Estatinas para el hígado graso no alcohólico y la esteatohepatitis no alcohólica
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008623.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 27 diciembre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Hepatobiliar
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Formulating the research question: Reza Malekzadeh (RM), Layli Eslami (LE), and Siavosh Nasseri‐Moghaddam (SNM).
Writing up the protocol: LE with comments from SNM and Shahin Merat (SM).
Searching: Cochrane Hepato‐Biliary Group (CHBG).
Selecting and reviewing eligible studies: LE and SNM.
Performing methodological assessment and extraction of data: LE.
Providing data entry and management: LE.
Performing data analysis: LE.
Preparing the manuscript: LE, Hermineh Aramin (HA), and SNM.
Sources of support
Internal sources
-
Tehran University of Medical Sciences (TUMS), Iran.
External sources
-
No sources of support supplied
Declarations of interest
None known.
Acknowledgements
Our special thanks goes to Dimitrinka Nikolova and Sarah Louise Klingenberg in The Cochrane Hepato‐Biliary Group (CHBG) for their unlimited help in preparing this review.
Peer reviewers: Mona H Ismail, Saudi Arabia; Yusuf Yilmaz, Turkey; Raj Vuppalanchi, USA.
Contact editor: Christian Gluud, Denmark.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Dec 27 | Statins for non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis | Review | Layli Eslami, Shahin Merat, Reza Malekzadeh, Siavosh Nasseri‐Moghaddam, Hermineh Aramin | |
| 2010 Jul 07 | Statins for non‐alcoholic steatohepatitis | Protocol | Layli Eslami, Shahin Merat, Reza Malekzadeh, Siavosh Nasseri‐Moghaddam, Hermineh Aramin | |
Differences between protocol and review
As the result of recent changes in Cochrane requirements, the order of outcomes in this review has been changed so that they reflect the needs of consumers.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Alanine Transaminase [blood];
- Atorvastatin;
- Fatty Liver [*drug therapy, enzymology];
- Fenofibrate [therapeutic use];
- Heptanoic Acids [therapeutic use];
- Hydroxymethylglutaryl‐CoA Reductase Inhibitors [*therapeutic use];
- Non‐alcoholic Fatty Liver Disease;
- Pyrroles [therapeutic use];
- Simvastatin [therapeutic use];
- gamma‐Glutamyltransferase [blood];
Medical Subject Headings Check Words
Humans;
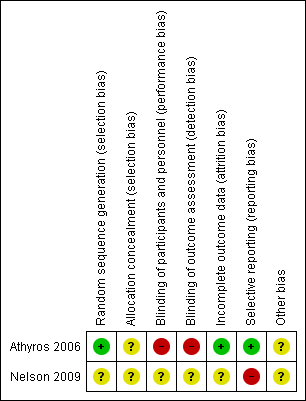
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Source of the search | Number of records |
| CHBG Controlled Trial Register | 51 |
| Cochrane Central Register of Controlled Trials | 78 |
| MEDLINE (Ovid) | 116 |
| EMBASE (Ovid) | 518 |
| Science Citation Index EXPANDED | 91 |
| Total number of references identified | 851 |
| Number of duplicates excluded | 198 |
| Number of references in final list | 653 |
|
| Statin (n = 10) | Placebo (n = 6) | ||||
| Before treatment | After treatment | Mean decrease | Before treatment | After treatment | Mean decrease | |
| ALT (U/L) | 70.4 | 49.5 | 20.9 | 66.8 | 75.3 | ‐8.5 |
| AST (U/L) | 43.3 | 36.5 | 6.8 | 42.8 | 49.3 | ‐6.5 |
| ALP (U/L) | 86.1 | 89.7 | ‐3.6 | 74.3 | 73 | 1.3 |
| TG (mg/dL) | 388.7 | 490 | ‐101.3 | 335.3 | 361.7 | ‐26.4 |
| ALT = alanine aminotransferase | ||||||
|
| Statin (n = 63) | Fenofibrate (n = 62) | ||||
| Before treatment | After treatment | Mean decrease | Before treatment | After treatment | Mean decrease | |
| ALT (U/L) | 54 | 32 | 22 | 52 | 36 | 16 |
| AST (U/L) | 38 | 25 | 13 | 39 | 27 | 12 |
| ALP (U/L) | 110 | 75 | 35 | 108 | 78 | 30 |
| TG (mg/dL) | 203.8 | 142.2 | 61.6 | 194.9 | 115.6 | 79.3 |
| ALT = alanine aminotransferase | ||||||
| Outcome | Biochemical improvement
| Radiological improvement | No improvement in histology | Serious adverse effects |
| Evidence | Low‐quality systematic review | Low‐quality randomised clinical trial | Small sample size in low‐quality randomised clinical trial | Low‐quality systematic review
|
| Level of evidence | 2 | 2 | 2 | 2 |
| Grade of recommendation | B | B | B | B |

