Glucocorticosteroides para lactantes con atresia biliar posterior a la portoenterostomía de Kasai
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Multicentre, double‐blind trial comparing glucocorticosteroid administration versus placebo | |
| Participants | 140 infants (70 in the treatment group and 70 in the placebo group) from multiple US centres Age in months at surgery, mean (standard deviation) Treatment: 2.3 (0.9) Placebo: 2.3 (0.8) Percentage of infants with biliary atresia splenic malformation syndrome Treatment: 3% Placebo: 4% Bilirubin, mean (standard deviation (SD)) [original units] Treatment: 128µmol/L (44) [7.5 mg/dl (2.6)] Placebo: 135µmol/L (48) [7.9 mg/dl (2.8)] Other biochemical indicators of liver injury and synthetic function were balanced between the 2 groups (exact values not stated). | |
| Interventions | Starting the first day after Kasai portoenterostomy, trial participants received either intravenous methylprednisolone (4 mg/kg/day) or oral prednisolone (4 mg/kg/day) for the first 2 weeks, then oral prednisolone (2 mg/kg/day) for 2 weeks, followed by a tapering protocol for 9 weeks (n = 70) or placebo (n = 70) initiated within 72 hours of Kasai portoenterostomy. | |
| Outcomes | Primary outcomes:
Secondary outcomes:
| |
| Notes | Subgroup of infants operated on at age of less than 70 days was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomized with equal probability to a 13‐week course of steroid therapy or matching placebo... The data coordinating center generated treatment randomization codes with permutated block sizes of 4 (stratified by site) and provided the central pharmacy with a list of assignments for each study site." |
| Allocation concealment (selection bias) | Low risk | Quote: "participants were randomized with equal probability to a 13‐week course of steroid therapy or matching placebo." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Study medications were labelled and put into a kit by the central pharmacy and distributed to study site research pharmacists who were instructed to dispense the kits to participants enrolled sequentially." |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: the authors confirmed that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Overall 5 infants were lost to follow‐up in the treatment group (2 withdrew and 3 were lost for other reasons) and 8 in the placebo group (4 withdrew and 4 were lost for other reasons). Imputation was used to account for missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: authors reported on all outcomes in accordance with their methods, and a study protocol was available. Some of the outcomes from the study protocol were reported in other publications but these were not of interest to our review. |
| Other bias | Low risk | None identified |
| Methods | Double‐blind trial comparing glucocorticosteroid administration versus placebo, in two UK centres | |
| Participants | 73 infants from 2 UK centres 34 male, 39 female infants were included. Age in days at surgery, median (interquartile range) Treatment: 60 (50 to 71) Placebo: 54 (45 to 70) Preoperative bilirubin (μmol/L), median (interquartile range (IQR)) Treatment: 132 (112 to 166) Placebo: 158 (125 to 183) Preoperative AST (IU/L), median (IQR) Treatment: 163 (118 to 202) Placebo: 54 (99 to 220) Preoperative ΓGT (IU/L), median (IQR) Treatment: 420 (275 to 898) Placebo: 54 (304 to 992) | |
| Interventions | Participants received either oral prednisolone 2 mg/kg/day on days 7 to 21 following Kasai portoenterostomy, then 1 mg/kg/day on days 22 to 28 following Kasai portoenterostomy (n = 34) or placebo (n = 37). | |
| Outcomes | Primary outcomes:
Secondary outcomes:
| |
| Notes | Two‐year native liver survival was reported in a graph and the exact values were confirmed with the author. Subgroup of infants who were operated on by day 70 of life was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participating infants were randomized to receive either oral prednisolone or placebo" Comment: sequence generation was performed by a centralised agency within the pharmacy and was performed independently of the study investigators. |
| Allocation concealment (selection bias) | Low risk | Comment: medications or placebo were prepared and concealed but the respective pharmacies of the hospitals included in the study. Investigators were unable to identify if glucocorticosteroid or placebo was being given to any particular patient. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double blinded" Comment: methodology was clarified upon contacting author and investigators and clinical personnel were blinded as the administered medication was not identifiable as placebo or glucocorticosteroids by clinical staff or investigators. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: the authors confirmed that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no significant incomplete reporting was identified. Two infants excluded from the trial due to the finding that they had factors which excluded them from eligibility after already being enrolled. |
| Selective reporting (reporting bias) | Low risk | Comment: all predefined outcomes from the protocol were reported on. |
| Other bias | Low risk | None identified |
AST: aspartate aminotransferase
IU/L: international units per litre
ΓGT: γ‐glutamyl‐transpeptidase
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Non‐randomised trial comparing groups of different glucocorticosteroid doses | |
| Meta‐analysis with no data from any new infants | |
| Non‐randomised trial | |
| Non‐randomised trial | |
| Review article | |
| Non‐randomised trial comparing groups of different glucocorticosteroid doses | |
| Non‐randomised trial | |
| Non‐randomised trial comparing groups of different glucocorticosteroid doses | |
| Randomised trial comparing groups of different glucocorticosteroid doses with no placebo control group | |
| Non‐randomised trial | |
| Non‐randomised trial | |
| Non‐randomised trial | |
| Non‐randomised trial | |
| Non‐randomised trial | |
| Groups not separated into glucocorticosteroid and placebo but were pooled together for outcome reporting. | |
| Groups not separated into glucocorticosteroid and placebo but were pooled together for outcome reporting. | |
| Review article | |
| Non‐randomised trial | |
| Review article with some prospective data |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The effect of adjuvant steroid therapy post‐Kasai portoenterostomy in biliary atresia |
| Methods | Single‐centre open label randomised parallel controlled trial |
| Participants | Aims to recruit 100 infants in each group (glucocorticosteroid and control) |
| Interventions | Methylprednisolone, unspecified dose, duration or weaning regimen |
| Outcomes | Study ongoing, none reported |
| Starting date | 1 January 2015 |
| Contact information | Clinical lead: Sah Zheng Address: 399 Wanyuan Rd, Shanghai, China, 201102 |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.14, 6.90] |
| Analysis 1.1 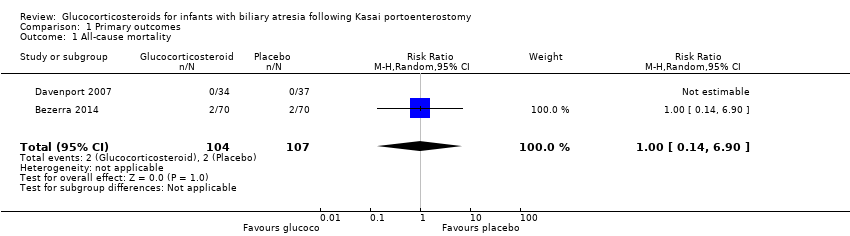 Comparison 1 Primary outcomes, Outcome 1 All‐cause mortality. | ||||
| 2 Serious adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.2 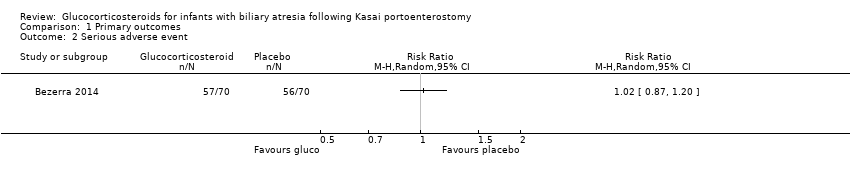 Comparison 1 Primary outcomes, Outcome 2 Serious adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infants who did not clear jaundice at six months Show forest plot | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.67, 1.17] |
| Analysis 2.1 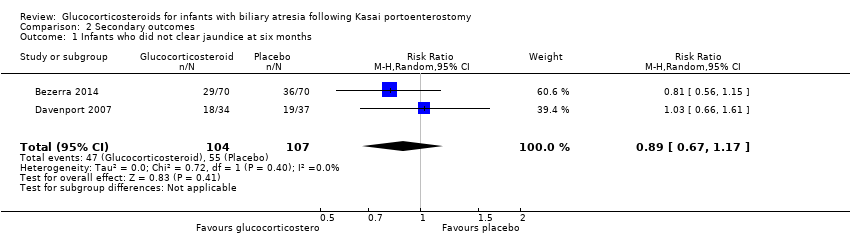 Comparison 2 Secondary outcomes, Outcome 1 Infants who did not clear jaundice at six months. | ||||
| 2 All‐cause mortality or liver transplantation at two years Show forest plot | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| Analysis 2.2  Comparison 2 Secondary outcomes, Outcome 2 All‐cause mortality or liver transplantation at two years. | ||||
| 3 Non‐serious adverse events | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Subgroup analysis of infants operated on at age of < 70 days who did not clear their jaundice by six months post KPE Show forest plot | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.46, 1.29] |
| Analysis 2.4  Comparison 2 Secondary outcomes, Outcome 4 Subgroup analysis of infants operated on at age of < 70 days who did not clear their jaundice by six months post KPE. | ||||
| 5 Subgroup analysis of infants operated on at age of > 69 days who did not clear their jaundice by six months post KPE Show forest plot | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.74, 1.62] |
| Analysis 2.5  Comparison 2 Secondary outcomes, Outcome 5 Subgroup analysis of infants operated on at age of > 69 days who did not clear their jaundice by six months post KPE. | ||||
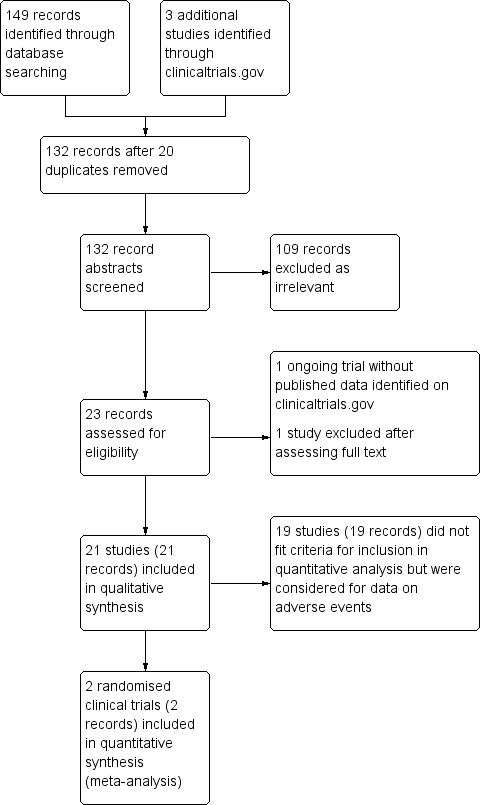
Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial Sequential Analysis of no clearance of jaundice by six months post Kasai portoenterostomy
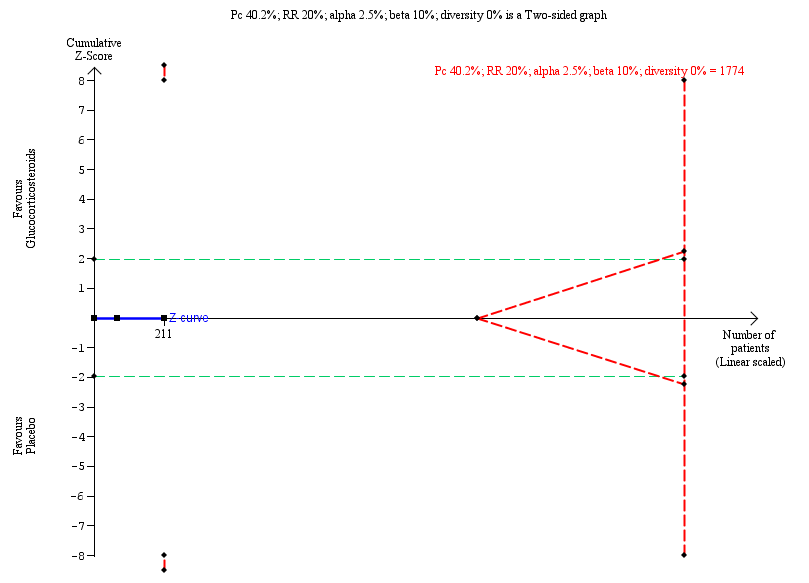
Trial Sequentil Analysis for all‐cause mortality or liver transplantation at two years
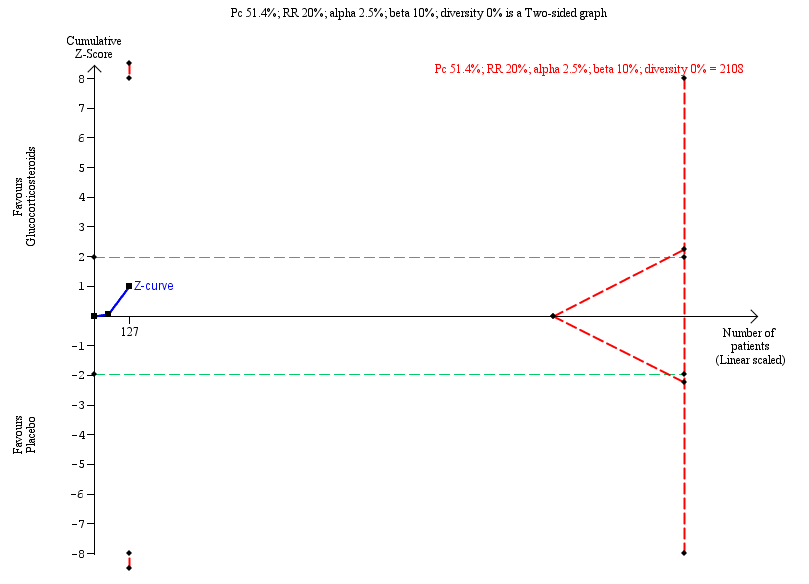
Trial Sequential Analysis of no clearance of jaundice at six months in the subgroup of infants who were less than 70 days old at the time of Kasai portoenterostomy

Comparison 1 Primary outcomes, Outcome 1 All‐cause mortality.

Comparison 1 Primary outcomes, Outcome 2 Serious adverse event.
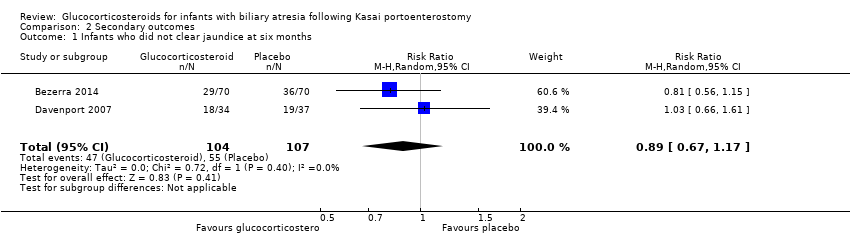
Comparison 2 Secondary outcomes, Outcome 1 Infants who did not clear jaundice at six months.
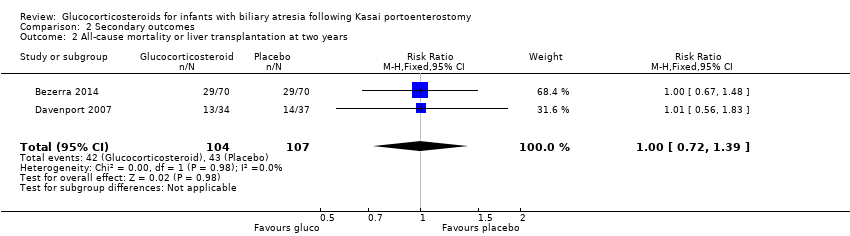
Comparison 2 Secondary outcomes, Outcome 2 All‐cause mortality or liver transplantation at two years.

Comparison 2 Secondary outcomes, Outcome 4 Subgroup analysis of infants operated on at age of < 70 days who did not clear their jaundice by six months post KPE.

Comparison 2 Secondary outcomes, Outcome 5 Subgroup analysis of infants operated on at age of > 69 days who did not clear their jaundice by six months post KPE.
| Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy | ||||||
| Patient or population: infants with biliary atresia Settings: hospitals Intervention: glucocorticosteroids Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Risk Ratio | Number (no) of infants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Glucocorticosteroids | |||||
| All‐cause mortality six months after Kasai portoenterostomy | 19 per 1000 | 19 per 1000 | 1.00 (0.14 to 6.90) | 104 placebo 107 treatment | ⊕⊕⊝⊝ | |
| Serious adverse event, two years follow‐up | 800 per 1000 | 814 per 1000 | 1.02 (0.87 to 1.20) | 70 placebo 70 treatment | ⊕⊕⊝⊝ | A significantly higher proportion of the treatment group had their first serious adverse event in the first 30 days after their Kasai portoenterostomy. |
| Health‐related quality of life | There are no data for this outcome in the included trials. | |||||
| Infants who did not clear jaundice at six months | 514 per 1000 | 452 per 1000 | 0.89 (0.67 to 1.17) | 107 placebo 104 treatment | ⊕⊕⊝⊝ | The required information size for significance for the Trial Sequential Analysis was 540. The number of infants included in this meta‐analysis was 211, corresponding to 39.1% of the required information size. |
| All‐cause mortality or liver transplantation at two years | 402 per 1000 | 404 per 1000 | 1.00 (0.72 to 1.39) | 107 placebo 104 treatment | ⊕⊕⊝⊝ | The required information size for significance for the Trial Sequential Analysis was 1774. The number of infants included in this meta‐analysis was 211, corresponding to 11.9% of the required information size. |
| Subgroup analysis of infants operated on at less than 70 days of age who did not clear their jaundice by six months after Kasai portoenterostomy | 516 per 1000 | 381 per 1000 | 0.75 (0.55 to 1.11) | 64 placebo 63 treatment | ⊕⊕⊝⊝ | The required information size for significance for the Trial Sequential Analysis was 538. The number of infants included in this meta‐analysis was 127, corresponding to 23.6% of the required information size. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence
| ||||||
| 1 Downgraded two levels due to imprecision of the evidence: Trial Sequential Analysis determined that the sample size was insufficient to detect a difference between the two groups. 2 Downgraded one level due to imprecision of the evidence and another level due to inconsistency of the evidence: there was heterogeneity between the trials and there were inconsistent assessments of what constituted a significant adverse event. Trial Sequential Analysis determined that the sample size was insufficient to detect a difference between the two groups. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.14, 6.90] |
| 2 Serious adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infants who did not clear jaundice at six months Show forest plot | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.67, 1.17] |
| 2 All‐cause mortality or liver transplantation at two years Show forest plot | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 3 Non‐serious adverse events | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Subgroup analysis of infants operated on at age of < 70 days who did not clear their jaundice by six months post KPE Show forest plot | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.46, 1.29] |
| 5 Subgroup analysis of infants operated on at age of > 69 days who did not clear their jaundice by six months post KPE Show forest plot | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.74, 1.62] |

