Acetaminofeno (paracetamol) para el resfriado común en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008800.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 01 julio 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Siyuan Li (SL), Jirong Yue (JY), Bi Rong Dong (BD), Xiufang Lin (FL), Taixiang Wu (TW) and Ming Yang (MY) were all involved in developing the protocol.
SL, JY, BD, MY were involved in selection of studies, extracting data and writing up findings.
TW provided statistical expertise.
Sources of support
Internal sources
-
Chinese Cochrane Centre, China.
External sources
-
No sources of support supplied
Declarations of interest
None known.
Acknowledgements
We thank Elizabeth Dooley, Managing Editor of the Cochrane Acute Respiratory Infections Group. We thank the Chinese Cochrane Centre for help in completing the protocol. We also wish to thank the following people for commenting on the draft protocol: Chanpen Choprapawon, Ann Fonfa, Nicholas Moore, Andrew Pothecary, Mark Griffin and Hans van der Wouden; and we thank the following people for commenting on the draft review: Noha Usama, Nicholas Moore, Andrew Pothecary, Robert Ware and Hans van der Wouden.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Jul 01 | Acetaminophen (paracetamol) for the common cold in adults | Review | Siyuan Li, Jirong Yue, Bi Rong Dong, Ming Yang, Xiufang Lin, Taixiang Wu | |
| 2010 Nov 10 | Acetaminophen (paracetamol) for the common cold in adults | Protocol | Siyuan Li, Jirong Yue, Bi Rong Dong, Ming Yang, Xiufang Lin, Taixiang Wu | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
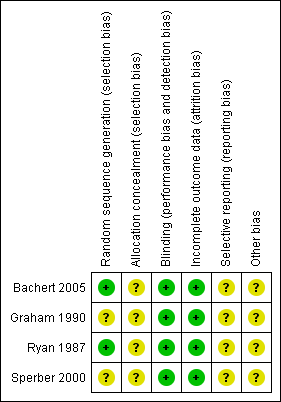
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

