk | "compared the efficacy and dose‐response relationship of albuterol delivered by MDI and NEB in a prospective randomized cross over study" (p1567) No further outcomes given |
| Other bias | High risk | "a wide variety of diseases requiring mechanical ventilation for reasons other than primary airflow obstruction" (p1568) Table 1: 2 different types of ventilator (p1568). All patients on different ventilator settings "meticulous attention was paid to counting only puffs that were entrained with inspiration and fewer than 10 puffs/100 needed to be repeated in any patient" (p1567) therefore dose with MDI was potentially different for these patients |
| Appropriate design? | Low risk | "All patients admitted to the University of Chicago Medical Center intensive care units in August and September of 1992, who required mechanical ventilation and had a difference of more than 15cm H2O between their peak (Ppeak) and pause (Ppause) airway pressures on tidal ‐ volume inflation" (p1597) "Patients were excluded if they had a history of symptomatic coronary artery disease in the 6 months prior to admission, or a history of haemodynamically significant arrhythmias" (p1597) |
| Order of treatments randomized? | Unclear risk | "patients who were randomized to receive albuterol by NEB first....patients who received albuterol by MDI first" (figure 2, p1569) |
| Free from carry‐over effects? | Low risk | "four hours were allowed for washout of the first course of albuterol. The patient was then crossed over to receive albuterol by alternative method" (p1567‐8) |
| Unbiased data available? | Unclear risk | "Individual responses of resistive pressure (ordinate) to cumulative doses of nebulized albuterol" (Figure 3, p1569) No paired analyses provided of nebulizer treatment response No dropouts or systematic differences between the two study periods |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| “All patients received intravenous steroids as part of their medical regime, and one patient received an Aminophylline infusion” (p818). Author contacted and confirmed the inclusion of this patient in the overall data analysis. No further study reports or raw data were available which exclude this patient, or would allow for re‐analysis of the study data. | |
| The study compared 2 different types of MDI to an intravenous bronchodilator (aminophylline), with no nebulizer comparison. | |
| Study primarily recorded percentage of deposition of drug given via MDI or nebulizer to the lung. Peak inspiratory pressure was measured at baseline, 5, 10, 15 and 30 minutes after administration of the bronchodilator (fenoterol) and the results presented as a percentage change from baseline over time. No further measurements were carried out which could enable calculation of the respiratory mechanics that are the primary outcomes of this review. | |
| Participants were randomized to receive bronchodilator aerosol from MDI, from one of four devices. No nebulizer comparison group. | |
| Patients were breathing spontaneously, not mechanically ventilated. | |
| Only limited data available from study abstract. Author contacted and responded with no further study reports or data available. | |
| Airway responses were not assessed or recorded. Efficacy of two different delivery methods evaluated through the measurement of total urinary excretion of albuterol. | |
| The study compared 2 different types of MDI and spacer, with no nebulizer comparison. |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
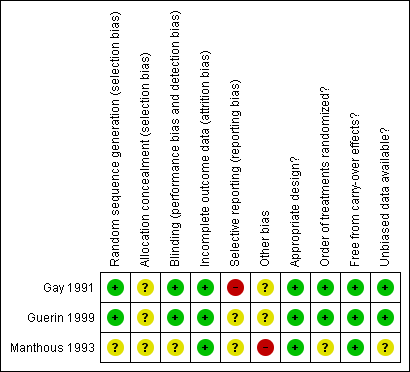
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Metered dose inhalers compared with nebulizers for aerosol bronchodilator delivery in mechanically ventilated adults | |||
| Patient or population: mechanically ventilated adults with need for aerosol bronchodilator therapy Settings: critical care units Intervention: metered dose inhalers Comparison: nebulizers | |||
| Outcomes | No of Participants | Quality of the evidence | Impact |
| Reduction in airway resistance Measured as a reduction in additional effective resistance (ΔRrs) and interrupter resistance (Rint) Assessed before treatment and 30 minutes after the end of each modality of administration | 28 | ⊕⊕⊕⊝1 | Both studies achieved a greater decrease in airway resistance using nebulizer |
| Mortality during critical care unit admission Measured using mortality rate in intervention and comparison groups During critical care admission | No studies found | N/A | |
| Duration of mechanical ventilation Measured as number of days | No studies found | N/A | |
| Adverse changes to haemodynamic observations Measured as a change in heart rate (beats per minute) Assessed before treatment and 30 minutes after the end of each modality of administration | 28 | ⊕⊕⊕⊝2 | Neither mode of delivery altered heart rate |
| GRADE Working Group grades of evidence | |||
| 1Downgraded for relatively few patients and events 2Downgraded for some selective outcome reporting in one study | |||

