Glucocorticosteroides para lactantes con atresia biliar posterior a la portoenterostomía de Kasai
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008735.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Hepatobiliar
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
AT prepared the updated protocol part of the review and the review.
PC prepared the updated protocol part of the review and the review.
MD prepared the updated protocol part of the review and the review.
All authors approved the final review version.
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
Athanasios Tyraskis has nothing to declare.
Christopher Parsons has nothing to declare.
Mark Davenport is the author of a randomised controlled trial on the use of prednisolone after the Kasai portoenterostomy (Davenport 2007).
Acknowledgements
Peer reviewers: A Floreani, Italy; Masato Shinkai, Japan.
Contact editor: Luit Penninga, Denmark.
Sign‐off editor: Christian Gluud, Denmark.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: The views and opinions expressed in this protocol are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 May 14 | Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy | Review | Athanasios Tyraskis, Christopher Parsons, Mark Davenport | |
| 2016 Aug 11 | Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy | Protocol | Athanasios Tyraskis, Christopher Parsons, Mark Davenport | |
| 2010 Oct 06 | Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy | Protocol | Christopher Parsons, Mark Davenport | |
Differences between protocol and review
The protocol part of this review is a major update. The original protocol was first published in 2010 (Parsons 2010). The changes have been made to focus on the study outcomes as well as the methods of executing the study and analysing the data. Specifically, the primary and secondary outcomes have been rearranged with a focus on harm. Furthermore, we included significantly more detail regarding the statistical analysis and the methodology of Trial Sequential Analysis.
New lead review protocol author.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Biliary Atresia [*drug therapy, etiology, mortality];
- Glucocorticoids [*therapeutic use];
- Liver Transplantation [statistics & numerical data];
- Portoenterostomy, Hepatic [*adverse effects, methods, mortality];
- Postoperative Complications [*drug therapy, etiology, mortality];
- Quality of Life;
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Humans; Infant;
PICO
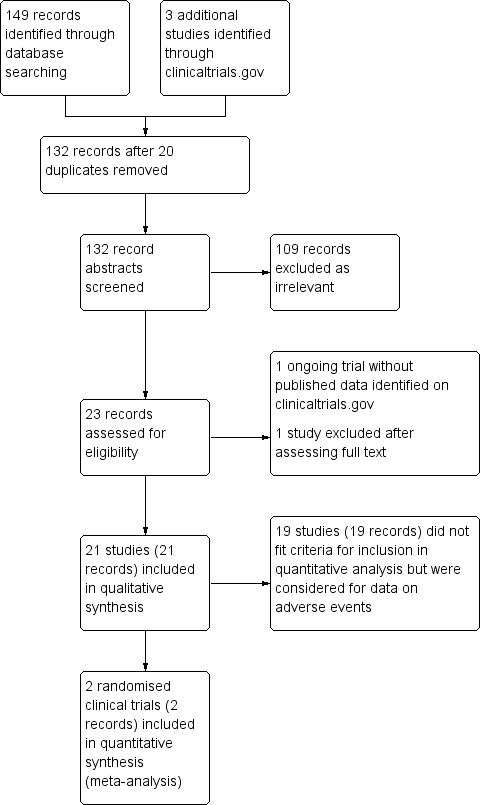
Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial Sequential Analysis of no clearance of jaundice by six months post Kasai portoenterostomy
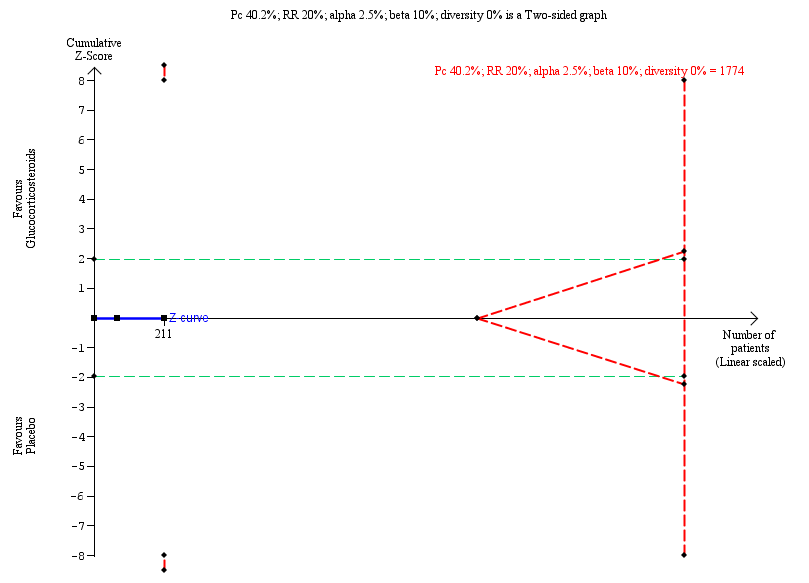
Trial Sequentil Analysis for all‐cause mortality or liver transplantation at two years
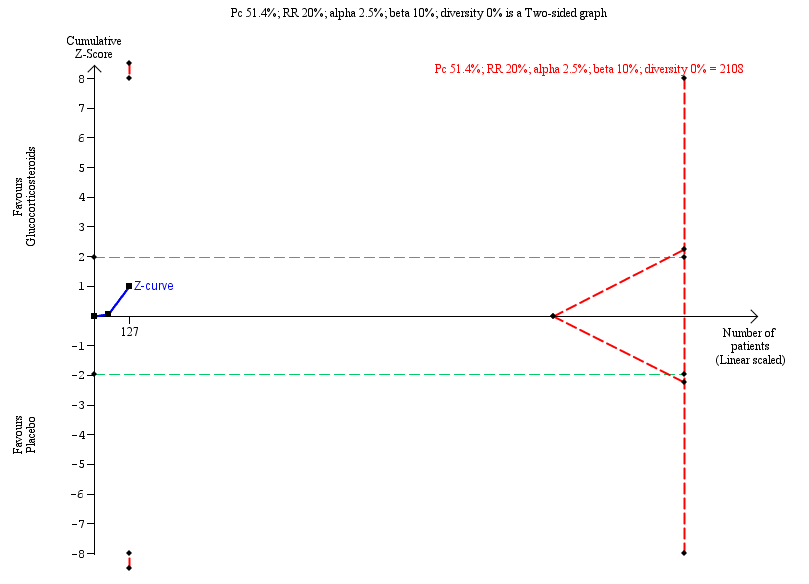
Trial Sequential Analysis of no clearance of jaundice at six months in the subgroup of infants who were less than 70 days old at the time of Kasai portoenterostomy

Comparison 1 Primary outcomes, Outcome 1 All‐cause mortality.

Comparison 1 Primary outcomes, Outcome 2 Serious adverse event.
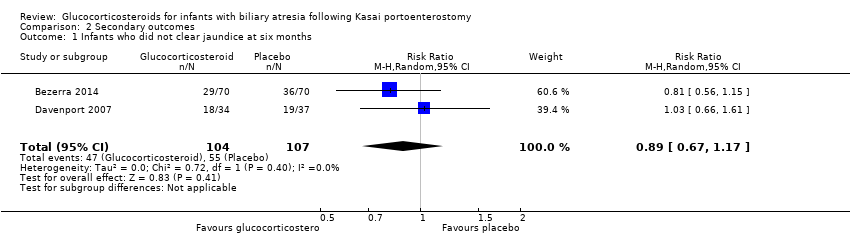
Comparison 2 Secondary outcomes, Outcome 1 Infants who did not clear jaundice at six months.
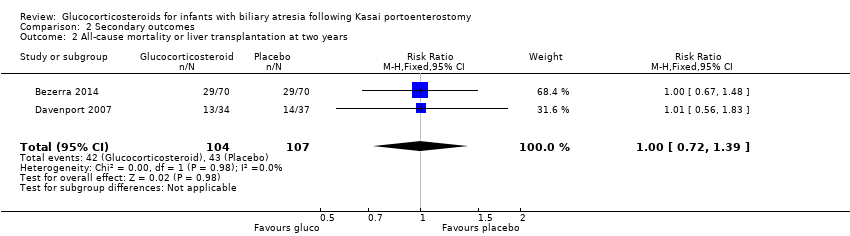
Comparison 2 Secondary outcomes, Outcome 2 All‐cause mortality or liver transplantation at two years.

Comparison 2 Secondary outcomes, Outcome 4 Subgroup analysis of infants operated on at age of < 70 days who did not clear their jaundice by six months post KPE.

Comparison 2 Secondary outcomes, Outcome 5 Subgroup analysis of infants operated on at age of > 69 days who did not clear their jaundice by six months post KPE.
| Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy | ||||||
| Patient or population: infants with biliary atresia Settings: hospitals Intervention: glucocorticosteroids Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Risk Ratio | Number (no) of infants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Glucocorticosteroids | |||||
| All‐cause mortality six months after Kasai portoenterostomy | 19 per 1000 | 19 per 1000 | 1.00 (0.14 to 6.90) | 104 placebo 107 treatment | ⊕⊕⊝⊝ | |
| Serious adverse event, two years follow‐up | 800 per 1000 | 814 per 1000 | 1.02 (0.87 to 1.20) | 70 placebo 70 treatment | ⊕⊕⊝⊝ | A significantly higher proportion of the treatment group had their first serious adverse event in the first 30 days after their Kasai portoenterostomy. |
| Health‐related quality of life | There are no data for this outcome in the included trials. | |||||
| Infants who did not clear jaundice at six months | 514 per 1000 | 452 per 1000 | 0.89 (0.67 to 1.17) | 107 placebo 104 treatment | ⊕⊕⊝⊝ | The required information size for significance for the Trial Sequential Analysis was 540. The number of infants included in this meta‐analysis was 211, corresponding to 39.1% of the required information size. |
| All‐cause mortality or liver transplantation at two years | 402 per 1000 | 404 per 1000 | 1.00 (0.72 to 1.39) | 107 placebo 104 treatment | ⊕⊕⊝⊝ | The required information size for significance for the Trial Sequential Analysis was 1774. The number of infants included in this meta‐analysis was 211, corresponding to 11.9% of the required information size. |
| Subgroup analysis of infants operated on at less than 70 days of age who did not clear their jaundice by six months after Kasai portoenterostomy | 516 per 1000 | 381 per 1000 | 0.75 (0.55 to 1.11) | 64 placebo 63 treatment | ⊕⊕⊝⊝ | The required information size for significance for the Trial Sequential Analysis was 538. The number of infants included in this meta‐analysis was 127, corresponding to 23.6% of the required information size. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence
| ||||||
| 1 Downgraded two levels due to imprecision of the evidence: Trial Sequential Analysis determined that the sample size was insufficient to detect a difference between the two groups. 2 Downgraded one level due to imprecision of the evidence and another level due to inconsistency of the evidence: there was heterogeneity between the trials and there were inconsistent assessments of what constituted a significant adverse event. Trial Sequential Analysis determined that the sample size was insufficient to detect a difference between the two groups. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.14, 6.90] |
| 2 Serious adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infants who did not clear jaundice at six months Show forest plot | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.67, 1.17] |
| 2 All‐cause mortality or liver transplantation at two years Show forest plot | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 3 Non‐serious adverse events | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Subgroup analysis of infants operated on at age of < 70 days who did not clear their jaundice by six months post KPE Show forest plot | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.46, 1.29] |
| 5 Subgroup analysis of infants operated on at age of > 69 days who did not clear their jaundice by six months post KPE Show forest plot | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.74, 1.62] |

