Inhaladores de dosis medida versus nebulizadores para la administración de broncodilatadores en aerosol en pacientes adultos que reciben asistencia respiratoria mecánica en unidades de cuidados intensivos
Appendices
Appendix 1. Search strategy for CENTRAL, T he Cochrane Library
#1 MeSH descriptor Metered Dose Inhalers explode all trees
#2 MeSH descriptor Nebulizers and Vaporizers explode all trees
#3 MeSH descriptor Bronchodilator Agents explode all trees
#4 MeSH descriptor Administration, Inhalation explode all trees
#5 MeSH descriptor Drug Delivery Systems explode all trees
#6 MeSH descriptor Nitric Oxide explode all trees
#7 metered‐dose inhaler*
#8 MDI:ti,ab
#9 Nebuliser
#10 (bronchodilat* near (therap* or strateg*))
#11 (heated near humidific*)
#12 (spacer near devic*)
#13 (helium near oxygen)
#14 ((nitric oxide or NO) near mixture*)
#15 (bronchodilator* near delivery)
#16 (aerosol near bronchodilat*)
#17 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16)
#18 MeSH descriptor Respiration, Artificial explode all trees
#19 mechanical near ventilat*
#20 (#18 OR #19)
#21 (#17 AND #20)
Appendix 2. Search strategy for MEDLINE (OvidSP)
1. exp Metered Dose Inhalers/
2. exp "Nebulizers and Vaporizers"/ or Bronchodilator Agents/
3. Administration, Inhalation/
4. Drug Delivery Systems/
5. Nitric Oxide/ad, tu, sd [Administration & Dosage, Therapeutic Use, Supply & Distribution]
6. metered‐dose inhaler*.mp.
7. MDI.ti,ab.
8. Nebuliser.mp.
9. (bronchodilat* adj6 (therap* or strateg*)).mp.
10. (heated adj3 humidific*).mp.
11. (spacer adj3 devic*).mp.
12. (helium adj3 oxygen).mp.
13. ((nitric oxide or NO) adj3 mixture*).ti,ab.
14. (bronchodilator* adj3 delivery).mp.
15. (aerosol adj6 bronchodilat*).mp.
16. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. exp Respiration, Artificial/
18. (mechanical adj3 ventilat*).mp.
19. 18 or 17
20. 19 and 16
21 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) and humans.sh.
22. 21 and 20
Appendix 3. Search strategy for EMBASE (OvidSP)
1 exp Metered Dose Inhaler/
2 exp Nebulizer/ or exp Medical Nebulizer/
3 exp Vaporizer/
4 exp Bronchodilating Agent/
5 exp Inhalational Drug Administration/
6 exp Drug Delivery System/
7 exp Nitric Oxide/dt, ad, do, ih [Drug Therapy, Drug Administration, Drug Dose, Inhalational Drug Administration]
8 metered‐dose inhaler*.mp.
9 MDI.ti,ab.
10 Nebuliser.mp.
11 (bronchodilat* adj6 (therap* or strateg*)).mp.
12 (heated adj3 humidific*).mp.
13 (spacer adj3 devic*).mp.
14 (helium adj3 oxygen).mp.
15 ((nitric oxide or NO) adj3 mixture*).ti,ab.
16 (bronchodilator* adj3 delivery).mp.
17 (aerosol adj6 bronchodilat*).mp.
18 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19 exp Artificial Ventilation/
20 (mechanical adj3 ventilat*).mp.
21 19 or 20
22 21 and 18
Appendix 4. Search strategy for CINAHL (EBSCOhost)
S26 S19 and S25
S25 S20 or S21 or S22 or S23 or S24
S24 AB trial* or random*
S23 (MM "Multicenter Studies")
S22 (MM "Placebos")
S21 (MM "Double‐Blind Studies") or (MM "Single‐Blind Studies") or (MM "Triple‐Blind Studies")
S20 (MM "Random Assignment") or (MH "Clinical Trials+")
S19 S15 and S18
S18 S16 or S17
S17 TX mechanical and ventilat*
S16 (MH "Respiration, Artificial+")
S15 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14
S14 TX aerosol and bronchodilat*
S13 TX bronchodilator* and delivery
S12 AB nitric oxide or NO
S11 TX helium and oxygen*
S10 AB spacer*
S9 TX heated and humidific*
S8 AB bronchodilat* and therap*
S7 TX Nebuliser
S6 TX metered‐dose inhaler*
S5 (MH "Nitric Oxide")
S4 (MH "Drug Delivery Systems+")
S3 (MM "Administration, Inhalation")
S2 (MH "Bronchodilator Agents+")
S1 (MM "Nebulizers and Vaporizers")
Appendix 5. Study quality assessment and data extraction form
| Study ID | Report ID | Review author name |
|
|
|
|
| First author | Full reference |
|
|
|
Study eligibility
| Type of study Is the study described as randomized? |
| Yes | Unclear | No |
|
|
| Next question | Next question | Exclude |
| Participants Were the participants mechanically ventilated and: ‐ defined as adult by trialists OR ‐ NOT identified as paediatric |
| Yes | Unclear | No |
|
|
| Next question | Next question | Exclude |
| Interventions Did the study contain at least two interventions, comparing any model of nebulizer to MDI for aerosol bronchodilation? |
| Yes | Unclear | No |
|
|
| Next question | Next question | Exclude |
| Was the difference in bronchodilator delivery device the only planned difference between the comparison interventions? |
| Yes | Unclear | No |
|
| Next question | Next question | Exclude | |
| Were the same bronchodilatory agents used in all comparison groups? |
| Yes | Unclear | No |
|
|
| Next question | Next question | Exclude |
| Were only bronchodilators delivered during the trial? (i.e. no other drug groups/agents mixed in with bronchodilator agent/s) |
| Yes | Unclear | No |
|
|
| Next question | Next question | Exclude |
| Was there any combination administration of bronchodilators of differing drug groups? |
| Yes | Unclear | No |
|
|
| Exclude | Next question | Next question |
| Outcomes Did the study record airway responses∗? |
| Yes | Unclear | No |
|
|
|
Include
| Include (subject to clarification of “unclear” points) | Exclude |
| Final decision |
| Include
| Unclear
| Exclude |
| If the study is to be excluded, record the reason and details to add to “Table of excluded studies”:
|
General information
| Authors | |
| Contact address |
|
| Country of study |
|
| Language of publication |
|
Any other published versions/reports of this trial?
All references to a trial need to be linked under one Study ID both on this form (p1) and in RevMan.
| Code | Authors | Full reference | Linked Study ID on p1? (tick) | Linked Study ID in RevMan? (tick) |
| A |
|
|
|
|
| B |
|
|
|
|
| C |
|
|
|
|
Add other additional lines/codes as required
Trial characteristics – Risk of bias assessment
| Sequence generation Was the allocation sequence adequately generated? | |
|
| Give text which enabled your decision, including page no: |
| “YES” if used: · Random number table · Computer random number generator · Coin tossing · Shuffling cards/envelopes · Throwing dice · Minimization |
|
| “No” if used non‐random method such as: · Odd / even D.O.B · Date of admission · Hospital/clinic number · Clinician judgement · Participant preference · Lab test results · Availability of intervention |
|
| “Unclear” if there is insufficient information to permit “Yes” or “No” judgement. |
|
|
|
|
| Allocation concealment Was the allocation adequately concealed?(i.e. participants/investigators enrolling participants could not foresee assignment) | |
|
| Give text which enabled your decision, including page no: |
| “YES” if used: · Central allocation · Sequentially numbered containers of identical appearance · Sequentially numbered opaque, sealed envelopes · Or equivalent method |
|
| “No” if investigators could potentially foresee allocation such as: · Open random allocation scheme e.g. random list · Envelopes without safeguards e.g. unsealed, non opaque · Alteration / rotation · Date of birth · Case record number · Other unconcealed procedure |
|
| “Unclear” if there is insufficient information to permit “Yes” or “No” judgement. |
|
| Blinding of participants, personnel and outcome assessors Was knowledge of allocated intervention adequately prevented during study? Note: Blinding of personnel not possible with current review, but consider if a lack of blinding has potentially influenced results | |
|
| Give text which enabled your decision, including page no: |
| “YES” if: · No blinding, but unlikely to influence results · Outcome assessment blinded |
|
| “No” if: · No blinding and is likely to influence result · Non‐blinding is likely to have introduced bias |
|
| “Unclear” if there is insufficient information to permit “Yes” or “No” judgement, OR study did not address this outcome |
|
| Incomplete outcome data Were incomplete outcome data adequately addressed? | |
|
| Give text which enabled your decision, including page no: |
| “YES” if missing data: · Complete ‐ none missing · Unlikely to be related to true outcome · Is balances across groups · Effect size not enough to have clinical relevance impact on observed effect size · Have been imputed appropriately |
|
| “No” if missing data: · Likely to be related to true outcome · Effect size enough to have clinical relevance impact on observed effect size · “as treated” analysis done with very different numbers than at outset · potentially inappropriate data imputation |
|
| “Unclear” if there is insufficient information to permit “Yes” or “No” judgement OR study did not address this outcome |
|
| Selective outcome reporting Are study reports free of selective outcome reporting? | |
|
| Give text which enabled your decision, including page no: |
| “YES” if: · Protocol available and pre‐set outcomes are reported in pre‐set way · No protocol, but clear published reports of all expected outcomes, including pre‐set ones |
|
| “No” if: · Not all pre‐set outcomes reported · 1/1+ of primary outcomes reported in different methods, units, subsets of participants to protocol · 1/1+ primary outcomes not pre‐set · 1/1+ outcomes reported incompletely · Report does not include key outcome which would be expected |
|
| “Unclear” if there is insufficient information to permit “Yes” or “No” judgement. |
|
|
|
|
| Other potential threats to validity Was the study free of anything else which may put it at risk of bias? | |
|
| Give text which enabled your decision, including page no: |
| “YES” if: · Appears free from other sources |
|
| “No” if other potential source of bias e.g.: · Study design · Stopped early · Extreme baseline imbalance · Claims to be fraudulent · Other problem |
|
| “Unclear” if there is insufficient information to permit “Yes” or “No” judgement. |
|
|
|
|
| Cross – over trials Consider these potential sources of bias if the study is a cross‐over design | |
|
| Give text which enabled your decision, including page no: |
| Was the design appropriate? |
|
| Order of receiving treatments randomized? |
|
| Not biased from carry‐over effects? |
|
| Unbiased data available? |
|
Trial characteristics
| Participants | |
| Age (mean, median, range)
|
|
| Sex (numbers/%)
|
|
| Any other ventilation/bronchodilation strategies? e.g.: · Heated humidification · Use of spacer devices · Helium oxygen mixtures · Nitric oxide mixtures |
|
| Pre‐existing lung pathology? e.g.: · COPD · Asthma |
|
| Other Include sources of funding, conflicts of interest and any unexpected findings
| |
Data extraction
| Outcomes | |
|
| Reported in study? |
| Airway response: |
|
| Airway resistance (Rrs min, Rrs max, ΔRrs) | Yes / No |
| Patient outcome: |
|
| Mortality | Yes / No |
| Duration of mechanical ventilation | Yes / No |
| Adverse changes to haemodynamic observations | Yes / No |
| Reduction in wheezing | Yes / No |
| Freedom from contamination | Yes / No |
| Practitioner satisfaction | Yes / No |
| Associated cost | Yes / No |
| Quality of life measures | Yes / No |
| Continuous Outcomes ‐ RCTs | ||||||
|
| Unit of measurement | Intervention | Control | Details if outcomes are only described | ||
|
|
| n | Mean (SD) | n | Mean (SD) |
|
| Airway resistance | ΔRrs |
|
|
|
|
|
|
| Rrs max |
|
|
|
|
|
|
| Rrs min |
|
|
|
|
|
| Duration of mechanical ventilation |
|
|
|
|
|
|
| Practitioner satisfaction |
|
|
|
|
|
|
| Continuous Outcomes – Cross over trials | ||||||||||
|
| Unit of measurement | Intervention | Control | Cross over trial data Record all that is available in the paper Note – it is the within patient differences that you need the SD, standard error and CI for | ||||||
|
|
| n | Mean (SD) | n | Mean (SD) | SD | Standard error | CI | t | P value |
| Airway resistance | ΔRrs |
|
|
|
|
|
|
|
|
|
| Rint | ||||||||||
|
| Rrs max |
|
|
|
|
|
|
|
|
|
|
| Rrs min |
|
|
|
|
|
|
|
|
|
| Duration of mechanical ventilation |
|
|
|
|
|
|
|
|
|
|
| Practitioner satisfaction |
|
|
|
|
|
|
|
|
|
|
| Dichotomous Outcomes | ||
|
| Intervention (n) Note: n = number of participants, NOT number of events | Control (n) Note: n = number of participants, NOT number of events |
| Mortality – during critical care unit admission |
|
|
| Adverse changes to haemodynamic observations |
|
|
| Reduction in wheezing |
|
|
| Freedom from contamination |
|
|
| Any other relevant information about results e.g. if data was obtained from the trialists, if results were estimated from graphs or are calculated by you (if so, state formula and calculations) |
|
|
| Freehand space for actions Please document any contact with study authors and changes here | |
|
| |
| Trial characteristics | |
| Single/multicentre? |
|
| Country/countries |
|
| Definition used of participant eligibility |
|
| How many people randomized? |
|
| Number of participants in each intervention group |
|
| Make and model of ventilator used |
|
| Ventilator settings used |
|
| Number of participants who received intended treatment |
|
| Number of participants who were analysed |
|
| Bronchodilator and make and model of each device used |
|
| Dose and frequency of administration |
|
| Detail administration process e.g. use of spacer device, position of nebulizer/MDI in circuit, patient positioning etc for each intervention |
|
| Duration of treatment |
|
| How was the decision to withdraw mechanical ventilation made? (i.e. protocol, clinical judgement or a combination) |
|
| Length of follow up reported for patient outcome |
|
| Time points when measurements were taken during the study |
|
| Time points reported |
|
| Time points you are using in RevMan |
|
| Any additional information | |
∗ measures to include airway resistance (Rrs min, Rrs max, ΔRrs, Rint) Remember – we are looking for recording of these outcomes; not reporting.
Appendix 6. Summary of primary outcome measures
| Outcome measure: Reduction in ΔRrs (H2O l‐1s) | ||||
| MDI | Nebulizer | |||
| Pre‐treatment | Post‐treatment | Pre‐treatment | Post‐treatment | |
| (n = 18) ± SEM | 11.46 ± 1.04 | 10.79 ± 0.88 | 12.80 ± 1.59 | 10.79 ± 1.11 |
| Not significant | P <0.01 | |||
| Outcome measure: Reduction in Rint (H20 l‐1 s) | ||||
| MDI | Nebulizer | |||
| Pre‐treatment | Post‐treatment | Pre‐treatment | Post‐treatment | |
| (n = 18) ± SEM | 5.03 ± 0.81 | 4.10 ± 0.60 | 5.23 ± 0.82 | 4.36 ± 0.62 |
| P <0.05 | Not significant | |||
| Outcome measure: Reduction in Rrs or Rint (cm H20) | ||||
| MDI | Nebulizer | |||
| Pre‐treatment | Post‐treatment | Pre‐treatment | Post‐treatment | |
| (n = 10) ± SEM | 18.9 ± 2.6 | 19.6 ± 4.7 (estimate from a published figure) | 21.5 ± 5.7 | 17.6 ± 5.4 |
| Not significant | P <0.01 | |||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
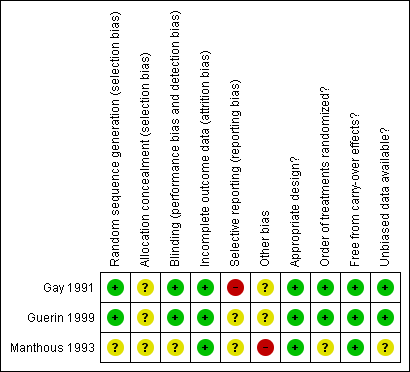
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Metered dose inhalers compared with nebulizers for aerosol bronchodilator delivery in mechanically ventilated adults | |||
| Patient or population: mechanically ventilated adults with need for aerosol bronchodilator therapy Settings: critical care units Intervention: metered dose inhalers Comparison: nebulizers | |||
| Outcomes | No of Participants | Quality of the evidence | Impact |
| Reduction in airway resistance Measured as a reduction in additional effective resistance (ΔRrs) and interrupter resistance (Rint) Assessed before treatment and 30 minutes after the end of each modality of administration | 28 | ⊕⊕⊕⊝1 | Both studies achieved a greater decrease in airway resistance using nebulizer |
| Mortality during critical care unit admission Measured using mortality rate in intervention and comparison groups During critical care admission | No studies found | N/A | |
| Duration of mechanical ventilation Measured as number of days | No studies found | N/A | |
| Adverse changes to haemodynamic observations Measured as a change in heart rate (beats per minute) Assessed before treatment and 30 minutes after the end of each modality of administration | 28 | ⊕⊕⊕⊝2 | Neither mode of delivery altered heart rate |
| GRADE Working Group grades of evidence | |||
| 1Downgraded for relatively few patients and events 2Downgraded for some selective outcome reporting in one study | |||

