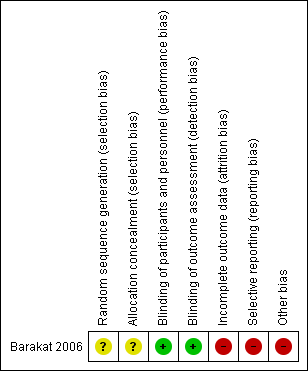
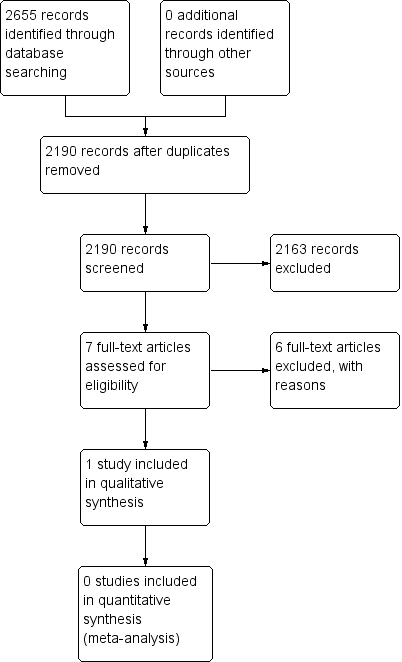
Contenido relacionado
Revisiones y protocolos relacionados
Alkistis Skalkidou, Theodoros N Sergentanis, Spyros P Gialamas, Marios K Georgakis, Theodora Psaltopoulou, Marialena Trivella, Charalampos S Siristatidis, Evangelos Evangelou, Eleni Petridou | 25 marzo 2017
Yunhai Chuaia, Ivana Rizzutoa, Xia Zhanga, Ying Li, Guanghai Dai, Sophie J Otter, Rasiah Bharathan, Alexandra Stewart, Aiming Wang | 4 marzo 2021
Kezia Gaitskell, Ewelina Rogozińska, Sarah Platt, Yifan Chen, Mohamed Abd El Aziz, Abigail Tattersall, Jo Morrison | 18 abril 2023
Khadra Galaal, Hannah Donkers, Andrew Bryant, Alberto D Lopes | 31 octubre 2018
Nungrutai Saeaib, Krantarat Peeyananjarassri, Tippawan Liabsuetrakul, Rakchai Buhachat, Eva Myriokefalitaki | 28 enero 2020
Jo Morrison, Clemens Thoma, Richard J Goodall, Thomas J Lyons, Kezia Gaitskell, Alison J Wiggans, Andrew Bryant | 15 octubre 2018
Jonathan A Frost, Katie E Webster, Andrew Bryant, Jo Morrison | 2 octubre 2017
Linda Rogers, Shing Shun N Siu, David Luesley, Andrew Bryant, Heather O Dickinson | 16 mayo 2012
Claire L Vale, Jayne Tierney, Sarah J Bull, Paul R Symonds | 15 agosto 2012
Chumnan Kietpeerakool, Apiwat Aue‐aungkul, Khadra Galaal, Chetta Ngamjarus, Pisake Lumbiganon | 12 febrero 2019