Terapia de reemplazo hormonal en pacientes con tratamiento previo para el cáncer endometrial
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008830.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer ginecológico, neurooncología y otros cánceres
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
KE was responsible for rewriting the protocol, developing the search strategy, extracting and analysing data, and developing and editing the review.
SR was responsible for data extraction and analysis and writing and editing the results and findings.
MH as an expert in menopause reviewed and edited the review as appropriate and assisted with data analysis.
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
KE: none known.
SR: none known.
MH: none known.
Acknowledgements
The authors thank Jo Morrison (Co‐ordinating Editor), Clare Jess (Managing Editor) and Jo Platt (Information Specialist) from Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers. We acknowledge Jing Fu, Xue Peng and Lina Hu who wrote the original draft of the protocol.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
We would like to thank the referees for many helpful suggestions and comments, some of these include Fani Kokka, Khadra Galaal, Ben Carter and Katharine Tylko‐Hill.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 May 15 | Hormone replacement therapy for women previously treated for endometrial cancer | Review | Katharine A Edey, Stuart Rundle, Martha Hickey | |
| 2016 Jul 26 | Hormone replacement therapy for women previously treated for endometrial cancer | Protocol | Katharine A Edey, Stuart Rundle, Martha Hickey | |
| 2010 Nov 10 | Hormone replacement therapy for women previously treated for endometrial cancer | Protocol | Xue Peng, Jing Fu, Hu Lina | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Breast Neoplasms [epidemiology];
- Early Termination of Clinical Trials;
- Endometrial Neoplasms [*chemically induced, epidemiology, surgery];
- Estrogen Replacement Therapy [*adverse effects];
- Neoplasm Recurrence, Local [*chemically induced, epidemiology];
- Neoplasms, Second Primary [epidemiology];
- Quality of Life;
Medical Subject Headings Check Words
Female; Humans;
PICO

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
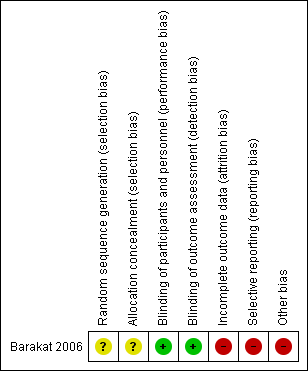
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
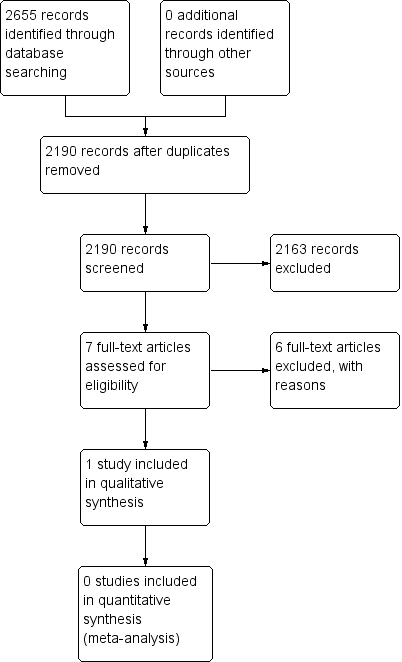
Study flow diagram.
| Oestrogen replacement therapy compared to placebo for women previously treated for endometrial cancer | ||||||
| Patient or population: women previously treated for endometrial cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty (quality) of the evidence | Comments | |
| Risk with placebo | Risk with oestrogen replacement therapy | |||||
| Rate of symptom relief | — | — | — | — | — | — |
| Rate of tumour recurrence | Study population | RR 1.17 (0.54 to 2.50) | 1236 | ⊕⊝⊝⊝ | — | |
| 19 per 1000 | 14 per 1000 | |||||
| Rate of appearance of a new malignancy | Study population | RR 0.80 (0.32 to 2.01) | 1236 | ⊕⊝⊝⊝ | — | |
| 16 per 1000 | 13 per 1000 | |||||
| Rate of survival: overall survival | — | — | — | — | — | The single study did not report overall survival of control and intervention groups individually, though it did report the percentage of participants alive at the end of follow‐up (median follow‐up: 35.7 months; 94.3% in the HRT group and 95.6% in the placebo group). |
| Rate of survival: progression‐free survival | — | — | — | — | — | The study did not report progression‐free survival of control and intervention groups individually, though it did report the percentage of participants alive, with no evidence of disease at the end of follow‐up (median follow‐up: 35.7 months; 95.8% in the HRT group and 96.9% in the placebo group). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level as the single RCT was closed prior to achieving its accrual goal. This was a serious departure from the study design and a serious risk of bias. 2Downgraded one level as there were insufficient data with respect to allocation concealment and description of the intervention, along with a significant risk of attrition bias. 3Downgraded one level for imprecision, as the single included study was underpowered to detect significant differences in the primary outcomes. | ||||||

