Auskultasi berselang‐seli (IA) kadar denyutan jantung janin semasa bersalin untuk kesejahteraan janin
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Prospective parallel randomised controlled trial. Funding: Grand Challenges Canada provided funding for the trial (grant number CRS1 0018) and Laerdal Foundation for the training module ‘Helping Babies Survive Labour’ (grant number 40038). | |
| Participants | Location: Nsambya teaching hospital in Kampala, Uganda. 1 centre. Inclusion criteria: women consented antenatal and reconfirmed in active labour. Women included were in established labour, with singleton pregnancy, cephalic presentation, > 37 weeks' gestation including post term, cervical dilation ≤ 7 cm, normal FHR on admission (120‐160). Exclusion criteria: women in second stage of labour or diagnosed as contraindicated for labouring when admitted (e.g. antepartum haemorrhage), intrauterine fetal death on admission or were having an elective caesarean. 1987 pregnant women were recruited. | |
| Interventions | Women in established labour received either: Intermittent FHR auscultation with hand‐held wind‐up Doppler for 1 minute (60 seconds) immediately after a contraction:
(n = 1000, 992 included in the analysis) or, Intermittent FHR auscultation with a Pinard for 1 minute (60 seconds) immediately after a contraction for the same time frames as above (n = 978, 979 included in the analysis). | |
| Outcomes | Primary outcomes: detection of FHR abnormality in labour (tachycardia > 160 bpm, bradycardia < 110 bpm, atypical variable, late or prolonged decelerations), intrapartum stillbirth and neonatal deaths in first 24 hours. Secondary outcomes: Apgar score < 7 at 5 minutes, admission to special care unit, diagnosis of neonatal encephalopathy (mild moderate or severe) and emergency caesarean section. | |
| Notes | Emailed A. Montgomery ([email protected]), identified as corresponding author in the trial about the availability and access to further outcome data of the trial. Responded that she will talk to the team and come back, but this did not happened by date of review submission. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomised’ equally to 1 of 2 groups, no other details. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and care providers were not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Low risk | Less than 1% attrition reported and reasons for losses were provided. 1987 enrolled; 987 to Pinard and 1000 to Doppler. Not analysed for Pinard n = 8 due to loss to follow‐up (n = 1), delivered before monitoring initiated (n = 1), breech birth (n = 2), multiple pregnancy (n = 2). For Doppler n = 8 not analysed; delivered before monitoring initiated (n = 3), breech birth (n = 3), multiple pregnancy (n = 2). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No other risk of bias identified. Groups appeared balanced at baseline. |
| Methods | Prospective parallel randomised controlled trial, 1 centre. Funding: this trial was funded by World Health Organization special programme for research, development and research training in human reproduction. | |
| Participants | Location: Harare maternity hospital, Zimbabwe, Africa. 1 centre. Inclusion criteria: women consented antenatal. Women included were > 37 weeks' gestation, with obstetric and medical risk factors, in established labour, with singleton baby in cephalic presentation, cervical dilation ≤ 7 cm, normal FHR on admission (120‐160). Exclusion criteria: women excluded from the trial were those presenting with placental abruption or eclampsia. 1255 pregnant women were recruited. | |
| Interventions | EFM group (n = 318): The intention was for a CTG with continuous trace via an external abdominal transducer for 10 minutes every half hour if normal tracing and every 20 minutes for 10 minutes if abnormal. Monitoring performed by a trained research midwife and doctor, strictly adhering to the research protocol.(*see notes) Doppler group (n = 312): intermittent auscultation using hand‐held Doppler (ultrasonography), listening for 1 minute, during the last 10 minutes of each half hour, during and immediately after a contraction. Auscultation performed by a research midwife, who had been educated about the trial and its intervention, strictly adhering to the research protocol. Intensive Pinard group (n = 310): intermittent auscultation with a Pinard stethoscope listening for 1 minute during the last 10 minutes of every half hour during and immediately after a contraction. Auscultation performed by a research midwife, who had been educated about the trial and its intervention, strictly adhering to the research protocol. Routine Pinard group (n = 315): intermittent auscultation with a Pinard stethoscope as was routine at the involved hospital, which expected the FHR to be auscultated for 1 minute during last 10 minutes of every half hour during and immediately after a contraction. Auscultation would be performed by the midwife on‐duty, who may care for other labouring women at the same time and would endeavour to follow the hospital recommendation for routine auscultation as much as possible. | |
| Outcomes | Baby: Apgar < 6 at 5 minutes of birth, stillbirth or neonatal death, meconium‐stained liquor, admission to NICU/NNU, seizures in neonatal unit, hypoxic ischaemic encephalopathy, prolonged early and late FHR decelerations, abnormal FHR. Mother: caesarean section for fetal distress, total caesarean section, operative (instrumental) vaginal delivery, spontaneous vaginal birth, spontaneous onset of labour, length of labour. The results were recorded as a single rate. | |
| Notes | * A 10 min tracing was done but not with all participants. In this arm of the study 18 women birthed too quickly and 24 women had an unreadable tracing (frequent loss of contact, paper got stuck). It appears the FH was recorded as one number but late decelerations were noted on the 10 min tracing when they were present assisting with clinical management decisions. There were a number of discrepancies in the way the interventions were described in different trial reports. In the 1992 article on page 460 there was a typo, it reads ' ... every 10 minutes if results were abnormal', should read "..every 20 minutes if..." (clarified via email with main trial author, Mahomed 1994) Furthermore, the 1992 article reported that the nurse in charge applied the intervention of the routine group for fetal auscultation with a Pinard stethoscope. The 1994 article reported that is was the midwife on‐duty (clarified via email with main trial author (Mahomed 1994 that latter statement is correct). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... Eligible women were randomly allocated to one of four methods of monitoring intrapartum... The randomisation was performed with a random permuted block of 16 numbers..." (p. 498). It is not stated how the random permuted blocks were generated. |
| Allocation concealment (selection bias) | Low risk | Quote: " ...by means of serially numbered sealed opaque envelopes containing the allocation..." (p. 498). |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not stated, however it is unlikely that blinding would be possible with the nature of the interventions, as they were visibly different. Therefore considered as high risk of bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Low risk | There seems to be no loss of follow‐up, as numbers remain consistent. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No other risk of bias identified. Groups appeared balanced at baseline. |
| Methods | Prospective parallel randomised controlled trial. Funding: no details provided. | |
| Participants | Location: 2 settings in the United Republic of Tanzania. An urban setting at the Muhimbili National Hospital and rural setting at the Haydom Lutheran Hospital. In all settings fetal blood gas sampling or epidural analgesia were not available. Inclusion criteria: women with low risk, who consented and were in active labour with singleton pregnancies, cephalic presentation, normal FHR (120‐160 bpm) and with a cervical dilatation of ≤ 7 cm. Exclusion criteria: women arriving in second stage, no other exclusion criteria described. It appears 1376 women at Muhimbili National Hospital and 1623 women at Haydom Lutheran Hospital were recruited. A total of 2999 women were randomised. | |
| Interventions | Intermittent auscultation with hand‐held Doppler (Doppler) comparing urban with rural setting (n = 1521 (not clear)). Frequency of intermittent auscultation and length of listening (timing) not described but stated as 'free play'. Unclear what 'free play' mean. Or intermittent auscultation with Pinard Feteoscope comparing urban with rural setting (n =1475 (not clear)). Frequency of intermittent auscultation and length of listening (timing) not described but stated as 'free play'. Unclear what 'free play' means. | |
| Outcomes | Outcomes are not reported separately for primary and secondary outcomes. Outcomes reported were caesarean section; abnormal FHR defined as < 120 or > 160 bpm; admission to NNU; 5 minute Apgar score < 7; bag mask ventilation; fresh stillbirth; neonatal death. | |
| Notes | Review author RM emailed Mdoe to seek further details to describe the interventions, in particular what 'free play' was. Clarification was also sought about the published data, as the outcome data presented did not correspond with the total number of participants identified. Mdoe responded stating that It is anticipated that the results of the trial will be published within the next 6 months and suggested to wait for this publication. No further details provided. This trial was only published as an abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | From email correspondence and abstract stated: "randomisation". No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | From email correspondence confirmed '1 to 1 open label'. Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not stated, however it is unlikely that blinding would be possible with the nature of the interventions, as they were visibly different. Therefore considered as high risk of bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No other details given. |
| Incomplete outcome data (attrition bias) | Unclear risk | This is not stated and the numerical data provided had major numerical inconsistencies in the publication and could not be used for meta‐analysis. Unclear which numbers were due to loss of follow‐up. |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes and the outcomes reported do not match. |
| Other bias | Unclear risk | This publication is only an abstract and it remains unclear if the groups were balanced at baseline. |
bpm: beats per minute
CTG: cardiotocograph
EFM: electronic fetal monitoring
FHR: fetal heart rate
NICU: neonatal intensive care unit
NNU: neonatal unit
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Compared intermittent ausculation with continuous monitoring, which was not one of the included comparisons. | |
| Compared intermittent ausculation with continuous monitoring, which was not one of the included comparisons. | |
| Compared intermittent ausculation with continuous monitoring, which was not one of the included comparisons. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.83] |
| Analysis 1.1 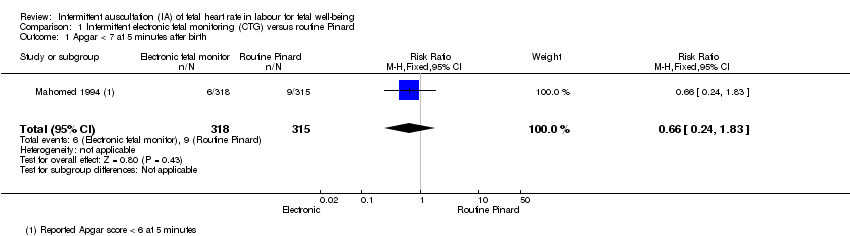 Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth. | ||||
| 2 Caesarean section for fetal distress Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.78, 4.80] |
| Analysis 1.2 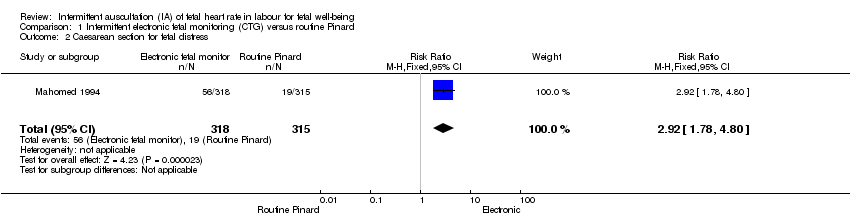 Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 2 Caesarean section for fetal distress. | ||||
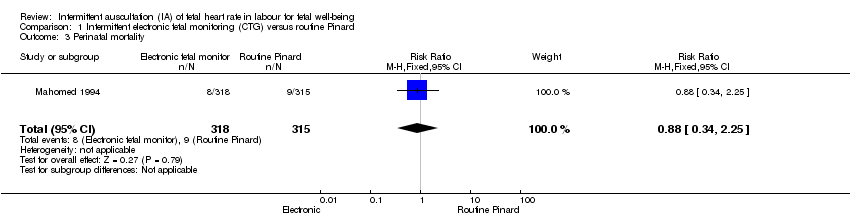
| 3 Perinatal mortality Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.34, 2.25] |
| Analysis 1.3  Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 3 Perinatal mortality. | ||||
| 4 Fetal heart rate abnormality detected Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.08 [4.21, 8.79] |
| Analysis 1.4 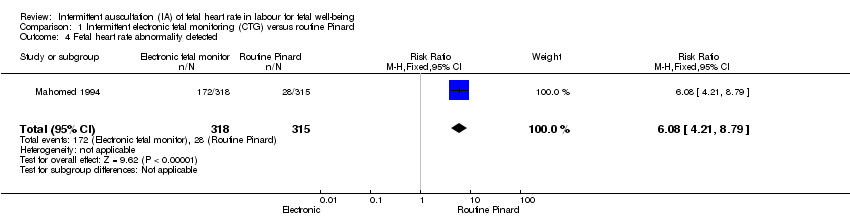 Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected. | ||||
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [1.82, 4.45] |
| Analysis 1.5 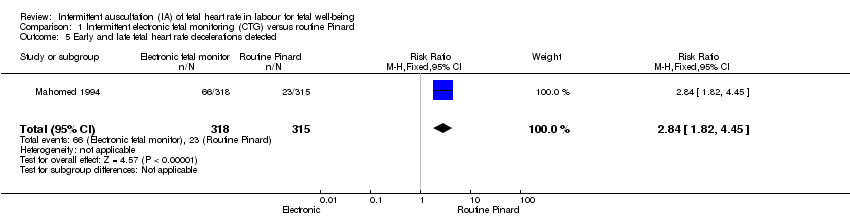 Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected. | ||||
| 6 Admission to NICU/NNU Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.25] |
| Analysis 1.6  Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 6 Admission to NICU/NNU. | ||||
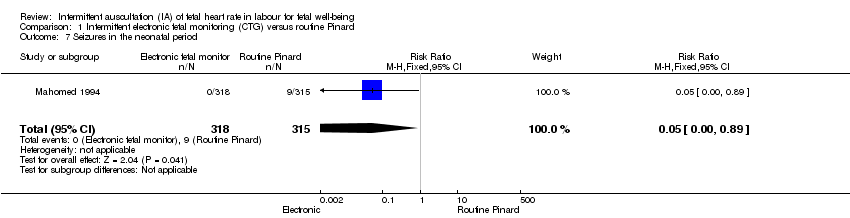
| 7 Seizures in the neonatal period Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.89] |
| Analysis 1.7  Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 7 Seizures in the neonatal period. | ||||
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| Analysis 1.8 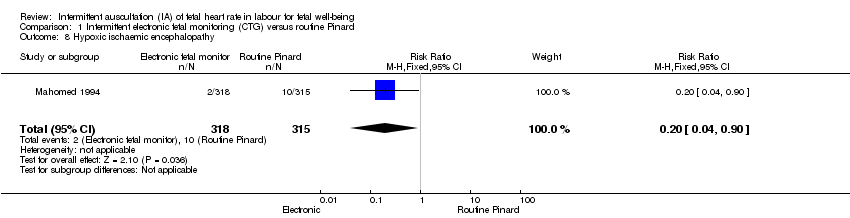 Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy. | ||||
| 9 Caesarean section Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.39, 2.64] |
| Analysis 1.9  Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 9 Caesarean section. | ||||
| 10 Instrumental vaginal birth Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.86, 2.49] |
| Analysis 1.10  Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 10 Instrumental vaginal birth. | ||||
| 11 Length of labour (hours) Show forest plot | 1 | 633 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.05, 1.85] |
| Analysis 1.11  Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 11 Length of labour (hours). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.20, 2.87] |
| Analysis 2.1  Comparison 2 Doppler versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth. | ||||
| 2 Caesarean section for fetal distress Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.64, 4.48] |
| Analysis 2.2  Comparison 2 Doppler versus routine Pinard, Outcome 2 Caesarean section for fetal distress. | ||||
| 3 Perinatal mortality Show forest plot | 2 | 2597 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.09, 5.40] |
| Analysis 2.3  Comparison 2 Doppler versus routine Pinard, Outcome 3 Perinatal mortality. | ||||
| 4 Fetal heart rate abnormality detected Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.09, 5.29] |
| Analysis 2.4  Comparison 2 Doppler versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected. | ||||
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.73, 4.28] |
| Analysis 2.5 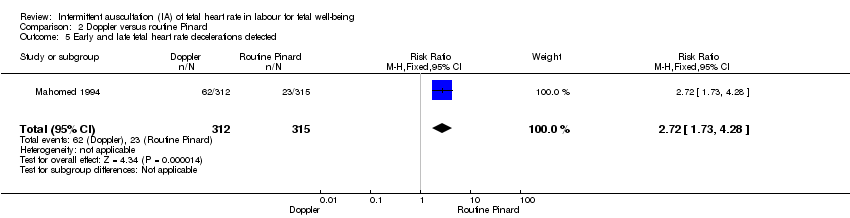 Comparison 2 Doppler versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected. | ||||
| 6 Admission to NICU/NNU Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.91] |
| Analysis 2.6  Comparison 2 Doppler versus routine Pinard, Outcome 6 Admission to NICU/NNU. | ||||
| 7 Seizures in the neonatal period Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.91] |
| Analysis 2.7 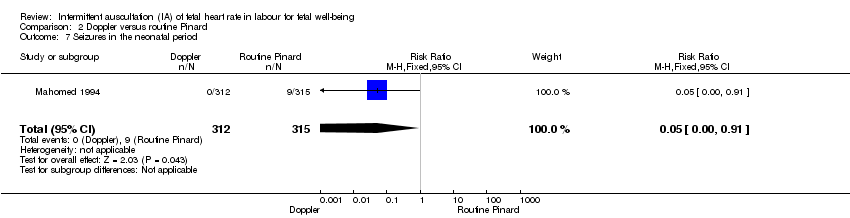 Comparison 2 Doppler versus routine Pinard, Outcome 7 Seizures in the neonatal period. | ||||
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.78] |
| Analysis 2.8  Comparison 2 Doppler versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy. | ||||
| 9 Caesarean section Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.81, 2.05] |
| Analysis 2.9  Comparison 2 Doppler versus routine Pinard, Outcome 9 Caesarean section. | ||||
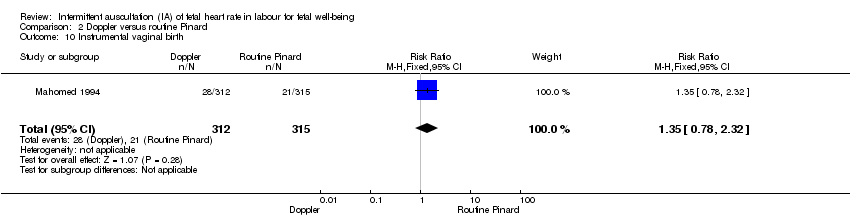
| 10 Instrumental vaginal birth Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.78, 2.32] |
| Analysis 2.10  Comparison 2 Doppler versus routine Pinard, Outcome 10 Instrumental vaginal birth. | ||||
| 11 Length of labour (hours) Show forest plot | 1 | 627 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.07, 1.07] |
| Analysis 2.11 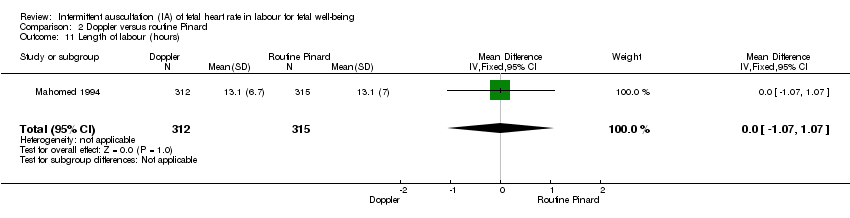 Comparison 2 Doppler versus routine Pinard, Outcome 11 Length of labour (hours). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.35, 2.31] |
| Analysis 3.1  Comparison 3 Intensive Pinard versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth. | ||||
| 2 Caesarean section for fetal distress Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.38] |
| Analysis 3.2  Comparison 3 Intensive Pinard versus routine Pinard, Outcome 2 Caesarean section for fetal distress. | ||||
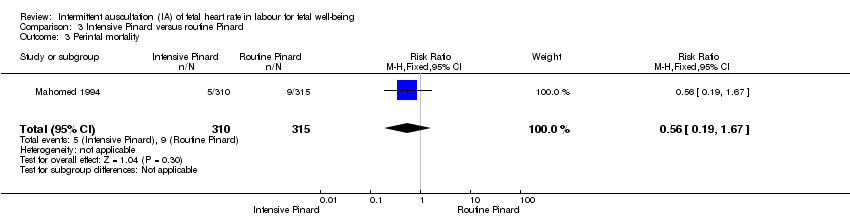
| 3 Perintal mortality Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.67] |
| Analysis 3.3 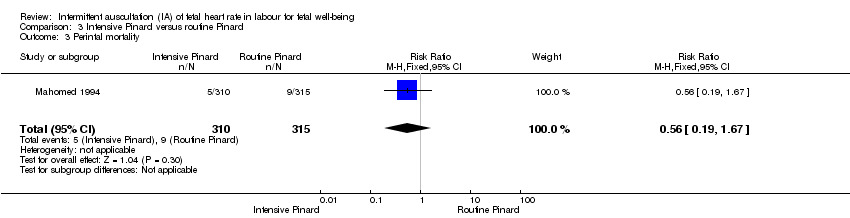 Comparison 3 Intensive Pinard versus routine Pinard, Outcome 3 Perintal mortality. | ||||
| 4 Fetal heart rate abnormality detected Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.10, 2.65] |
| Analysis 3.4 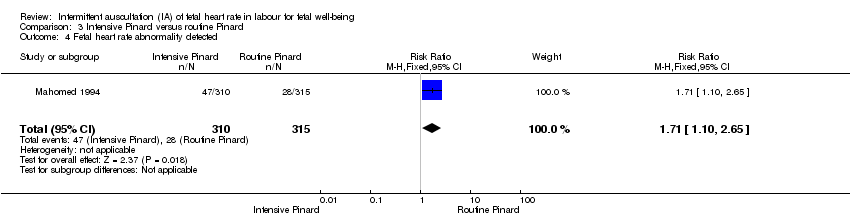 Comparison 3 Intensive Pinard versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected. | ||||
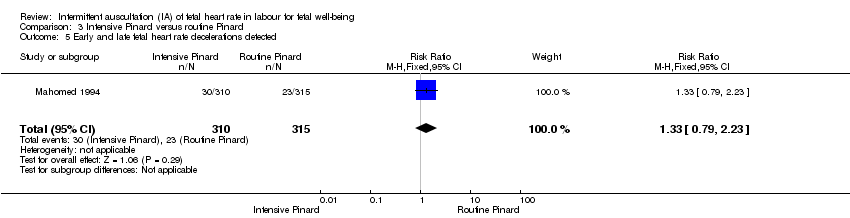
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.79, 2.23] |
| Analysis 3.5 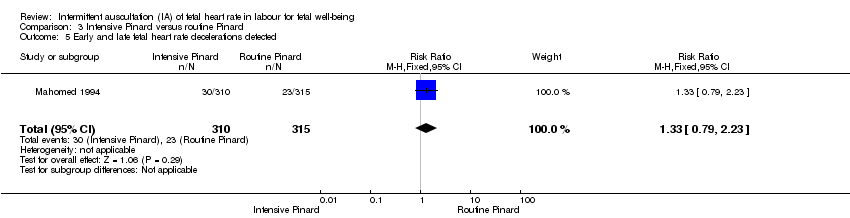 Comparison 3 Intensive Pinard versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected. | ||||
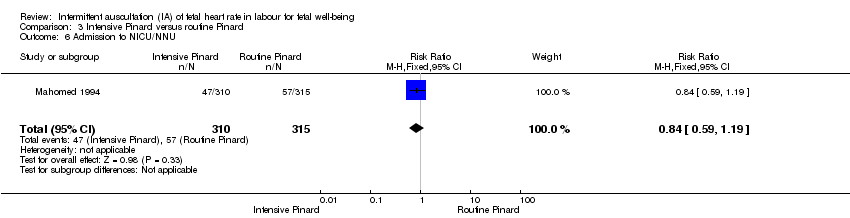
| 6 Admission to NICU/NNU Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.19] |
| Analysis 3.6  Comparison 3 Intensive Pinard versus routine Pinard, Outcome 6 Admission to NICU/NNU. | ||||
| 7 Seizures in the neonatal period Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.24, 1.88] |
| Analysis 3.7  Comparison 3 Intensive Pinard versus routine Pinard, Outcome 7 Seizures in the neonatal period. | ||||
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.84] |
| Analysis 3.8 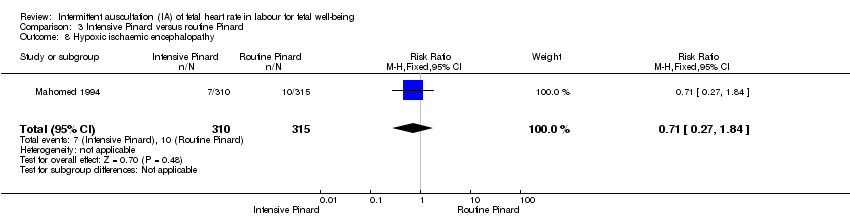 Comparison 3 Intensive Pinard versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy. | ||||
| 9 Caesarean section Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.46, 1.08] |
| Analysis 3.9 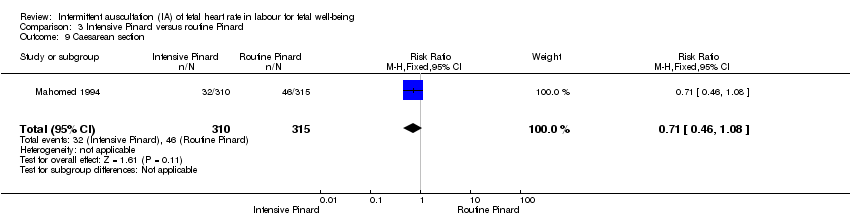 Comparison 3 Intensive Pinard versus routine Pinard, Outcome 9 Caesarean section. | ||||
| 10 Instrumental vaginal birth Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.69, 2.11] |
| Analysis 3.10  Comparison 3 Intensive Pinard versus routine Pinard, Outcome 10 Instrumental vaginal birth. | ||||
| 11 Length of labour (hours) Show forest plot | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.52, 1.52] |
| Analysis 3.11  Comparison 3 Intensive Pinard versus routine Pinard, Outcome 11 Length of labour (hours). | ||||
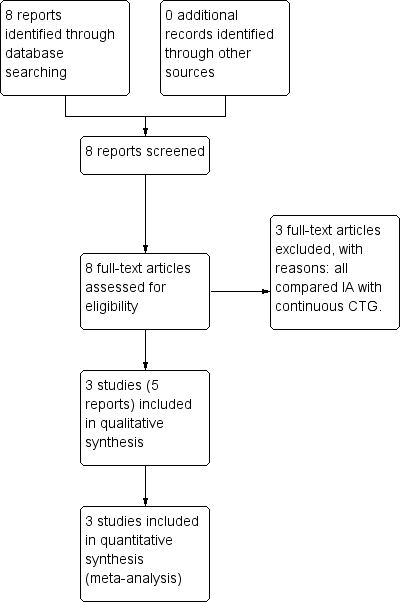
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included trial.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 3 Perinatal mortality.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 6 Admission to NICU/NNU.
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 7 Seizures in the neonatal period.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 9 Caesarean section.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 10 Instrumental vaginal birth.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 11 Length of labour (hours).

Comparison 2 Doppler versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.

Comparison 2 Doppler versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
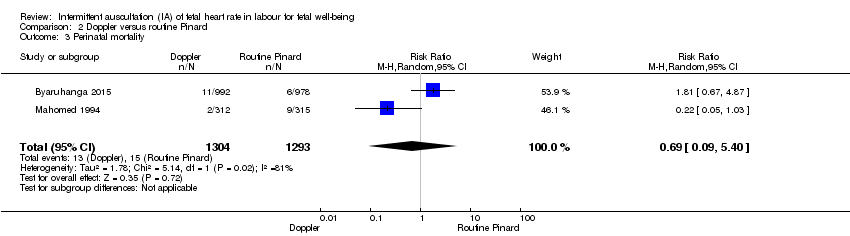
Comparison 2 Doppler versus routine Pinard, Outcome 3 Perinatal mortality.

Comparison 2 Doppler versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.
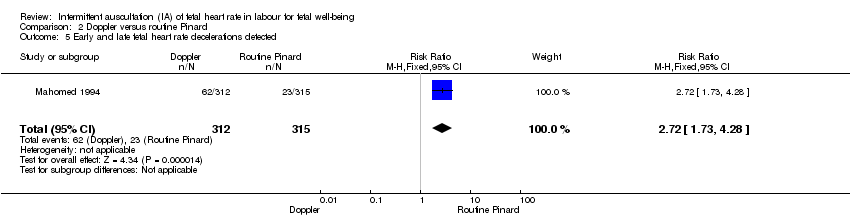
Comparison 2 Doppler versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.

Comparison 2 Doppler versus routine Pinard, Outcome 6 Admission to NICU/NNU.
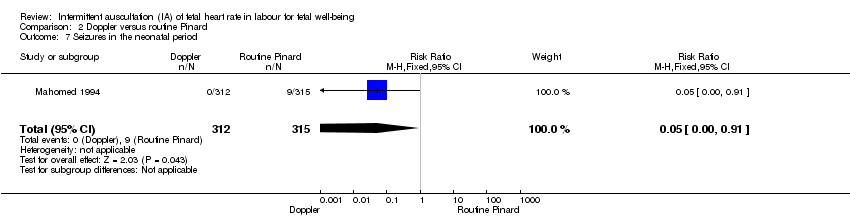
Comparison 2 Doppler versus routine Pinard, Outcome 7 Seizures in the neonatal period.
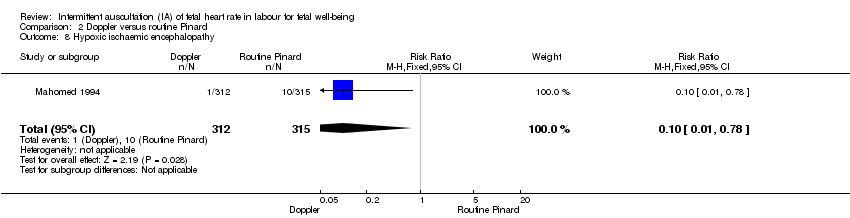
Comparison 2 Doppler versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.

Comparison 2 Doppler versus routine Pinard, Outcome 9 Caesarean section.
Comparison 2 Doppler versus routine Pinard, Outcome 10 Instrumental vaginal birth.

Comparison 2 Doppler versus routine Pinard, Outcome 11 Length of labour (hours).

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 3 Perintal mortality.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 6 Admission to NICU/NNU.
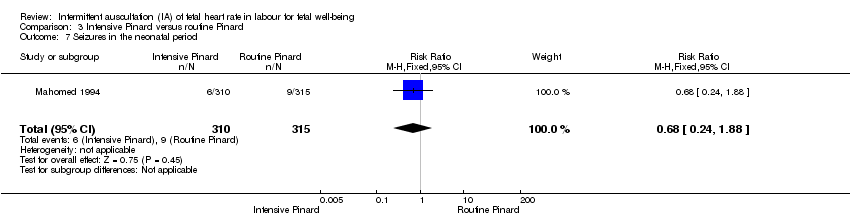
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 7 Seizures in the neonatal period.
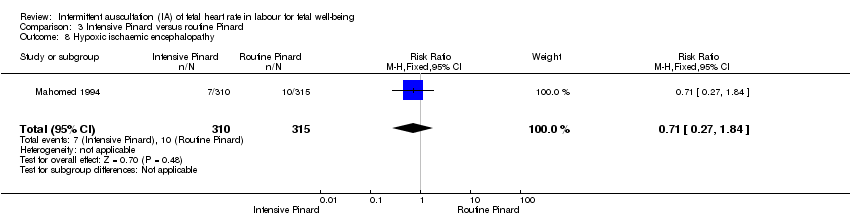
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 9 Caesarean section.
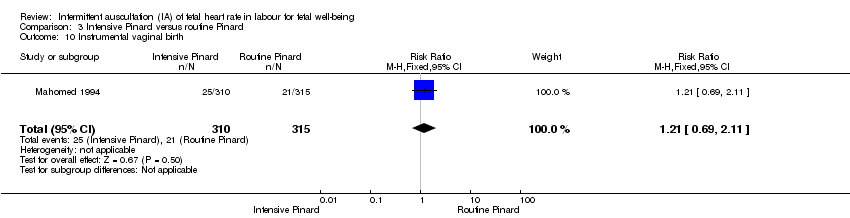
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 10 Instrumental vaginal birth.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 11 Length of labour (hours).
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus routine Pinard (outcomes for the baby). | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 19 per 1000 | RR 0.66 | 633 | ⊕⊕⊝⊝ | Low event rate. Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 1 per 1000 | RR 0.05 | 633 | ⊕⊕⊝⊝ LOW 1,3 | Low event rates. Routine Pinard group (9/315) compared to the intermittent EFM (CTG) group (0/318). |
| Perinatal mortality | 29 per 1000 | 25 per 1000 | RR 0.88 | 633 | ⊕⊝⊝⊝ | Neonatal deaths included, unable to separate out from reported data. Low event rates 8/318 for intermittent EFM (CTG) group and 9/315 for routine Pinard group. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies at either 6 months or 1 year. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision; single trial with low event rate and wide 95% CI crossing the line of no effect. Downgraded two levels. 3 Evidence of imprecision, evidence based on a single trial with low event rates. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus Routine Pinard (outcomes for the mother). | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 176 per 1000 | RR 2.92 | 633 | ⊕⊕⊕⊝ MODERATE 1, | |
| Instrumental vaginal birth | 67 per 1000 | 97 per 1000 | RR 1.46 | 633 | ⊕⊕⊝⊝ LOW 1,2, | |
| Maternal mortality | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for any pharmacological or non‐ pharmacological analgesia use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision with wide confidence intervals. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Routine Pinard | Risk with Doppler | |||||
| Apgar < 7 at 5 minutes | 20 per 1000 | 15 per 1000 | RR 0.76 | 2598 | ⊕⊝⊝⊝ | One of the studies contributing data reported Apgar score < 6. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Seizures in the neonatal period | 29 per 1000 | 1 per 1000 | RR 0.05 | 627 | ⊕⊝⊝⊝ | Event rates are low 0/312 for Doppler and 9/315 for routine Pinard. |
| Perinatal mortality | 12 per 1000 | 8 per 1000 | RR 0.69 | 2597 | ⊕⊕⊝⊝ | Event rates 13/1304 for Doppler and 15/1293 for routine Pinard. Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision with wide 95% CI crossing the line of no effect. Downgraded one level. 3 There was high heterogeneity for this outcome. 4 Evidence of imprecision, with wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels. 5 There was high heterogeneity for this outcome. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Doppler | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 163 per 1000 | RR 2.71 | 627 | ⊕⊕⊕⊝ | |
| Instrumental vaginal birth | 67 per 1000 | 90 per 1000 | RR 1.35 | 627 | ⊕⊕⊝⊝ | |
| Maternal mortality | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for any pharmacological or non‐pharmacological use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Wide confidence interval. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 26 per 1000 | RR 0.90 | 625 | ⊕⊝⊝⊝ | Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 19 per 1000 | RR 0.68 | 625 | ⊕⊝⊝⊝ | |
| Perinatal mortality | 29 per 1000 | 16 per 1000 | RR 0.56 | 625 | ⊕⊝⊝⊝ | Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included trial. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded 1 level. 2 Evidence was imprecise; wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 42 per 1000 | RR 0.70 | 625 | ⊕⊕⊝⊝ | |
| Instrumental vaginal birth | 67 per 1000 | 81 per 1000 | RR 1.21 | 625 | ⊕⊕⊝⊝ | |
| Maternal morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal morbidity in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No reported data for any pharmacological or non‐pharmacological analgesia use excluding epidural. | |
| Epidural anaesthesia for pain relief excluding caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for post natal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Some imprecision with wide CI crossing the line of no effect. Downgraded one level. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.83] |
| 2 Caesarean section for fetal distress Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.78, 4.80] |
| 3 Perinatal mortality Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.34, 2.25] |
| 4 Fetal heart rate abnormality detected Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.08 [4.21, 8.79] |
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [1.82, 4.45] |
| 6 Admission to NICU/NNU Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.25] |
| 7 Seizures in the neonatal period Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.89] |
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 9 Caesarean section Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.39, 2.64] |
| 10 Instrumental vaginal birth Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.86, 2.49] |
| 11 Length of labour (hours) Show forest plot | 1 | 633 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.05, 1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.20, 2.87] |
| 2 Caesarean section for fetal distress Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.64, 4.48] |
| 3 Perinatal mortality Show forest plot | 2 | 2597 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.09, 5.40] |
| 4 Fetal heart rate abnormality detected Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.09, 5.29] |
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.73, 4.28] |
| 6 Admission to NICU/NNU Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.91] |
| 7 Seizures in the neonatal period Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.91] |
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.78] |
| 9 Caesarean section Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.81, 2.05] |
| 10 Instrumental vaginal birth Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.78, 2.32] |
| 11 Length of labour (hours) Show forest plot | 1 | 627 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.07, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.35, 2.31] |
| 2 Caesarean section for fetal distress Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.38] |
| 3 Perintal mortality Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.67] |
| 4 Fetal heart rate abnormality detected Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.10, 2.65] |
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.79, 2.23] |
| 6 Admission to NICU/NNU Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.19] |
| 7 Seizures in the neonatal period Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.24, 1.88] |
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.84] |
| 9 Caesarean section Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.46, 1.08] |
| 10 Instrumental vaginal birth Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.69, 2.11] |
| 11 Length of labour (hours) Show forest plot | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.52, 1.52] |

