Acetaminofeno (paracetamol) para el resfriado común en adultos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial | |
| Participants | Setting: out‐patient, conducted in Ukraine and Russia Study period: 6 hours Age: mean age 37.4 years (range 18 to 65) Sex: male/female: 201/191 Diagnostic criteria: participants with an oral temperature between 38.5 and 40°C and other symptoms of URTI which have lasted for no more than 5 days Inclusion criteria: participants between 18 and 65 years with an acute, uncomplicated, febrile URTI of suspected viral origin Exclusion criteria: bacterial infection of the respiratory tract; current antibiotic treatment or taken the previous week; asthma or hypersensitivity to acetaminophen and aspirin; peptic ulceration; gastric bleeding, haemorrhagic diathesis, hepatic and/or renal dysfunction, Gilbert's disease; patients have participated in other studies during the previous 4 weeks | |
| Interventions | Participants received a single dose of aspirin 500 or 1000 mg, acetaminophen 500 or 1000 mg, or placebo | |
| Outcomes | Primary outcome: the AUC for the change in orally measured body temperature from baseline to 4 hours after dosing Secondary outcomes: the maximum temperature difference between baseline and the lowest measured body temperature, the time to the maximum temperature difference, the temperature difference between baseline and each measured time point after dosing, the intensity of the URTI symptoms (from 0 = none to 10 = severe) at baseline and again at 2, 4 and 6 hours after treatment, adverse events | |
| Notes | The researchers conducted the study on behalf of Bayer Company and received remuneration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data |
| Blinding (performance bias and detection bias) | Low risk | Identical colour, size and shape of tablet blinded to participants and investigators or study nurses |
| Incomplete outcome data (attrition bias) | Low risk | 6 excluded for incomplete data for the primary endpoint at 4 hours after dosing, ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Details not reported |
| Other bias | Unclear risk | The researchers conducted the study on behalf of Bayer Company and received remuneration for participation |
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | Age: mean age: 20.1 years (range 18 to 30) Sex: male/female: 34/26 Diagnostic criteria: if 2 of the 3 following criteria were met: a total symptom score ≥ 6 above the baseline level (day 0); increased nasal discharge for 3 consecutive days; and the subjective impression that after the first 6 days after the challenge that he or she had a common cold, similar to a previous naturally acquired illness Inclusion criteria: healthy young adult volunteers free of URTI (runny or blocked noses, sore throat or cough for 2 days) for 2 weeks before virus challenge Exclusion criteria: took aspirin, acetaminophen, ibuprofen or other related drugs 2 weeks before the virus challenge Conducted in Australia Aetiology: experimental cold | |
| Interventions | After intranasal challenge with RV2, the volunteers received medication on the first day of upper respiratory symptoms or on day 3 if no symptoms had developed. Medications were 4 g of aspirin (4 doses of 2 capsules, 500 mg per capsule), 4 g of acetaminophen (4 doses of 2 capsules, 500 mg per capsule), 1.2 g of ibuprofen (3 doses of 2 capsules and 1 dose of 2 placebo capsules, 200 mg per capsule), or placebo for 7 days | |
| Outcomes | Primary outcome: the common cold symptom scores (0 to 3) for day 0 to 14 Secondary outcome: side effect symptom scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sixty healthy volunteers were randomised within three strata based on their initial serum antibody titer to RV2" Common: insufficient information to judge |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data |
| Blinding (performance bias and detection bias) | Low risk | Identical capsules |
| Incomplete outcome data (attrition bias) | Low risk | 4 uninfected participants were excluded from further analysis. ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data |
| Other bias | Unclear risk | Insufficient data |
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | Setting: out‐patient, conducted in USA Age: acetaminophen group range 17 to 62; fenoprofen group: range 18 to 60; placebo group: range 24 to 60 Sex: male/female: 25/71 Diagnostic criteria: participants with systemic symptoms of malaise/aches judged to produce moderate pain and an oral temperature of at least 100 Inclusion criteria: the criteria for subject enrolment include systemic symptoms of malaise/aches judged to produce moderate pain and an oral temperature of at least 100 Exclusion criteria: pregnant or had language or intellectual barriers; clinical symptoms indicative of bacterial infections such as pneumonia, meningitis, streptococcal pharyngitis or tonsillitis; blood dyscrasias, carcinoma, serious kidney, liver, cardiac, gastrointestinal or metabolic disease; recent major surgery, history of drug hypersensitivity to study drugs; anticoagulant therapy; had administered another analgesic‐antipyretic drug prior to study | |
| Interventions | Each treatment group administered a single oral dose: acetaminophen 650 mg, fenoprofen 200 mg or placebo | |
| Outcomes | Primary outcomes: rectal temperatures and intensity of pain on a 0 to 3 scale at zero time and 1, 2, 3, 4 hours following administration of drug Secondary outcome: adverse medication experiences during and at the end of the 4‐hour study interval | |
| Notes | Funding source: supported in part by the Dista Products Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was assigned a chronological study number. These study number had been previously assigned to one of three treatment groups via a computer‐generated random numbers tables" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Each dose of medication was dispensed in identically appearing capsules in a double‐blind (patient and assessors) method" Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data |
| Other bias | Unclear risk | Quote: "This research was supported in part by the Dista Products Company" Comment: it is unclear if the funding source did or did not have any role in the results |
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | Setting: Medical University, conducted in the United States Study period: 6 hours Age: acetaminophen group: mean age 27.6 years; placebo group: mean age 28.6 years, range 18 to 65 years Sex: female/male: 285/145 Inclusion criteria: participants had cold symptoms of 48 hours or less, and reported at least moderate symptom severity in response to the question, "Overall, how would you rate the severity of your sinus symptoms? Absent, mild, moderate, moderately severe, or severe." Exclusion criteria: pregnant, diastolic blood pressure greater than 90 mmHg, participants with illnesses might be exacerbated by sympathomimetic drugs or affect the assessment of common cold symptoms, receiving drugs that might interact with sympathomimetic drugs | |
| Interventions | Participants were assigned either 60 mg of pseudoephedrine plus 1000 mg of acetaminophen or placebo tablets. The second dose was self administered 6 hours after the first dose | |
| Outcomes | Primary outcome: common cold symptom scores (0 to 4) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data |
| Blinding (performance bias and detection bias) | Low risk | Identically appearing tablet |
| Incomplete outcome data (attrition bias) | Low risk | 18 participants did not complete the study. ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data |
| Other bias | Unclear risk | Insufficient data |
AUC = area under curve
ITT = intention‐to‐treat
RV2 = rhinovirus type 2
URTI = upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No placebo comparison | |
| No placebo comparison | |
| No placebo comparison |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | We cannot retrieve the abstract of the study |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
| Methods | Randomised, double‐blind, placebo‐controlled, multi‐centre, parallel design clinical trial |
| Participants | Eligible participants had to have at least moderate nasal congestion and a runny nose, at least mild cough and at least mild pain with one or more of the following: sore throat, sore chest, headache or body pain/aches |
| Interventions | A single night‐time dose of a syrup containing 15 mg dextromethorphan hydrobromide, 7.5 mg doxylamine succinate, 600 mg paracetamol and 8 mg ephedrine sulfate |
| Outcomes | The primary endpoint (composite of nasal congestion/runny nose/cough/pain relief scores 3 hours post‐dosing) |
| Notes |
| Methods | We cannot retrieve the abstract of the study |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
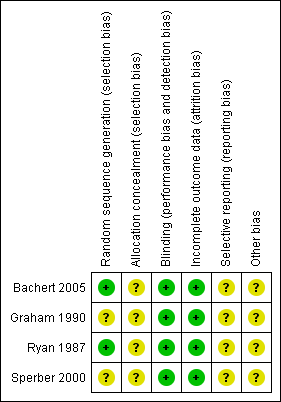
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

