Auskultasi berselang‐seli (IA) kadar denyutan jantung janin semasa bersalin untuk kesejahteraan janin
Abstract
Background
The goal of fetal monitoring in labour is the early detection of a hypoxic baby. There are a variety of tools and methods available for intermittent auscultation (IA) of the fetal heart rate (FHR). Low‐ and middle‐income countries usually have only access to a Pinard/Laënnec or the use of a hand‐held Doppler device. Currently, there is no robust evidence to guide clinical practice on the most effective IA tool to use, timing intervals and length of listening to the fetal heart for women during established labour.
Objectives
To evaluate the effectiveness of different tools for IA of the fetal heart rate during labour including frequency and duration of auscultation.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (19 September 2016), contacted experts and searched reference lists of retrieved articles.
Selection criteria
All published and unpublished randomised controlled trials (RCTs) or cluster‐RCTs comparing different tools and methods used for intermittent fetal auscultation during labour for fetal and maternal well‐being. Quasi‐RCTs, and cross‐over designs were not eligible for inclusion.
Data collection and analysis
All review authors independently assessed eligibility, extracted data and assessed risk of bias for each trial. Data were checked for accuracy.
Main results
We included three studies (6241 women and 6241 babies), but only two studies are included in the meta‐analyses (3242 women and 3242 babies). Both were judged as high risk for performance bias due to the inability to blind the participants and healthcare providers to the interventions. Evidence was graded as moderate to very low quality; the main reasons for downgrading were study design limitations and imprecision of effect estimates.
Intermittent Electronic Fetal Monitoring (EFM) using Cardiotocography (CTG) with routine Pinard (one trial)
There was no clear difference between groups in low Apgar scores at five minutes (reported as < six at five minutes after birth) (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.24 to 1.83, 633 babies, very low‐quality evidence). There were no clear differences for perinatal mortality (RR 0.88, 95% CI 0.34 to 2.25; 633 infants, very low‐quality evidence). Neonatal seizures were reduced in the EFM group (RR 0.05, 95% CI 0.00 to 0.89; 633 infants, very low‐quality evidence). Other important infant outcomes were not reported: mortality or serious morbidity (composite outcome), cerebral palsy or neurosensory disability. For maternal outcomes, women allocated to intermittent electronic fetal monitoring (EFM) (CTG) had higher rates of caesarean section for fetal distress (RR 2.92, 95% CI 1.78 to 4.80, 633 women, moderate‐quality evidence) compared with women allocated to routine Pinard. There was no clear difference between groups in instrumental vaginal births (RR 1.46, 95% CI 0.86 to 2.49, low‐quality evidence). Other outcomes were not reported (maternal mortality, instrumental vaginal birth for fetal distress and or acidosis, analgesia in labour, mobility or restriction during labour, and postnatal depression).
Doppler ultrasonography with routine Pinard (two trials)
There was no clear difference between groups in Apgar scores < seven at five minutes after birth (reported as < six in one of the trials) (average RR 0.76, 95% CI 0.20 to 2.87; two trials, 2598 babies, I2 = 72%, very low‐quality evidence); there was high heterogeneity for this outcome. There was no clear difference between groups for perinatal mortality (RR 0.69, 95% CI 0.09 to 5.40; 2597 infants, two studies, very low‐quality evidence), or neonatal seizures (RR 0.05, 95% CI 0.00 to 0.91; 627 infants, one study, very low‐quality evidence). Other important infant outcomes were not reported (cord blood acidosis, composite of mortality and serious morbidity, cerebral palsy, neurosensory disability). Only one study reported maternal outcomes. Women allocated to Doppler ultrasonography had higher rates of caesarean section for fetal distress compared with those allocated to routine Pinard (RR 2.71, 95% CI 1.64 to 4.48, 627 women, moderate‐quality evidence). There was no clear difference in instrumental vaginal births between groups (RR 1.35, 95% CI 0.78 to 2.32, 627 women, low‐quality evidence). Other maternal outcomes were not reported.
Intensive Pinard versus routine Pinard (one trial)
One trial compared intensive Pinard (a research midwife following the protocol in a one‐to‐one care situation) with routine Pinard (as per protocol but midwife may be caring for more than one woman in labour). There was no clear difference between groups in low Apgar score (reported as < six this trial) (RR 0.90, 95% CI 0.35 to 2.31, 625 babies, very low‐quality evidence). There were also no clear differences identified for perinatal mortality (RR 0.56, 95% CI 0.19 to 1.67; 625 infants, very low‐quality evidence), or neonatal seizures (RR 0.68, 95% CI 0.24 to 1.88, 625 infants, very low‐quality evidence)). Other infant outcomes were not reported. For maternal outcomes, there were no clear differences between groups for caesarean section or instrumental delivery (RR 0.70, 95% CI 0.35 to 1.38, and RR 1.21, 95% CI 0.69 to 2.11, respectively, 625 women, both low‐quality evidence)) Other outcomes were not reported.
Authors' conclusions
Using a hand‐held (battery and wind‐up) Doppler and intermittent CTG with an abdominal transducer without paper tracing for IA in labour was associated with an increase in caesarean sections due to fetal distress. There was no clear difference in neonatal outcomes (low Apgar scores at five minutes after birth, neonatal seizures or perinatal mortality). Long‐term outcomes for the baby (including neurodevelopmental disability and cerebral palsy) were not reported. The quality of the evidence was assessed as moderate to very low and several important outcomes were not reported which means that uncertainty remains regarding the use of IA of FHR in labour.
As intermittent CTG and Doppler were associated with higher rates of caesarean sections compared with routine Pinard monitoring, women, health practitioners and policy makers need to consider these results in the absence of evidence of short‐ and long‐term benefits for the mother or baby.
Large high‐quality randomised trials, particularly in low‐income settings, are needed. Trials should assess both short‐ and long‐term health outcomes, comparing different monitoring tools and timing for IA.
PICO
Ringkasan bahasa mudah
Apakah cara yang paling berkesan untuk mendengar secara berselang‐seli jantung bayi semasa bersalin untuk meningkatkan kesejahteraan bayi?
Apakah isunya?
Salah satu kaedah memantau kesejahteraan bayi ialah mendengar degupan jantung janin dan coraknya secara berselang‐seli semasa bersalin (auskultasi berselang‐seli). Terdapat beberapa cara untuk mengukur kadar denyutan jantung bayi. Sesetengah alat untuk mendengar jantung bayi diperbuat daripada kayu, plastik atau aluminium (Pinard, Laennec dan fetoscope), dan terdapat juga alat elektronik dengan kecanggihan yang pelbagai, termasuk pegangan tangan (bateri atau dikendalikan kunci) Doppler ultrasound (Doppler) dan cardiotocogram (CTG), yang kadangkala dirujuk sebagai pemantauan janin elektronik (EFM).
Mengapakah hal ini penting?
Matlamat pemantauan adalah supaya bayi yang mengalami kesukaran dapat dikenal pasti dengan tepat dan intervensi (seperti pembedahan caesarean atau kelahiran melalui vagina) boleh digunakan untuk meningkatkan hasil bagi bayi.
Apakah bukti yang kami dapati?
Kami ingin melihat jenis alat mendengar dan masa untuk auskultasi berselang‐seli yang paling berkesan. Kami menganggap peranti mendengar pegangan tangan, contohnya Doppler pegangan tangan dan pelbagai stetoskop Pinard. Kami mencari kajian (19 September 2016) dan menemui tiga kajian rawak terkawal dari Afrika, melibatkan 6241 wanita dalam proses bersalin. Data daripada salah satu kajian adalah tidak konsisten dan kami tidak dapat memasukkannya ke dalam keputusan. Ini bermakna 3242 wanita dan bayi mereka telah dimasukkan dalam analisis. Keputusan kajian mungkin berat sebelah kerana tidak mungkin membutakan wanita dan kakitangan, dan secara keseluruhan kualiti bukti dinilai sebagai berkualiti sederhana hingga sangat rendah.
Satu kajian membandingkan EFM berselang‐seli dengan Pinard rutin dan tidak menunjukkan perbezaan yang jelas antara kumpulan dalam skor Apgar bayi yang rendah pada lima minit selepas kelahiran (bukti berkualiti sangat rendah) atau dalam kematian perinatal (bukti berkualiti rendah) walaupun sawan neonatal dikurangkan dalam kumpulan EFM (bukti berkualiti rendah). Hasil penting lain berkaitan bayi (seperti cerebral palsy) adalah tidak dilaporkan. Wanita yang mengalami EFM berselang‐seli mempunyai kadar pembedahan caesarean yang lebih tinggi untuk gangguan janin (bukti berkualiti sederhana), tetapi tidak terdapat perbezaan yang jelas antara kumpulan dalam kelahiran vagina instrumental (bukti berkualiti rendah). Hasil penting lain untuk wanita tidak dilaporkan (kematian ibu, analgesia semasa bersalin, mobiliti atau sekatan semasa bersalin, dan kemurungan selepas bersalin).
Dua kajian membandingkan ultrasonografi Doppler dengan Pinard rutin. Tiada perbezaan yang jelas antara kumpulan dalam skor Apgar rendah pada lima minit selepas kelahiran (sangatbukti berkualiti rendah) atau untuk kematian perinatal (sangatbukti berkualiti rendah), atau sawan neonatal (bukti kualiti sangat rendah). Hasil penting lain berkaitan bayi adalah tidak dilaporkan. Hanya satu kajian melaporkan hasil untuk wanita. Mereka yang menjalani ultrasonografi Doppler mempunyai kadar pembedahan caesarean yang lebih tinggi untuk gangguan janin berbanding dengan Pinard rutin (bukti berkualiti sederhana). Tiada perbezaan yang jelas dalam kelahiran vagina instrumental antara kumpulan (bukti berkualiti rendah). Hasil yang lain berkaitan ibu adalah tidak dilaporkan.
Satu kajian membandingkan Pinard intensif (bidan penyelidikan dalam situasi penjagaan satu sama satu) dengan Pinard rutin (bidan mungkin telah menjaga lebih daripada seorang wanita yang bersalin). Tiada perbezaan yang jelas antara kumpulan dalam skor Apgar rendah (bukti berkualiti sangat rendah), kematian perinatal (bukti berkualiti sangat rendah), atau sawan neonatal (bukti berkualiti sangat rendah)). Hasil lain berkaitan bayi adalah tidak dilaporkan. Bagi wanita, tiada perbezaan yang jelas antara kumpulan untuk pembedahan caesarean atau kelahiran instrumental (kedua‐dua bukti berkualiti rendah)) Hasil lain adalah tidak dilaporkan.
Apakah maksudnya?
Memandangkan EFM dan Doppler yang berselang‐seli dikaitkan dengan kadar pembedahan caesarean yang lebih tinggi berbanding dengan pemantauan rutin Pinard, wanita, pengamal kesihatan dan pembuat dasar perlu mempertimbangkan keputusan ini sekiranya tiada bukti manfaat jangka pendek dan panjang untuk ibu atau bayi.
Kajian berkualiti tinggi yang besar membandingkan alat pemantauan yang berbeza dan masa untuk auskultasi berselang‐seli adalah diperlukan. Kajian harus menilai hasil kesihatan jangka pendek dan jangka panjang, dan harus mengumpulkan maklumat tentang pandangan wanita.
Authors' conclusions
Summary of findings
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus routine Pinard (outcomes for the baby). | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 19 per 1000 | RR 0.66 | 633 | ⊕⊕⊝⊝ | Low event rate. Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 1 per 1000 | RR 0.05 | 633 | ⊕⊕⊝⊝ LOW 1,3 | Low event rates. Routine Pinard group (9/315) compared to the intermittent EFM (CTG) group (0/318). |
| Perinatal mortality | 29 per 1000 | 25 per 1000 | RR 0.88 | 633 | ⊕⊝⊝⊝ | Neonatal deaths included, unable to separate out from reported data. Low event rates 8/318 for intermittent EFM (CTG) group and 9/315 for routine Pinard group. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies at either 6 months or 1 year. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision; single trial with low event rate and wide 95% CI crossing the line of no effect. Downgraded two levels. 3 Evidence of imprecision, evidence based on a single trial with low event rates. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus Routine Pinard (outcomes for the mother). | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 176 per 1000 | RR 2.92 | 633 | ⊕⊕⊕⊝ MODERATE 1, | |
| Instrumental vaginal birth | 67 per 1000 | 97 per 1000 | RR 1.46 | 633 | ⊕⊕⊝⊝ LOW 1,2, | |
| Maternal mortality | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for any pharmacological or non‐ pharmacological analgesia use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision with wide confidence intervals. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Routine Pinard | Risk with Doppler | |||||
| Apgar < 7 at 5 minutes | 20 per 1000 | 15 per 1000 | RR 0.76 | 2598 | ⊕⊝⊝⊝ | One of the studies contributing data reported Apgar score < 6. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Seizures in the neonatal period | 29 per 1000 | 1 per 1000 | RR 0.05 | 627 | ⊕⊝⊝⊝ | Event rates are low 0/312 for Doppler and 9/315 for routine Pinard. |
| Perinatal mortality | 12 per 1000 | 8 per 1000 | RR 0.69 | 2597 | ⊕⊕⊝⊝ | Event rates 13/1304 for Doppler and 15/1293 for routine Pinard. Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision with wide 95% CI crossing the line of no effect. Downgraded one level. 3 There was high heterogeneity for this outcome. 4 Evidence of imprecision, with wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels. 5 There was high heterogeneity for this outcome. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Doppler | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 163 per 1000 | RR 2.71 | 627 | ⊕⊕⊕⊝ | |
| Instrumental vaginal birth | 67 per 1000 | 90 per 1000 | RR 1.35 | 627 | ⊕⊕⊝⊝ | |
| Maternal mortality | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for any pharmacological or non‐pharmacological use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Wide confidence interval. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 26 per 1000 | RR 0.90 | 625 | ⊕⊝⊝⊝ | Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 19 per 1000 | RR 0.68 | 625 | ⊕⊝⊝⊝ | |
| Perinatal mortality | 29 per 1000 | 16 per 1000 | RR 0.56 | 625 | ⊕⊝⊝⊝ | Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included trial. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded 1 level. 2 Evidence was imprecise; wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 42 per 1000 | RR 0.70 | 625 | ⊕⊕⊝⊝ | |
| Instrumental vaginal birth | 67 per 1000 | 81 per 1000 | RR 1.21 | 625 | ⊕⊕⊝⊝ | |
| Maternal morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal morbidity in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No reported data for any pharmacological or non‐pharmacological analgesia use excluding epidural. | |
| Epidural anaesthesia for pain relief excluding caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for post natal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Some imprecision with wide CI crossing the line of no effect. Downgraded one level. | ||||||
Background
Description of the condition
Auscultation of the fetal heart during labour became a universal 'standard of care' during the first half of the 20th century to monitor fetal well‐being (Schmidt 2000; Hindley 2005). The fetal heart rate (FHR) and patterns of the FHR are used in the assessment of fetal well‐being in an effort to identify those fetuses who might have, or be at risk of, developing hypoxaemia. There are currently two main methods of monitoring the FHR in labour: continuous and intermittent. Continuous FHR monitoring consists of the continuous and simultaneous monitoring of the FHR and maternal uterine contractions onto a paper tracing called a cardiotocograph (CTG). Intermittent auscultation (IA) involves listening to the fetal heart beat periodically with a Pinard or fetal stethoscope or hand‐held Doppler device and recording a single measure of the heart rate at that time. IA during labour can mean listening to the fetal heart for anywhere from five‐ to 30‐minute intervals before and/or during and/or immediately after a contraction.
In low‐ and middle‐income countries, IA is commonly practiced with a Pinard or fetal stethoscope (also known as an obstetric stethoscope or fetoscope, Pinard or Laënnec stethoscope), or sometimes with an ordinary stethoscope if no other auscultation tool is available. Hand‐held Doppler devices are also used to auscultate the fetal heart, particularly in high‐income countries. However, as with other electronic fetal monitoring (EFM) devices, such as CTG, the disadvantages of hand‐held Doppler devices are considerable for low‐ and middle‐income countries because of cost, access to reliable source of electricity or batteries, and maintenance issues (Irwig 1998; Arasaratnam 2013). Recently in Uganda, a prototype of a wind‐up Doppler ultrasound FHR monitor (or wind‐up fetal Doppler) has been tested by PowerFree Education Technology, a South Africa based not‐for‐profit organisation, in collaboration with Philips. A wind‐up fetal Doppler is not reliant on battery or electricity and may make this device more accessible to low‐ and middle‐income countries (PowerFree Education Technology 2014).
EFM devices, in particular CTG machines with or without access to fetal scalp blood and cord blood testing are not commonly available in low‐ and middle‐income countries. Continuous EFM use in labour, usually with a CTG, has been the subject of two other Cochrane reviews (Alfirevic 2013; Neilson 2015). The results from these reviews indicated that continuous CTG monitoring during labour is associated with a reduction in neonatal seizures, but there are no significant differences in cerebral palsy or infant mortality or other neonatal assessments (e.g. Apgar score). Continuous CTG was associated with an increase in caesarean sections and instrumental vaginal births. It is difficult how to interpret these results. With the limited evidence of the effectiveness of continuous CTG, there is also a renewed interest worldwide in IA during normal labour, not only for low‐ and middle‐income countries (Goodwin 2000, Albers 2001; Sholapurkar 2015). This review evaluates the effectiveness of different tools for intermittent ausculation of the FHR during labour including frequency and duration of auscultation.
Historical context
Fetal heart sounds were first described in the 17th century in poetry (Freeman 1991). Direct FHR auscultation for fetal well‐being has been practiced for many centuries, mainly with the practitioner's ear placed on the mother's abdomen (Gultekin‐Zootzmann 1975). Mayor, a Swiss surgeon, first reported direct FHR auscultation in 1818 (Solt 2005). The first medical texts to describe and discuss auscultation were written in 1819 by Laennec and in 1822 by Keregaredec (Solt 2005; Maude 2010), followed by DeLee‐Hills in 1922 (Chez 2000), and by Pinard in the late 1880s (Mainstone 2004). Their auscultation tools are currently still in use and have undergone little change since their early development.
Description of the intervention
IA is the auditory technique for sampling and counting the FHR at particular intervals with the human ear. It uses bone conduction to assist in hearing the opening and closing of the valves of the fetal heart and is often practised by listening and counting the fetal heart sounds through the mother's abdominal wall for one minute (ACNM 2007). The FHR is best heard over the fetal back/anterior shoulder and therefore it is good practice to perform auscultation of the FHR as part of a comprehensive abdominal examination, which should include inspection, palpation and auscultation (RANZCOG 2006; NCCWCH 2008; NICE 2014). Clinical practice guidelines vary in their recommendations of IA and with recommendations for duration of auscultation of the fetal heart rate ranging from 15 to 30 to 60 seconds, after a contraction, in the first stage of labour and from auscultating after every contraction to every five minutes in the second stage of labour (ACOG 1995; Liston 2002; NZCOM 2005; RANZCOG 2006; NICE 2014). The FHR is recorded as a single number.
In 1893, von Winckel, a German physician, identified clinical criteria for identification of the potentially compromised fetus, based on work also done by Schatz in 1885 and Kehrer in 1867 (Goodlin 1979). Von Winckel identified the normal range of FHR as 120 to 160 beats per minute (bpm) and identified fetal bradycardia as less than 120 bpm and fetal tachycardia greater than 160 bpm (Goodlin 1979). Seitz in 1903 (Gultekin‐Zootzmann 1975), and later Cox in 1950 (Schmidt 2000), identified further possible indicators of fetal distress by describing and differentiating between three types of FHR: early decelerations, late decelerations and decelerations during uterine contractions. Early decelerations refer to the transient decrease of the FHR with the onset of the deceleration at the onset of the contraction, and late decelerations of the transient decrease of the FHR beginning at or after the peak of the contraction phase. Accelerations of the FHR that are transient are regarded as a reassuring sign of fetal well‐being (Freeman 2012; Saxena 2013). It is recognised that during labour natural alterations of the FHR pattern can occur. For example, FHR patterns may differ depending on whether the fetus is undergoing a period of rest or activity or a period of stress induced by maternal uterine activity. Maternal opioids, in particular pethidine and meptazinol have been reported to impact on the FHR pattern causing fetal acidosis (Anderson 2011; Kranke 2013; Goodson 2014). Most clinical guidelines today indicate the normal baseline FHR range as 110 to 160 bpm (RANZCOG 2006; NICE 2014).
How the intervention might work
Auscultation tools have been used since the 1800s and how they work is described in detail below. These individual tools were introduced on the premise that they would facilitate early detection of abnormal FHR patterns thought to be associated with hypoxia and therefore identify the babies who may require more intensive heart rate monitoring through, for example, continuous CTG monitoring or require to be delivered, usually by emergency caesarean section. There are several auscultation tools in use including the following.
Laënnec
In 1818, Rene‐Theophile‐Hyacinthe Laënnec, a French physician, devised a straight wooden tube for listening to breathing sounds of the human chest through his ear after having observed two children playing with sticks listening to the ends of a long piece of wood that transmitted the sounds of pin scratches. He built a 25 cm by 2.5 cm hollow wooden 'stick'. This was the first mono‐aural device built and he called it the stethoscope (stetho (Greek), meaning chest) (Laënnec 1819). While it was initially used for listening to adult heart and chest sounds, JA Le Jumeau and Viscount de Kergaradec soon described auscultation of the fetal heart with a Laënnec stethoscope (Solt 2005). The Laënnec used in obstetrics today is still about 25 cm long with a 2.5 cm hollow diameter with a small flat bell curve opening at one end. The bell curve is placed onto the mother's abdomen and the end with the smaller diameter into the practitioner's ear (Chez 2000).
Pinard
In the late 1880s, A. Pinard, a French physician, developed a mono‐aural fetoscope to listen to fetal heart sounds. It became known as the Pinard or Pinard horn. It is shaped like a trumpet and its flat end is placed on the practitioner's ear while the horn part is placed on the pregnant mother's abdomen to listen to the FHR. The horn/bell shaped end is produced with a variety of diameters from 12.5 cm to 16.5 cm, depending on the choice of the individual Pinard maker (Gapultos 2008). The early Pinard was produced out of wood. More recently, it is also produced in plastic and aluminium, with the wooden option still preferred by many practitioners. While Laënnec and Pinard are inexpensive and readily available in many countries, they may be difficult to be used in certain labouring and birthing position (Lewis 2015).
Fetal stethoscope or fetoscope or DeLee‐Hillis
In 1917, D Hillis, an American physician (Hillis 1917), and in 1922 JB DeLee (Goodlin 1979), also an American physician, both developed and described an identical fetoscope for obtaining better fetal heart sounds. This simultaneous development caused some controversy, which was eventually resolved by naming the instrument DeLee‐Hillis. It uses the practitioner's forehead to conduct sound and is made from metal and plastic. Shaped much like the stethoscope, two tubes extend out of the main long plastic tube; these are inserted into the practitioner's ears. Similar to the Pinard, on the other end, the fetoscope fans out into a bell/horn shape that is placed on the mother's abdomen. The diameter of the bell/horn is smaller than that of a Pinard. It is still in use today, mainly in the UK and USA (Chez 2000). Some DeLee‐Hillis also come with a head strap, which enables the practitioner to have both hands free. Similarly to Pinard and Laënnec, DeLee is inexpensive but may be difficult to be used in certain labouring and birthing position (Lewis 2015).
Stethoscope
The stethoscope is a common tool used in clinical practice today. In 1852, G Cammann, an American physician, invented the binaural stethoscope, building on Laënnec's earlier invention. It consisted of a Y‐shaped tube, with the longer section fitted with interchangable wide and narrow ends intended for lung and heart examination. While initially made from wood it quickly changed to plastic, rubber and metal. The stethoscope can also be used for fetal auscultation and is often used in the absence of any other tool to ascertain the FHR (Bishop 1980), with the diaphragm or small bell placed onto the mother's abdomen. While relatively inexpensive, using a stethoscope requires access to spare parts for replacements of the ear pieces and diaphragm, which may not be available in all countries.
Hand‐held fetal Doppler/Sonic Aid device
A hand‐held Doppler device uses ultrasound technology to bounce sound waves off the fetal heart valves or walls and converts it into sound. This is then heard and/or displayed as a representation of the fetal cardiac cycle/fetal heart beat (Goodwin 2000). The device comes in a range of portable sizes as well as waterproof options. It requires the use of conducting gel, similar to the practice when using other ultrasound technology. Older models require the practitioner to count the FHR, as there is no display unit. When the FHR is read from a display unit during IA, it needs to be clearly stated that this is the case. Listening with a hand‐held Doppler is more comfortable for the woman as she can be in any labouring and birthing positions. The FHR is audible for everyone present during the fetal auscultation. This device is considerably more expensive than other IA tools and requires access to spare parts, as well as batteries or access to reliable electricity for rechargeable batteries (Lewis 2015). With the recent development of a wind‐up fetal Doppler this may reduce costs, as no batteries are needed (PowerFree Education Technology 2014).
Electronic fetal monitoring (EFM) with a cardiotocograph (CTG)
Sometimes the external transducer of an EFM device (most commonly from a CTG) alone is used for IA. However, this is not recommended as such practice results in intermittent CTG not IA (Feinstein 2000; Goodwin 2000). Most CTG machines use autocorrelation where the FHR is frequently averaged and displayed as digital interface and printed on a CTG paper trace. This is not the same as counting the fetal heart for a minute or other recommended periods of time. Therefore, intermittent CTG should not be confused with the counting technique of IA or incorrectly labelled as such (Feinstein 2000; Goodwin 2000).
Why it is important to do this review
Current evidence from a Cochrane review (Alfirevic 2013) on the use of continuous CTG as a form of EFM showed that compared to IA, continuous CTG does not significantly reduce perinatal death but is associated with a reduction in neonatal seizures. There is, however, a significant increase in caesarean sections and assisted vaginal births associated with continuous CTG . There were no differences between the two groups in relation to Apgar scores, neonatal admission or hypoxic ischaemic encephalopathy.
Many clinical practice guidelines recommend continuous CTG for high‐risk women with access to fetal scalp blood sampling (Walsh 2007; Walsh 2008; NICE 2014). This indicates an increased need for the practice of IA of the FHR during labour for pregnant women identified as 'low risk' of intrapartum fetal hypoxia. However, the optimal tool, timing and methods of performing IA is unknown.
Apart from Alfirevic's review (Alfirevic 2013), there are several other Cochrane reviews that relate to assessing fetal well‐being during labour including a review of fetal electrocardiogram (ECG) for fetal monitoring during labour (Neilson 2015), fetal pulse oximetry for fetal assessment in labour (East 2014) and vibroacoustic stimulation for fetal assessment in labour in the presence of a non‐reassuring FHR trace (East 2013). Devane assessed CTG versus IA of fetal heart on admission to the labour ward for assessment of fetal well‐being (Devane 2012) and found a probability that admission CTG increases the caesarean section rate by approximately 20% when monitoring normal labour without detecting important differences in perinatal mortality. However, Devane 2012 also comments that the data lacked power to identify these differences significantly. While some of these reviews compare intermittent with continuous monitoring, none address the issue of optimal tools and timing for IA. With the explosion of digital computer technology referred to often as artificial intelligence, expert systems (ESs) have been promoted to be useful in labour rooms. ESs can assist in complex clinical decision‐making and potentially serve as a mechanism to improve interpretation of FHR tracings. The Lutomski 2015 review concluded that no recommendations for clinical practice can be made for the use of ES for FHR interpretation for intermittent and continuous fetal auscultation as there is currently no strong evidence available and high‐quality trials are needed.
Low‐income countries have limited access to any electronic auscultation tool that can be used for ausculting the FHR continuously for women with high risk in labour or when concerns for the FHR arises. In all maternity settings clinicians want to know the evidence for the use of IA tools and timing for intermittently monitoring the FHR in labour.
Objectives
To evaluate the effectiveness of different tools for intermittent ausculation (IA) of the fetal heart rate (FHR) during labour including frequency and duration of auscultation.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials (RCTs) or cluster‐RCTs comparing different tools and methods used for intermittent fetal auscultation for fetal and maternal well‐being. Quasi‐RCTs, and cross‐over designs were not eligible for inclusion.
Types of participants
Pregnant women in labour and their babies.
Types of interventions
Different methods of intermittent fetal heart auscultation during labour in which one method was compared to another, or different timings of the same method were compared.
Types of outcome measures
Main outcomes for the baby
Primary outcome
-
Apgar < seven at five minutes.
-
Infant mortality or serious morbidity (composite outcome).
Secondary outcomes
-
Perinatal mortality (stillbirth, and neonatal deaths excluding lethal congenital anomalies).
-
Meconium liquor.
-
Fetal bradycardia (as specified by trial authors).
-
Fetal tachycardia (as specified by trial authors).
-
Early decelerations (as specified by trial authors).
-
Decelerations with contractions (as specified by trial authors).
-
Late decelerations (as specified by trial authors).
-
Apgar < seven at 10 minutes.
-
Cord blood acidosis (as specified by the trial authors; but most likely a cord blood pH ≤ 7.10).
-
Neonatal infections.
-
Breastfeeding.
-
Admissions to the neonatal intensive care unit (NICU) or neonatal unit (NNU).
-
Length of stay in NICU or NNU.
-
Seizures in the neonatal period, either clinically apparent or detected by electro‐encephalographic (EEG) recordings.
-
Hypoxic ischaemic encephalopathy.
-
Cerebal palsy.
-
Neurodevelopmental disability assessed at six months of age.
-
Neurodevelopmental disability assessed at 12 months of age.
Main outcomes for the mother
Primary outcomes
-
Caesarean section for fetal distress and/or fetal acidosis.
-
Instrumental vaginal birth for fetal distress and/or fetal acidosis.
Secondary outcomes
-
Death.
-
Caesarean section.
-
Instrumental vaginal birth.
-
Vaginal birth.
-
Length of labour.
-
Use of pain relief (pharmacological or non‐pharmacological).
-
Use of epidural analgesia.
-
Amniotomy ‐ artificial rupture of membranes.
-
Duration of ruptured membranes.
-
Group B streptococcus status of the mother.
-
Maternal temperature during labour.
-
Mobility or restriction during labour.
-
Episiotomy.
-
Perineal trauma requiring repair.
-
Maternal acidosis.
-
Maternal satisfaction with auscultation tool during labour.
-
Maternal satisfaction with timing intervals of auscultation in labour.
-
Overall satisfaction with the labour.
-
Skin‐to‐skin contact.
-
Postpartum depression.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (19 September 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
Searching other resources
We searched the reference lists of relevant papers. We contacted the author of Mahomed 1994 for additional information about clarifying the timing intervals for the intervention, which we received. We also contacted Mdoe 2015 about randomisation, inclusion criteria, intervention description of 'free play' and inconsistency with published outcome data. Only clarifying information about randomisation and inclusion criteria was received.
We did not apply any language or date restrictions.
Data collection and analysis
The methodology for data collection and analysis is based on the Cochrane Handbook of Systematic Reveiws of Intervention (Higgins 2011).
Selection of studies
Four authors, Ruth Martis (RM), Detty Nurdiati (DN), Ova Emilia (OE) and Julie Brown (JB), independently examined the results of the search to ascertain if the studies met the inclusion criteria. The reason for excluded studies have been set out in the Characteristics of excluded studies table. We did not encounter any disagreements.
Data extraction and management
RM, DN and OE independently extracted data using a purposefully designed data extraction form. We resolved any discrepancies through discussion. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy by RM and DN. JB and RM extracted data independently for the Byaruhanga 2015 and Mdoe 2015 trials which were added after the most recent literature search. No disagreements occurred.
Mahomed 1994, the author of one of the included studies was contacted to clarify discrepancies between the two publications of the trial. The author confirmed that the trial methods were correctly reported in the 1994 publication. In addition, the author also confirmed that the duration and frequency of auscultation was for one minute during and immediately after a contraction in every 10‐minute period.
Clarification was also sought and obtained from Mdoe 2015 on randomisation and on inclusion criteria. Further clarification was requested for the description of the intervention 'free play' and discrepancies in the published outcome data as it did not correspond with the total number of participants identified. The author suggested waiting until the trial results would be published in approximately six months. This meant that the inconsistent numbers from Mdoe 2015 could not contribute outcome data for this review.
Assessment of risk of bias in included studies
Four review authors (RM, DN, OE, JB) independently assessed the risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and contained in RevMan (RevMan 2014). We did not have any disagreement.
(1) Sequence generation (checking for possible selection bias)
We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the method as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the included studies the method used to blind trial participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We were aware that blinding women and health professionals in which different auscultation tools were used was not possible.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For the included studies we described the methods used for blinding of outcome assessment to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes. We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for the included studies the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported; the numbers included in the analysis at each stage (compared with the total randomised participants); reasons for attrition or exclusion where reported; and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting bias
We investigated the possibility of selective reporting bias by identifying all outcomes reported in the methods section of the results publication and cross‐checking to see if these were reported in the results section of the trial publications.
We assessed the risk of bias for selective reporting as:
-
low risk of bias (where it was clear that all of the trial’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
-
high risk of bias (where not all the trial’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; trial failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for the included studies any important concerns we had about other possible sources of bias. We assessed whether each trial was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there was risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis below.
Assessment of the quality of the evidence using the GRADE approach
The quality of the evidence of the included trial was assessed using the GRADE approach as outlined in the GRADE Handbook (chapter 5). We produced 'Summary of findings' tables using the GRADEpro Guideline Development Tool. A summary of the intervention effect and a measure of quality for each of the outcomes was produced using the GRADE approach. GRADEpro uses five criteria (trial limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. We chose seven maternal and seven infant outcomes (seven is the maximum number of outcomes permitted with the GRADEpro software), as listed below. The comparisons were different methods/tools of intermittent fetal heart auscultation during labour in which one method/tool was compared to another, or different timings of the same method were compared.
For the included studies the infant and maternal outcomes assessed for quality using the GRADE approach were Apgar < seven at five minutes, neonatal seizures, perinatal mortality, composite of mortality and serious morbidity, caesarean section for fetal distress and/or fetal acidosis and instrumental vaginal birth for fetal distress and/or fetal acidosis. No other data were available for the other listed outcomes. summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6.
Outcomes for the baby
-
Apgar < seven at five minutes.
-
Cord blood acidosis.
-
Neonatal seizures.
-
Perinatal mortality.
-
Infant mortality or serious morbidity (composite outcome).
-
Cerebral palsy.
-
Neurosensory disability.
Outcomes for the mother
-
Caesarean section for fetal distress and/or fetal acidosis.
-
Instrumental vaginal birth for fetal distress and/or fetal acidosis*.
-
Maternal mortality.
-
Any pharmacological or non‐pharmacological analgesia use excluding epidural.
-
Epidural anaesthesia for pain relief excluding for caesarean section.
-
Mobility or restriction during labour.
-
Postnatal depression.
(*Reasons for instrumental vaginal births were not reported in the trials contributing data for this outcome; therefore in the 'Summary of findings' tables, we have reported results for all instrumental vaginal births which is one of our secondary outcomes.)
Measures of treatment effect
Dichotomous data
Dichotomous data were presented as a summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference with 95% confidence intervals.
Unit of analysis issues
Cluster‐randomised trials
There were no cluster‐randomised trials included for analysis for this review. In future updates of this review, if we include cluster‐randomised trials in the analyses, we will adjust their sample sizes using the methods described in the Handbook using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, then we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little clinical and statistical heterogeneity between the trial designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
Multi‐arm‐randomised trials
For the one multi‐arm trial (Mahomed 1994), we made pair‐wise comparisons to avoid double counting data.
Cross‐over trials
Cross‐over trials were not included for analysis as this would not be an appropriate trial design for this intervention.
Dealing with missing data
We noted the levels of attrition for included studies. We explored, using sensitivity analysis, the impact of including studies with high levels of missing data in the overall assessment of treatment effect.
Intention‐to‐treat analysis (ITT)
For all outcomes, we analysed the data, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing. We analysed data on all participants with available data in the group to which they are allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we attempted to restore them to the correct group.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial where the Tau² was greater than zero and either an I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
This review included three studies. In future updates we will investigate reporting biases (such as publication bias) using funnel plots for outcomes with 10 or more studies in the meta‐analysis and plan to assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We used a fixed‐effect meta‐analysis for combining data where we considered it was reasonable to assume that the studies were estimating a similar underlying treatment effect such that the trials were examining the same intervention and that the population under investigation and the research methods were similar. We used the Review Manager software (RevMan 2014) to perform these meta‐analyses.
However, where there was clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects might differ between trials, we used random‐effects meta‐analysis representing the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We were unable to carry out our prespecified subgroup analyses because of insufficient data. In future updates, if more data become available, we will carry out the following subgroup analysis, using interaction tests as described by Deeks 2001.
-
Low‐risk versus high‐risk pregnancy.
-
Gestation at least 37 weeks versus less than 36+6 weeks.
-
First stage versus second stage of labour.
-
Singleton pregnancy with cephalic presentation versus singleton pregnancy with breech presentation versus twin pregnancy.
-
Primiparity versus multiparity
Subgroup analyses will be restricted to this review's primary outcomes.
In future updates, we will assess potential subgroup differences by subgroup interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality for the primary outcomes in the review. Where there was risk of bias associated with a particular aspect of trial quality (e.g. inadequate allocation concealment), this was explored by sensitivity analyses. In future updates, we will carry out sensitivity analysis to investigate the effect of the randomisation unit if we combine data from cluster‐RCTS along with data with individually‐randomised trials.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
See: Figure 1.

Study flow diagram.
We identified eight reports of six trials from our search (Haverkamp 1979; Wood 1981; MacDonald 1985; Mahomed 1994, Byaruhanga 2015, Mdoe 2015). Some information regarding trial methods, interventions and results were reported differently in Mahomed's two publications. Review author R Martis contacted the author who confirmed that the 1994 publication reported these correctly. Of the seven publications, three studies were eligible for inclusion (Mahomed 1994; Byaruhanga 2015; Mdoe 2015) (see Included studies) and three studies were excluded (Haverkamp 1979; Wood 1981; MacDonald 1985) (see Excluded studies).
Included studies
Three randomised controlled trials involving a total of 6241 women and 6241 babies were included into this review (Mahomed 1994; Byaruhanga 2015; Mdoe 2015). Two trials were included for meta‐analysis (Mahomed 1994; Byaruhanga 2015) with a total of 3242 women and their babies. Mdoe 2015 had numerical inconsistencies in its publication and could not be used for meta‐analysis. Mdoe clarified that results would be published at the end of 2016. It is anticipated the results will be included once they are published in the next update of this review.
Design
Mahomed 1994 is a parallel four‐armed randomised controlled trial. Both Byaruhanga 2015 and Mdoe 2015 studies are parallel randomised controlled trials with two arms each.
Sample size
Of the three included studies, Mdoe 2015 was the largest trial with 2999 women enrolled. Byaruhanga 2015 recruited 1987 women and Mahomed 1994 included 1255 women.
Setting
All three studies were conducted in Africa. Mahomed 1994 was conducted in Zimbabwe (Harare) at a referral maternity hospital. Byaruhanga 2015 was conducted at the Nsambya teaching hospital in Uganda (Kampala) and Mdoe 2015's trial involved two settings in the United Republic of Tanzania i.e. an urban setting at the Muhimbili National hospital and a rural setting at the Haydom Lutheran Hospital. In all settings, fetal blood gas and cord blood sampling or epidural analgesia were not available.
Participants
All studies included women in active labour with a singleton pregnancy, cephalic presentation, ≥ 37 weeks' gestation, with a cervical dilatation ≤ 7 cm and normal fetal heart rate (FHR) at admission (120 to 160 bpm) recorded by the health professional attending the admission. Byaruhanga 2015 and Mdoe 2015 only included women identified with low‐risk factors. Mahomed 1994 included women with obstetric and medial risk factors, but with normal FHR on admission to the hospital. In all trials, women were excluded if they arrived at the trial sites in the second stage of labour. Additionally, Byaruhanga 2015 excluded women with antepartum haemorrhage, intrauterine fetal death and women having an elective caesarean. Mahomed 1994 reported that the only high‐risk women excluded from the trial were those presenting with placental abruption or eclampsia. Mdoe 2015 did not publish any additional exclusion criteria other than excluding women who arrived in second stage.
Mahomed 1994 and Byaruhanga 2015 described exclusion and inclusion criteria for participants and identified the maternal characteristics clearly in their publications. Mdoe 2015's publication did not provide clear descriptions of these. See Characteristics of included studies.
Interventions
We did not anticipate that one trial would include electronic fetal monitoring (EFM) with a cardiotocograph (CTG) as an intermittent auscultation (IA) intervention (Mahomed 1994). After discussion, we decided to include this arm of the intervention as well, as it appears that only the FHR was auscultated with the CTG transducer and used for clinical management decisions.
Mahomed 1994 compared intermittent electronic fetal monitoring using a CTG abdominal transducer by a research midwife or doctor, Doppler ultrasonography by a research midwife, intensive Pinard by a research midwife with routine use of Pinard by the midwife on‐duty. The intervention protocol outlined listening intermittently for one minute (60 seconds) to the FHR during and immediately after a contraction occurring in the last 10 minutes of every half hour for all groups. If the fetal heart pattern was found to be abnormal, this changed to listening for one minute (60 seconds) during and immediately after a contraction occurring in the last 10 minutes of every 20 minutes and a doctor was informed.
Byaruhanga 2015 auscultated the FHR intermittently for one minute (60 seconds) with a hand‐held wind‐up Doppler immediately after a contraction every 30 minutes in the first stage of labour, every 15 minutes in the second stage before pushing and every five minutes in the second stage when pushing.
Mdoe 2015 auscultated intermittently FHR with a hand‐held Doppler in urban and rural settings. Frequency of IA and length of listening of FHR was not described but stated as 'free play'. It is unclear what this means.
Comparisons
In the Mahomed 1994 trial, women in the control group were randomised to receive IA of the FHR with a Pinard as was the routine at the hospital, which expected the FHR to be auscultated for one minute during the last 10 minutes of every half hour during and immediately after a contraction by the midwife on‐duty, who may care for other labouring women at the same time and would endeavour to follow the hospital recommendation for routine auscultation as much as possible. This did mean that some women did not receive one‐to‐one care, as indicated for the intervention groups. It is not described how many labouring women were in this situation.
Byaruhanga 2015 compared intermittent FHR auscultation for one minute (60 seconds) using a Pinard immediately after a contraction every 30 minutes in the first stage of labour, every 15 minutes in the second stage before pushing and every five minutes in the second stage when pushing.
Mdoe 2015 also compared its intervention to intermittent FHR auscultation using a Pinard and Doppler, but did not describe the frequency and length of listening to the FHR.
Outcomes
Mahomed 1994 reported data for immediate maternal, fetal and neonatal outcomes. Our prespecified outcomes included Apgar score < seven at five minutes after the birth. However, in the Mahomed 1994 trial the outcome reported was Apgar < six at five minutes. As only two studies contributed data to the review, we decided that although this was not the same as out pre‐specified cut‐off, we would include it as it provided some indication of the condition of the infants soon after the birth.
Mahomed 1994 reported "stillbirth or neonatal death" as a single outcome but did not clearly report the number of stillbirths and neonatal deaths separately for each arm of the trial.
Byaruhanga 2015 and Mdoe 2015 reported clinical measures focusing primarily on neonatal outcomes and both reported data for caesarean section but did not provide the indications for these. Byaruhanga 2015 reported "intrapartum stillbirth" and "neonatal death prior to discharge" and we combined these outcomes for our perinatal mortality outcome.
Mdoe 2015 listed a further three neonatal outcomes (admission to neonatal unit (NNU), stillbirth and neonatal deaths) in the publication, but did not provide outcome data.
All included studies documented the FHR as a single rate.
None of the included studies reported long‐term outcomes for the mother and the baby.
It was not possible from the available outcome data to separate IA for first and second stage of labour or to perform subgroup analyses according to risk status or parity.
Excluded studies
Three studies comparing IA with continuous electronic FHR monitoring were excluded, as CTG monitoring was not one of the included comparisons for this review (Haverkamp 1979; Wood 1981; MacDonald 1985). See the Characteristics of excluded studies table.
Risk of bias in included studies
See 'Risk of bias' table for the included studies in Characteristics of included studies, Figure 2 and Figure 3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included trial.
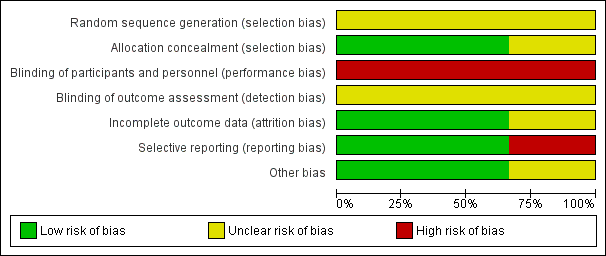
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation ‐ none of the included studies stated how their randomisation was generated. Therefore, all studies identified as having an unclear risk of bias for sequence generation.
Allocation concealment ‐ Mahomed 1994 and Byaruhanga 2015 both reported using sequentially numbered, sealed, opaque envelopes, the risk of bias for allocation concealment was judged to be low. Further information on methods of allocation used in the Mdoe 2015 trial was provided by trial authors through email correspondence as it was not clear from the published abstract. Authors stated that the trial was "one to one open label" and was judged to be of unclear risk of bias for allocation concealment.
Blinding
Performance bias ‐ Mahomed 1994 and Byaruhanga 2015 did not report on blinding of participants and clinicians. Mdoe 2015 identified through email correspondence that open‐label randomisation was used in their trial i.e. participants and clinicians knew which group women had been randomised to. It is highly unlikely that blinding of the intervention for clinicians and participants was achieved in the Mahomed 1994 and Byaruhanga 2015 trials as the intervention tool used is impossible to conceal. Performance bias was therefore judged to be of high risk for all three studies.
Detection bias ‐ Mahomed 1994, Byaruhanga 2015 and Mdoe 2015 did not report on blinding of outcome assessors. Therefore detection bias was judged to be unclear. Additionally, blinding may also be important for the assessment of the outcomes. Although some outcomes were objectively measured and unlikely to be affected by knowledge of group assignment, some outcomes were judged to have some degree of subjectivity and may possibly be at high risk of detection bias. These would include Apgar scores, early and late FHR decelerations and neonatal infections.
Incomplete outcome data
Mahomed 1994's trial reported there were no losses to follow‐up and no drop out. Eighteen of the women allocated to the EFM (CTG) group did not receive the intervention, as birth was either too rapid or was prevented by technical problems with the machine. For a further 24 women in the EFM (CTG) group, traces (the printed record produced by the CTG) were of poor quality, usually because of frequent loss of contact, machine failure, or poor marking on the paper. However, outcomes for all women were measured as planned, and included in the statistical analysis on an intention‐to‐treat basis. We judged this trial to be at a low risk for attrition bias. In the Byaruhanga 2015 trial, eight women in the Pinard group were excluded from the analysis (one woman was lost to follow‐up, one woman birthed before the partogram was started, two women had undiagnosed breech births and four women had undiagnosed multiple births), and equally data from eight women for the Doppler group were not analysed (three women birthed before their partograms were started, three women had undiagnosed breech births and two women had undiagnosed multiple births). Both studies Mahomed 1994and Byaruhanga 2015 were judged to be of low risk for attrition. Mdoe 2015 did not report how incomplete outcome data were addressed but the numerical data published are inconsistent and pending clarification on publication of main trial results. Attrition bias for this trial was judged to be at unclear risk of attrition bias.
Selective reporting
All intended outcomes appear to have been reported for Mahomed 1994 and Byaruhanga 2015. Both studies Mahomed 1994 and Byaruhanga 2015 were judged to be of low risk for selective reporting. In the Mdoe 2015 trial the pre‐specified outcomes (admission to neonatal unit, five‐minute Apgar score less than seven, death and fresh stillbirth) did not match the reported outcomes except for Apgar score less than seven. We judged this trial of high risk for reporting bias. No trial protocols of any trials were available.
Other potential sources of bias
No other potential sources of bias were identified for Mahomed 1994 and Byaruhanga 2015. Mdoe 2015 was assessed as 'unclear risk' of other bias ‐ this trial report was only an abstract, and it remains unclear if the groups were balanced at baseline.
Effects of interventions
See: Summary of findings for the main comparison Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) versus routine Pinard (outcomes for the baby); Summary of findings 2 Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) versus routine Pinard (outcomes for the mother); Summary of findings 3 Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus routine Pinard (outcomes for the baby); Summary of findings 4 Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus routine Pinard (outcomes for the mother); Summary of findings 5 Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus routine Pinard (outcomes for the baby); Summary of findings 6 Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus routine Pinard (outcomes for the mother)
We included three studies (Mahomed 1994;Byaruhanga 2015; Mdoe 2015) with usable data available from two studies (Mahomed 1994;Byaruhanga 2015) involving 3242 women and 3242 babies.
Mdoe 2015's trial had numerical inconsistencies in its publication and clarification is pending. Therefore it could not be used for analysis.
1.0 Intermittent electronic fetal monitoring (EFM) cardiotocograph (CTG) versus routine Pinard
We did not anticipate that one trial would include EFM with a CTG as an IA intervention (Mahomed 1994). After discussion, we decided to include this arm of the intervention as well, as it appears that only the FHR was auscultated with the CTG transducer and used for clinical management decisions.
One trial compared intermittent EFM (CTG) with routine Pinard (Mahomed 1994).summary of findings Table 2
Neonatal primary outcome
1.1 There was no clear difference in Apgar scores < seven at five minutes after birth (reported as six at five minutes after birth in the Mahomed 1994 trial) between the intermittent EFM (CTG) and the routine Pinard groups (risk ratio (RR) 0.66, 95% CI 0.24 to 1.83, one trial, 633 babies, very low‐quality evidence,Analysis 1.1). The evidence was downgraded due to lack of blinding and imprecision (evidence being based on a single trial with low event rates). (summary of findings Table for the main comparison). Infant mortality or serious morbidity (composite outcome) was not reported).
Maternal primary outcomes
1.2 Women allocated to intermittent EFM (CTG) had significantly higher rates of caesarean section for fetal distress (RR 2.92, 95% CI 1.78 to 4.80, one trial, 633 women, heterogeneity not applicable, moderate‐quality evidence,Analysis 1.2) compared to women allocated to routine Pinard. Evidence was downgraded due to study design limitations (summary of findings Table 2).
Instrumental vaginal birth for fetal distress and/or acidosis was not reported.
Neonatal secondary outcomes
1.3 The results for perinatal mortality (stillbirth and neonatal death) showed no clear difference between the intermittent EFM (CTG) group compared to the routine Pinard group (RR 0.88, 95% CI 0.34 to 2.25, one trial, 633 babies, very low‐quality evidence, Analysis 1.3). Evidence was downgraded due to imprecision (wide 95% CIs and low event rates), and study design limitations (summary of findings Table for the main comparison).
1.4 Intermitent EFM (CTG) was associated with increased likelihood of detecting abnormal FHR patterns (bradycardia and tachycardia) compared with the routine Pinard (RR 6.08, 95% CI 4.21 to 8.79, one trial, 633 babies, Analysis 1.4). Evidence should be interpreted with caution due to wide 95% CIs.
1.5 Intermitent EFM (CTG) was associated with increased detection of early and late decelerations compared to routine Pinard (RR 2.84, 95% CI 1.82 to 4.45, one trial, 633 babies, Analysis 1.5). Evidence should be interpreted with caution due to wide 95% CIs.
1.6 There was no difference in neonatal intensive care unit/neonatal unit (NICU/NNU) admission rates between routine Pinard (control) and EFM (CTG) (RR 0.89, 95% CI 0.63 to 1.25, one trial, 633 babies, Analysis 1.6).
1.7 There were no seizures in the neonatal period in the intermittent EFM (CTG) group (0/318) compared with nine in the routine Pinard group (9/315) (RR 0.05, 95% CI 0.00 to 0.89, one trial, 633 babies, very low‐quality evidence, Analysis 1.7). Evidence was downgraded due to imprecision (wide CIs and low event rates), and bias associated with study design.
1.8 The incidence of hypoxic ischaemic encephalopathy was lower in the intermittent EFM (CTG) group (2/318) compared to the routine Pinard group (10/315) (RR 0.20, 95% CI 0.04 to 0.90, one trial, 633 babies, Analysis 1.8). Evidence should be interpreted with caution due to wide 95% CIs and low event rates.
The included trial did not report on other listed neonatal secondary review outcomes (meconium liquor, declarations with contractions, Apgar < seven at 10 minutes, cord blood acidosis, neonatal infections, breastfeeding, cerebral palsy, or neurodevelopmental disability at six months or one year).
Maternal secondary outcomes
1.9 Birth by caesarean section (without indications identified) was more likely in the intermittent EFM (CTG) than in the routine Pinard monitoring group (RR 1.92, 95% CI 1.39 to 2.64, one trial, 633 women, Analysis 1.9).
1.10 There was no significant difference in instrumental vaginal births between the intermittent EFM (CTG) group and the routine Pinard group (RR 1.46, 95% CI 0.86 to 2.49, one trial, 633 women, low‐quality evidence, Analysis 1.10). The reasons for instrumental births (e.g. fetal distress) were not reported in the trial contributing data. Evidence was downgraded due to imprecision, and study design limitations (summary of findings Table 2).
1.11 There was no clear difference in the length of labour (in hours) between the intermittent EFM (CTG) and routine Pinard groups (mean difference (MD) 0.90 hours, 95% CI ‐0.05 to 1.85, one trial, 633 women, Analysis 1.11).
The trial did not report on other listed maternal secondary review outcomes (death, vaginal birth, use of pain relief (pharmacological or non‐pharmacological), use of epidural analgesia, amniotomy, duration of ruptured membranes, Group B streptococcus status of the mother, maternal temperature during labour, mobility or restriction during labour, episiotomy, perineal trauma requiring repair, maternal acidosis, maternal satisfaction with auscultation tool during labour, maternal satisfaction with timing intervals of auscultation in labour, overall satisfaction with the labour, skin‐to‐skin contact, postpartum depression).
2.0 Doppler ultrasonography versus routine Pinard
Two studies Mahomed 1994 and Byaruhanga 2015 published usable data for analysis comparing Doppler ultrasonography with routine Pinard use.
Neonatal primary outcomes
2.1 Both Mahomed 1994 and Byaruhanga 2015 identified there was no clear evidence of a difference in Apgar scores < 7 at five minutes after birth (reported as < six in Mahomed 1994's trial) among infants of women in the Doppler ultrasonography group compared to the routine Pinard group (average RR 0.76, 95% CI 0.20 to 2.87; two trials, 2598 babies,Tau2 = 0.69, I2 = 72%, very low‐quality evidenceAnalysis 2.1). The evidence was downgraded for performance bias and imprecision, and I2 results indicate substantial heterogeneity (summary of findings Table 3). Infant mortality or serious morbidity (composite outcome) was not reported).
Maternal primary outcomes
Only Mahomed 1994 reported on the maternal primary outcomes of this review.
2.2 Women allocated to the Doppler ultrasonography had higher rates of caesarean section for fetal distress than women allocated to routine Pinard monitoring group (RR 2.71, 95% CI 1.64 to 4.48, one trial, 627 women, heterogeneity not applicable, moderate‐quality evidenceAnalysis 2.2). Evidence was downgraded due to performance bias in the trials contributing data (summary of findings Table 4).
Instrumental vaginal birth for fetal distress and/or acidosis was not reported.
Neonatal secondary outcomes
2.3 There was no clear evidence of a difference in perinatal mortality (stillbirth or neonatal death) between Doppler ultrasonography and routine Pinard (average RR 0.69, 95% CI 0.09 to 5.40, two trials, 2597 babies,Tau2 = 1.78; I2 = 81%, very low‐quality evidenceAnalysis 2.3). Evidence was downgraded due to imprecision with wide 95% CI, and study design limitations caused by inability to blind participants and health professionals (summary of findings Table 3) and I2 indicates substantial heterogeneity.
2.4 Doppler ultrasonography was also associated with being more likely to detect abnormal FHR patterns (bradycardia and tachycardia) compared to routine Pinard (average RR 2.40, 95% CI 1.09 to 5.29; two trials, 2598 babies,Tau2 = 0.29, I2 = 89%, Analysis 2.4). Evidence should be interpreted with caution due to wide 95% CI suggesting imprecision and Í2 indicates substantial heterogeneity.
2.5 Doppler ultrasonography was associated with increased detection of early and late fetal heart rate decelerations compared to the routine Pinard (RR 2.72, 95% CI 1.73 to 4.28, one trial, 627 women, Analysis 2.5). Evidence should be interpreted with caution due to wide 95% CI suggesting imprecision.
2.6 There were no clear evidence of a difference with NICU/NNU admissions between the Doppler ultrasonography group and the routine Pinard group (average RR 0.89, 95% CI 0.41 to 1.91; two trials, 2598 babies,Tau2 = 0.26, I2 = 86%, Analysis 2.6).
2.7 There was an increased risk of neonatal seizures in the routine Pinard group (9/315) compared to the Doppler ultrasonography group (0/312) (one trial, 627 babies, RR 0.05, 95% CI 0.00 to 0.91, very low‐quality evidence, Analysis 2.7). Evidence from this single trial should be interpreted with caution due to low event rates (summary of findings Table 3).
2.8 The incidence of hypoxic ischaemic encephalopathy was less in the Doppler sonography (1/312) group compared to the routine Pinard group (10/315) (RR 0.10, 95% CI 0.01 to 0.78, one trial, 627 babies, heterogeneity not applicable, Analysis 2.8). Evidence from this single trial should be interpreted with caution due to low event rates. and one trial.
The studies did not report on other listed neonatal secondary review outcomes (meconium liquor, declarations with contractions, Apgar < seven at 10 minutes, cord blood acidosis, neonatal infections, breastfeeding, cerebral palsy, or neurodevelopmental disability at six months or one year).
Maternal secondary outcomes
2.9 Birth by caesarean section (without indications identified) did not clearly differ between groups (average RR 1.29, 95% CI 0.81 to 2.05; two studies, 2598 women,Tau2 = 0.09, I2 = 83%, Analysis 2.9).
2.10 There was no clear evidence of a difference in instrumental vaginal births between women in the Doppler ultrasonography group compared to the routine Pinard auscultation group (RR 1.35, 95% CI 0.78 to 2.32, one trial, 627 women, heterogeneity not applicable, low‐quality evidenceAnalysis 2.10). Reasons for instrumental delivery were not reported. Evidence was downgraded due to imprecision and study design limitations (summary of findings Table 4).
2.11 There was no evidence of a difference in the length of labour > 18 (in hours) between women in the Doppler ultrasonography and routine Pinard groups (MD 0.00 hours, 95% CI ‐1.07 to 1.07, one trial, 627 women, heterogeneity not applicable, Analysis 2.11).
The studies did not report on other maternal secondary review outcomes as listed in this review (death, vaginal birth, use of pain relief (pharmacological or non‐pharmacological), use of epidural analgesia, amniotomy, duration of ruptured membranes, Group B streptococcus status of the mother, maternal temperature during labour, mobility or restriction during labour, episiotomy, perineal trauma requiring repair, maternal acidosis, maternal satisfaction with auscultation tool during labour, maternal satisfaction with timing intervals of auscultation in labour, overall satisfaction with the labour, skin‐to‐skin contact, postpartum depression).
3.0 Intensive Pinard versus routine Pinard
Only one trial (Mahomed 1994) compared intensive Pinard (a research midwife following the protocol in a one‐to‐one care situation) with routine Pinard (as per protocol but midwife may be caring for more than one women in labour).
Neonatal primary outcomes
3.1 There was no clear evidence of a difference in low Apgar score at five minutes after birth (reported as six in Mahomed 1994's trial) between the intensive Pinard and routine Pinard groups (RR 0.90, 95% CI 0.35 to 2.31, one trial, 625 babies, very low‐quality evidenceAnalysis 3.1). Evidence was downgraded for performance bias and imprecision. Infant mortality or serious morbidity (composite outcome) was not reported).
Maternal primary outcomes
3.2 There was no clear evidence of a difference in the incidence of caesarean section for fetal distress between women allocated to the intensive Pinard group and women allocated to the routine Pinard group (RR 0.70, 95% CI 0.35 to 1.38, one trial, 625 women, heterogeneity not applicable, low‐quality evidence, Analysis 3.2). Evidence was downgraded for performance bias and imprecision.
Instrumental vaginal birth for fetal distress and/or acidosis was not reported.
Neonatal secondary outcomes
3.3 There was no clear evidence of a difference in perinatal morality between the intensive Pinard and routine Pinard groups (RR 0.56, 95% CI 0.19 to 1.67, one trial, 625 babies, very low‐quality evidence, Analysis 3.3). Evidence was downgraded for performance bias and imprecision.
3.4 The intensive Pinard method was more likely to detect abnormal FHR patterns (bradycardia and tachycardia) than routine Pinard (RR 1.71, 95% CI 1.10 to 2.65, one trial, 625 babies, heterogeneity not applicable, Analysis 3.4).
3.5 There was no clear evidence of a difference between the intensive Pinard and routine Pinard groups in detection of early and late decelerations (RR 1.33, 95% CI 0.79 to 2.23, one trial, 625 babies, heterogeneity not applicable Analysis 3.5).
3.6 There was no clear evidence of a difference in NICU/NNU admission rates between the intensive Pinard and routine Pinard groups (RR 0.84, 95% CI 0.59 to 1.19, one trial, 625 babies, Analysis 3.6).
3.7 There was no clear evidence of a difference in the incidence of neonatal seizures between the intensive Pinard group the routine Pinard group (RR 0.68, 95% CI 0.24 to 1.88, one trial, 625 babies, very low‐quality evidence, Analysis 3.7). Evidence was downgraded for performance bias and imprecision.
3.8 There was no clear difference in the incidence of hypoxic ischaemic encephalopathy between the intensive Pinard and routine Pinard groups (RR 0.71, 95% CI 0.27 to 1.84, one trial, 625 babies, Analysis 3.8).
The studies did not report on other listed neonatal secondary review outcomes (meconium liquor, declarations with contractions, Apgar ≤ seven at 10 minutes, cord blood acidosis, neonatal infections, breastfeeding, cerebral palsy, or neurodevelopmental disability at six months or one year).
Maternal secondary outcomes
3.9 There was no clear evidence of a difference between the intensive Pinard and routine Pinard groups in the incidence of birth by caesarean section (without indications identified) (RR 0.71, 95% CI 0.46 to 1.08, one trial, 625 women, Analysis 3.9).
3.10 There was no clear evidence of a difference in instrumental vaginal births between women allocated to the intensive Pinard group and women allocated to the routine Pinard group (RR 1.21, 95% CI 0.69 to 2.11, one trial, 625 women, low‐quality evidence, Analysis 3.10). Reasons for instrumental birth were not reported. Evidence was downgraded for performance and bias and imprecision.
3.11 There was no clear difference between the intensive Pinard and routine Pinard groups in the length of labour (in hours) (MD 0.50 hours, 95% CI ‐0.52 to 1.52, heterogeneity not applicable, one trial, 625 women, Analysis 3.11).
The studies did not report on other maternal secondary review outcomes as listed in this review (death, vaginal birth, use of pain relief (pharmacological or non‐pharmacological), use of epidural analgesia, amniotomy, duration of ruptured membranes, Group B streptococcus status of the mother, maternal temperature during labour, mobility or restriction during labour, episiotomy, perineal trauma requiring repair, maternal acidosis, maternal satisfaction with auscultation tool during labour, maternal satisfaction with timing intervals of auscultation in labour, overall satisfaction with the labour, skin‐to‐skin contact, postpartum depression).
Discussion
Summary of main results
The review assessed intermittent auscultation (IA) of the fetal heart rate FHR) in labour for fetal well‐being. We identified three studies (involving 6241 women) that met the inclusion criteria for this systematic review (Mahomed 1994; Byaruhanga 2015; Mdoe 2015). All women were ≥ 37 weeks' gestation, in established labour with ≤ 7 cm dilatation, had a singleton pregnancy and had normal FHR (120 to 160 bpm) on admission to the hospital. All three studies were conducted in Africa and at the participating hospitals fetal scalp blood sampling or cord blood assessment was not available. Byaruhanga 2015 and Mdoe 2015 reported that only women identified with low‐risk factors were included into their studies. Byaruhanga 2015 reported exclusion criteria for participants as women contraindicated for labouring when admitted (e.g. antepartum haemorrhage, intrauterine fetal death on admission, or were having an elective caesarean section) and Mdoe 2015 did not publish any additional exclusion criteria other than excluding women who arrived in second stage. Mahomed 1994 included women with obstetric and medial risk factors and only excluded women with high risks from the trial when they presented with placental abruption or eclampsia. It was not possible from the available data to separate IA for subgroup analysis according to risk status, parity or if women were in first or second stage of labour. Therefore, it is not clear how or if this may have impacted intervention effects.
Intermittent auscultation for FHR was identified as listening every 30 minutes immediately after a contraction for one minute (60 seconds) in first stage of labour in Mahomed 1994 and Byaruhanga 2015 studies, whereas Mdoe 2015 identified intermittent listening as 'free play' in the trial with no further clarification. Byaruhanga 2015 identified that the fetal heart was auscultated every five minutes immediately after a contraction in the second stage of labour with pushing. None of the included studies reported if the FHR was auscultated over the anterior shoulder, the optimum place to hear the FHR.
We examined three comparisons: intermittent EFM (CTG) versus routine Pinard, Doppler ultrasonography versus routine Pinard, and Intensive Pinard versus routine Pinard.
One trial compared intermittent EFM (CTG) with routine Pinard. There was no clear difference between groups in low Apgar scores at five minutes (reported as < six at five minutes after birth) (very low‐quality evidence) or in perinatal mortality (low‐quality evidence). Neonatal seizures were reduced in the EFM group (low‐quality evidence). Other important infant outcomes were not reported: mortality or serious morbidity (composite outcome), cerebral palsy or neurosensory disability. For maternal outcomes, women allocated to intermittent EFM (CTG) had higher rates of caesarean section for fetal distress (moderate‐quality evidence) compared with women allocated to routine Pinard. There was no clear difference between groups in instrumental vaginal births (low‐quality evidence). Other outcomes were not reported (maternal mortality, analgesia in labour, mobility or restriction and postnatal depression).
Two studies compared Doppler ultrasonography with routine Pinard. There was no clear evidence of a difference between groups in Apgar scores < 7 at five minutes after birth (reported as < six in one of the trials) (very low‐quality evidence), in perinatal mortality (very low‐quality evidence), or neonatal seizures (very low‐quality evidence). Other important infant outcomes were not reported: mortality or serious morbidity (composite outcome), cerebral palsy or neurosensory disability. Only one study reported maternal outcomes. Women allocated to the Doppler ultrasonography had higher rates of caesarean section for fetal distress compared with those allocated to routine Pinard (moderate‐quality evidence). There was no clear evidence of a difference in instrumental vaginal births between groups (low‐quality evidence). Other maternal outcomes were not reported (mortality, analgesia in labour, mobility or restriction and postnatal depression).
One trial compared intensive Pinard (a research midwife in a one‐to‐one care situation) with routine Pinard (midwife may be caring for more than one women in labour). There was no evidence of a difference between groups in low Apgar score (reported as < six this trial) (very low‐quality evidence), perinatal mortality (very low‐quality evidence), or neonatal seizures (very low‐quality evidence). Other infant outcomes were not reported. For maternal outcomes, there were no clear differences between groups for caesarean section or instrumental delivery (both low‐quality evidence)) Other outcomes were not reported.
Any ausculation of the FHR during labour attempts to identify those babies at risk of intrapartum hypoxia (Arulkumaran 2000). Apgar scores (< seven) have been used as a surrogate measure for birth asphyxia and possible long‐term adverse neurodevelopmental outcomes. Lie 2010 has published results of a large population‐based cohort trial confirming a strong association between low Apgar score and cerebral palsy in both low and normal birthweight infants. This review found no significant difference in low Apgar scores with different interventions for monitoring FHR. None of the included studies provided data for long‐term follow‐up assessing for neurodevelopmental disability or cerebral palsy.
There were more caesarean sections performed for fetal distress and caesarean sections without indications in the intermittent CTG and Doppler groups compared to the intensive Pinard and routine Pinard groups. This is understandable as the intermittent CTG and Doppler groups also detected significantly higher fetal distress interpreted as early and late fetal heart rate decelerations and brady‐ or tachycardia. Fetal hypoxia/acidosis was not confirmed with fetal scalp blood or umbilical cord blood sampling. It is difficult to interpret these findings in the absence of significant differences for maternal and fetal well‐being.
Furthermore, a Pinard requires the practitioner to have sound hearing ability (no full or partial deafness). It requires the listener to put an ear to one end of the tool and press it into the woman's abdomen. This requires close proximity, as most Pinards are around 15 cm long. Detecting the baby's anterior shoulder for optimal fetal heart sound is often only possible if the woman is reclined or semi‐reclined. This may result in missing significant abnormal fetal heart rate patterns or potentially induce maternal supine hypotension which may cause abnormal fetal heart rate patterns (Thurlow 2002), particularly when the trial protocols required the ausculation to last one minute immediately after a contraction. This would also be difficult to maintain with frequent contractions.
There was no clear evidence of a difference between the groups for NICU/NNU admissions and perinatal death although the caesarean section rate was higher for the CTG (one trial) and Doppler groups (two studies) compared to routine Pinard and the incidence of hypoxic ischaemic encephalopathy significantly higher in the routine Pinard group (Mahomed 1994). It is unclear why the NICU/NNU admission rate does not reflect this. Although the underlying causes for hypoxic ischaemic encephalopathy are not well understood, the condition has often long‐term consequences either physically or neuro‐psychologically and comprehensive long‐term follow‐up of these babies is needed to clarify the association. As noted before, no long‐term follow‐up data are available to assess if these findings are clinically significant and therefore caution needs to be applied when interpreting the findings.
The results of this review demonstrates that IA for FHR in labour with a hand‐held battery or wind‐up Doppler and intermittent CTG transducer (without paper tracing) identifies more FHR abnormalities (tachycardia, bradycardia, early and late decelerations), but leads to an increase in caesarean sections compared to using a Pinard as a listening device without showing significance differences in low Apgar scores at five minutes after the birth, perinatal death and admission to NICU/NNU. There are well‐known risks for the mother having a caesarean section particularly in Africa, the setting of all included studies, (Souza 2010) and these must also be considered when making decisions about methods of monitoring the fetal heart during labour. The findings suggest that intermittent FHR auscultation with a Pinard is comparable to a hand‐held Doppler as an effective listening tool, but this needs to be interpreted with caution as the confidence intervals are wide for FHR abnormality detection and no long‐term follow‐up data for the babies involved are available.
Only one trial Mahomed 1994, reported on neonatal seizures, identified as occurring in the neonatal unit. There were six seizures in the intensive Pinard group, nine in the routine Pinard group and no seizures in the Doppler and intermittent CTG group. These differences did not reach statistical significance and as no long‐term follow‐up outcome data were available it is unclear what these seizures mean in terms of infant well‐being. Mahomed 1994 on page 499, reported "that there were five neonatal deaths from hypoxia caused by delays in the delivery, either because of a delayed decision to operate or because the operating theatre was not available". It is questioned whether this situation could have also contributed to live babies who experienced neonatal seizures in NICU born by delayed emergency caesarean section and as a result of the delay experiencing prolonged fetal hypoxia. However, with the limited data available this could not be confirmed.
While results for perinatal death did not reach statistical significance, most deaths in the Mahomed 1994 trial resulted from a delay in birthing the babies due to either a delay in the decision to operate or the unavailability of the operating theatre, as reported above. This highlights the importance of understanding a trial's context when interpreting the findings and considering applicability across different practice settings.
Overall completeness and applicability of evidence
The inadequate and inconsistent reporting of the data from the Mdoe 2015 trial meant that it could not be included for assessment into this review. In all three included studies, the lack of reporting of a number of secondary outcomes for the mother and the baby, as well as the lack of long‐term follow‐up data for the infant limits the applicability of the evidence.
All trial settings were in Africa, identified as a low‐income country, therefore the results may not be generalisable or transferable to middle‐ or high‐income countries. In low‐income countries, a Pinard or a hand‐held Doppler device might be the only tool available to assess fetal well‐being during labour.
Most of our pre‐specified outcomes were not reported in the included trials. None of the included studies reported on longer‐term outcomes for the baby. In addition, only one of the trials reported on maternal outcomes. One of our important outcomes was instrumental vaginal birth for fetal distress of fetal acidosis, which was not reported. The number of women undergoing instrumental birth was reported in the trial contributing data, the reasons for the intervention were not, and although fetal distress is the most likely reason for assisted delivery, there may have been other reasons such as prolonged labour or maternal exhaustion. In the absence of other data, we included instrumental vaginal birth (for whatever reason) in our 'Summary of findings' tables.
Quality of the evidence
The overall quality of the evidence has been assessed using the GRADE approach as moderate to very low quality and has been summarised in the summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6. The main reasons for downgrading evidence were study design limitations (due to lack of blinding) and imprecision (often due to low event rates).
Potential biases in the review process
We have searched the available literature for published and unpublished studies related to IA. There were no restrictions to language or date of publication. We used Cochrane methodology and two review authors independently identified potential studies for inclusion, extracted data and entered data for the review. We believe we have minimised potential biases in the review process.
Agreements and disagreements with other studies or reviews
Given the limited evidence available in this area, we did not identify any major disagreements with other studies or reviews. The NICE 2014 guidelines for intrapartum care recommend IA of the fetal heart during established labour for women without risk factors using either a Pinard stethoscope or Doppler ultrasound immediately after a contraction listening for at least one minute at least every 15 minutes. This recommendation is based on results from reviews and observational studies comparing continuous CTG with IA, not comparing different intermittent listening devices with each other and/or different timing intervals. The recommended timing intervals in the studies included in this review was 30 minutes for the first stage of labour.
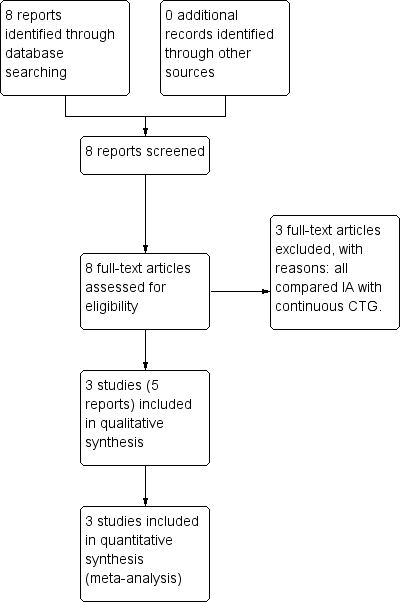
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included trial.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
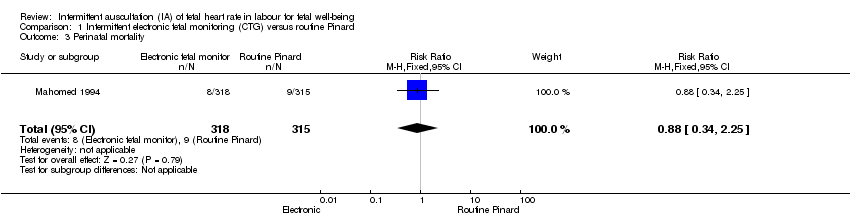
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 3 Perinatal mortality.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 6 Admission to NICU/NNU.
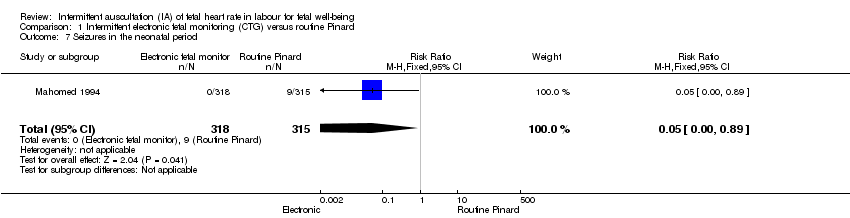
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 7 Seizures in the neonatal period.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 9 Caesarean section.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 10 Instrumental vaginal birth.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 11 Length of labour (hours).

Comparison 2 Doppler versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.

Comparison 2 Doppler versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
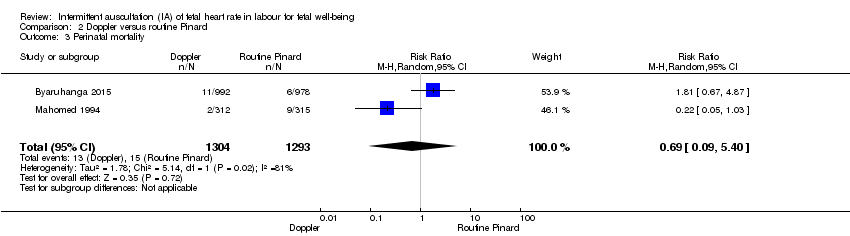
Comparison 2 Doppler versus routine Pinard, Outcome 3 Perinatal mortality.

Comparison 2 Doppler versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.
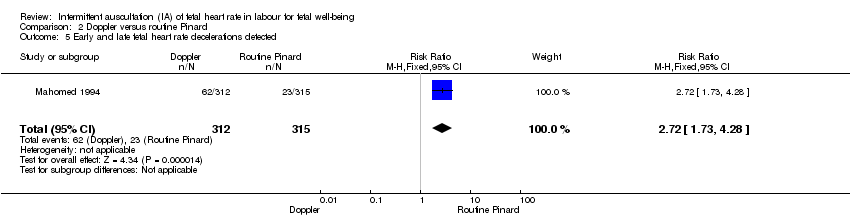
Comparison 2 Doppler versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.

Comparison 2 Doppler versus routine Pinard, Outcome 6 Admission to NICU/NNU.
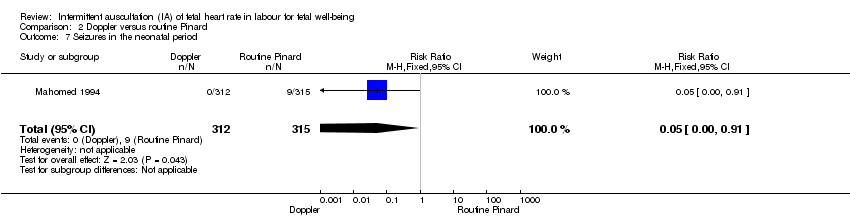
Comparison 2 Doppler versus routine Pinard, Outcome 7 Seizures in the neonatal period.
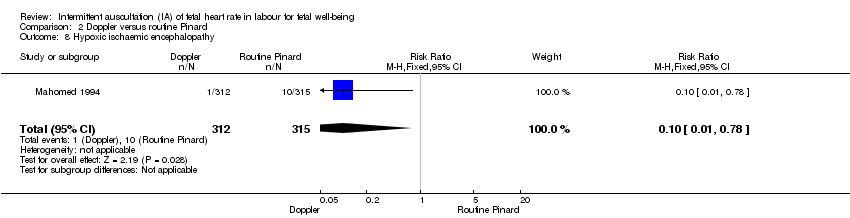
Comparison 2 Doppler versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.

Comparison 2 Doppler versus routine Pinard, Outcome 9 Caesarean section.
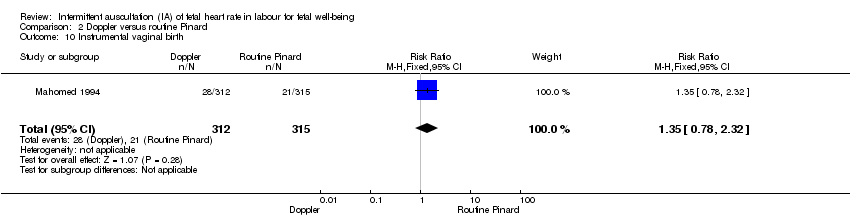
Comparison 2 Doppler versus routine Pinard, Outcome 10 Instrumental vaginal birth.

Comparison 2 Doppler versus routine Pinard, Outcome 11 Length of labour (hours).

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
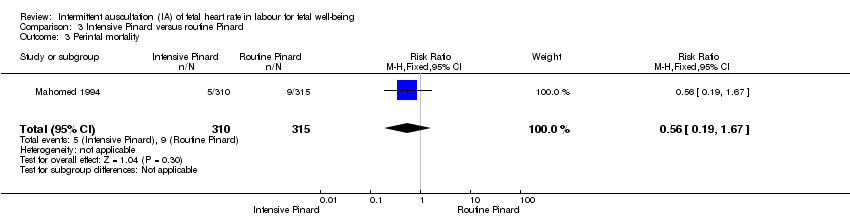
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 3 Perintal mortality.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.
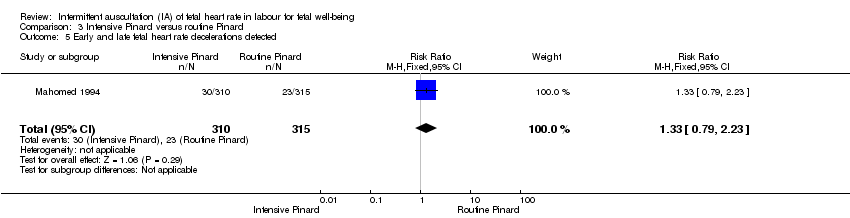
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.
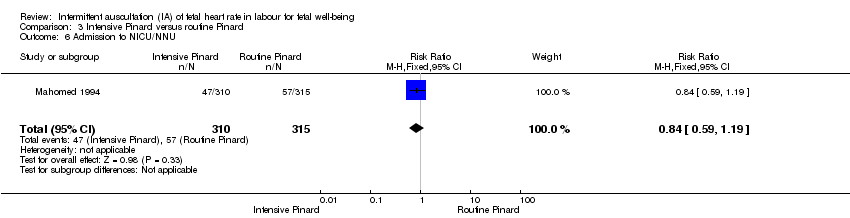
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 6 Admission to NICU/NNU.
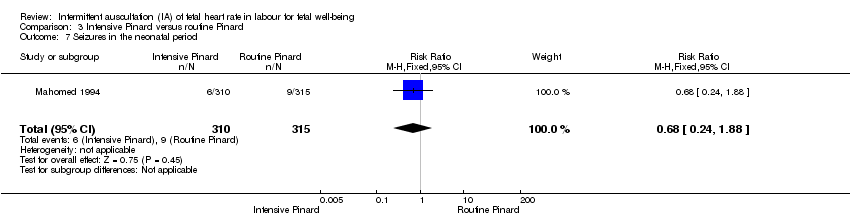
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 7 Seizures in the neonatal period.
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 9 Caesarean section.
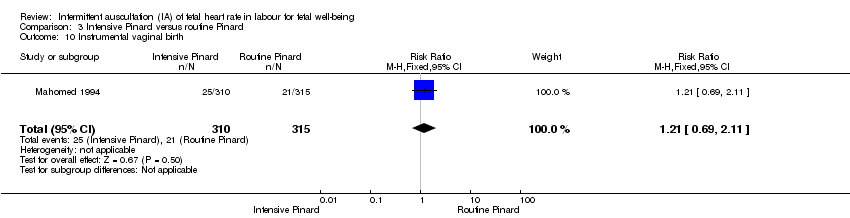
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 10 Instrumental vaginal birth.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 11 Length of labour (hours).
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus routine Pinard (outcomes for the baby). | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 19 per 1000 | RR 0.66 | 633 | ⊕⊕⊝⊝ | Low event rate. Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 1 per 1000 | RR 0.05 | 633 | ⊕⊕⊝⊝ LOW 1,3 | Low event rates. Routine Pinard group (9/315) compared to the intermittent EFM (CTG) group (0/318). |
| Perinatal mortality | 29 per 1000 | 25 per 1000 | RR 0.88 | 633 | ⊕⊝⊝⊝ | Neonatal deaths included, unable to separate out from reported data. Low event rates 8/318 for intermittent EFM (CTG) group and 9/315 for routine Pinard group. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies at either 6 months or 1 year. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision; single trial with low event rate and wide 95% CI crossing the line of no effect. Downgraded two levels. 3 Evidence of imprecision, evidence based on a single trial with low event rates. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus Routine Pinard (outcomes for the mother). | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 176 per 1000 | RR 2.92 | 633 | ⊕⊕⊕⊝ MODERATE 1, | |
| Instrumental vaginal birth | 67 per 1000 | 97 per 1000 | RR 1.46 | 633 | ⊕⊕⊝⊝ LOW 1,2, | |
| Maternal mortality | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for any pharmacological or non‐ pharmacological analgesia use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision with wide confidence intervals. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Routine Pinard | Risk with Doppler | |||||
| Apgar < 7 at 5 minutes | 20 per 1000 | 15 per 1000 | RR 0.76 | 2598 | ⊕⊝⊝⊝ | One of the studies contributing data reported Apgar score < 6. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Seizures in the neonatal period | 29 per 1000 | 1 per 1000 | RR 0.05 | 627 | ⊕⊝⊝⊝ | Event rates are low 0/312 for Doppler and 9/315 for routine Pinard. |
| Perinatal mortality | 12 per 1000 | 8 per 1000 | RR 0.69 | 2597 | ⊕⊕⊝⊝ | Event rates 13/1304 for Doppler and 15/1293 for routine Pinard. Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision with wide 95% CI crossing the line of no effect. Downgraded one level. 3 There was high heterogeneity for this outcome. 4 Evidence of imprecision, with wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels. 5 There was high heterogeneity for this outcome. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Doppler | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 163 per 1000 | RR 2.71 | 627 | ⊕⊕⊕⊝ | |
| Instrumental vaginal birth | 67 per 1000 | 90 per 1000 | RR 1.35 | 627 | ⊕⊕⊝⊝ | |
| Maternal mortality | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for any pharmacological or non‐pharmacological use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Wide confidence interval. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 26 per 1000 | RR 0.90 | 625 | ⊕⊝⊝⊝ | Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 19 per 1000 | RR 0.68 | 625 | ⊕⊝⊝⊝ | |
| Perinatal mortality | 29 per 1000 | 16 per 1000 | RR 0.56 | 625 | ⊕⊝⊝⊝ | Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included trial. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded 1 level. 2 Evidence was imprecise; wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 42 per 1000 | RR 0.70 | 625 | ⊕⊕⊝⊝ | |
| Instrumental vaginal birth | 67 per 1000 | 81 per 1000 | RR 1.21 | 625 | ⊕⊕⊝⊝ | |
| Maternal morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal morbidity in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No reported data for any pharmacological or non‐pharmacological analgesia use excluding epidural. | |
| Epidural anaesthesia for pain relief excluding caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for post natal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Some imprecision with wide CI crossing the line of no effect. Downgraded one level. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.83] |
| 2 Caesarean section for fetal distress Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.78, 4.80] |
| 3 Perinatal mortality Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.34, 2.25] |
| 4 Fetal heart rate abnormality detected Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.08 [4.21, 8.79] |
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [1.82, 4.45] |
| 6 Admission to NICU/NNU Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.25] |
| 7 Seizures in the neonatal period Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.89] |
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 9 Caesarean section Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.39, 2.64] |
| 10 Instrumental vaginal birth Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.86, 2.49] |
| 11 Length of labour (hours) Show forest plot | 1 | 633 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.05, 1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.20, 2.87] |
| 2 Caesarean section for fetal distress Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.64, 4.48] |
| 3 Perinatal mortality Show forest plot | 2 | 2597 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.09, 5.40] |
| 4 Fetal heart rate abnormality detected Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.09, 5.29] |
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.73, 4.28] |
| 6 Admission to NICU/NNU Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.91] |
| 7 Seizures in the neonatal period Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.91] |
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.78] |
| 9 Caesarean section Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.81, 2.05] |
| 10 Instrumental vaginal birth Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.78, 2.32] |
| 11 Length of labour (hours) Show forest plot | 1 | 627 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.07, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.35, 2.31] |
| 2 Caesarean section for fetal distress Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.38] |
| 3 Perintal mortality Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.67] |
| 4 Fetal heart rate abnormality detected Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.10, 2.65] |
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.79, 2.23] |
| 6 Admission to NICU/NNU Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.19] |
| 7 Seizures in the neonatal period Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.24, 1.88] |
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.84] |
| 9 Caesarean section Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.46, 1.08] |
| 10 Instrumental vaginal birth Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.69, 2.11] |
| 11 Length of labour (hours) Show forest plot | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.52, 1.52] |

