Antidepresivos de segunda generación para el trastorno afectivo estacional
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial. One‐week placebo wash‐out phase with exclusion of responders, then randomisation and a five‐week treatment period. | |
| Participants | N=68. Outpatients at five centres in Canada suffering recurrent major depressive episodes with a seasonal pattern (DSM‐IIIR). Enrollment over two years between 1991 and 1993. Average age approximately 39 years, 66% female. | |
| Interventions | Fluoxetine 20 mg per day versus placebo. | |
| Outcomes | Depression response (baseline versus end results and a greater than 50% improvement in baseline score) as per the 29‐item SIGH‐SAD, 21‐item HDRS, and the BDI. Safety measures: weight, pulse, blood pressure and adverse effects. | |
| Notes | Funded by Eli Lilly, Canada, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear what method of sequence generation was used: "eligible patients were randomly assigned to either fluoxetine, 20 mg daily, or an identical placebo capsule". |
| Allocation concealment (selection bias) | Unclear risk | This is not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The number of participants who did not complete the trial is not reported, however, the authors state that they conduct analyses "using the intention‐to‐treat last‐observation‐carried‐forward method". |
| Selective reporting (reporting bias) | Unclear risk | Although no study protocol is available, the authors report all of the outcomes that are described in the methods section clearly and in detail. |
| Other bias | Low risk | None. |
| Blinding of participants and personnel (performance bias) | Unclear risk | This is unclear. The trial is described as being "double‐blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | This is unclear. The trial is described as being "double‐blind". |
| Methods | Randomised controlled trial. One‐week washout of placebo responders with subsequent randomisation and an eight week treatment period. | |
| Participants | N=96. Outpatients at four centres in Canada suffering recurrent major depressive episodes with a seasonal pattern (DSM‐IV SCID). Enrollment over three years between 2000 and 2003. Average age approximately 39 years, 66% female. Outpatients aged 18‐65 years, DSM‐IV criteria for major depressive episodes with a seasonal pattern. | |
| Interventions | Light therapy: 10,000 Lux, 14 inches between screen and cornea for 30 minutes as soon as possible after waking (between 7:00am and 8:00am) with a placebo capsule versus fluoxetine 20 mg/day taken between 7:00am and 8:00am and placebo light box of 100 Lux. | |
| Outcomes | Depression response and remission. Response is defined as an improvement of 50% or more on the 24‐item HAM‐D (consisting of the 17‐item HAM‐D scale, plus seven atypical symptoms). Remission is clinical response plus an end score of eight or less. Other outcome measures include the CGI and the BDI. Adverse effects were captured using a pre‐defined list of 32 adverse effects and both the severity and frequency of treatment‐emergent adverse effects was captured. | |
| Notes | This is the CAN‐SAD study, from which multiple publications have been produced, including Michalak 2007 on quality of life outcomes. This study was funded by a grant (CT62962) from the Canadian Institutes of Health Research (CIHR) and a CIHR/Wyeth Postdoctoral Fellowship Award to Dr Michalak. Light boxes were supplied by Uplift Technologies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | |
| Other bias | Low risk | |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Open‐label observational study (drug surveillance) | |
| Participants | Twenty participants with SAD (DSM‐IV‐TR criteria) with a score of 20 or higher on the Structured Interview Guide for the Hamilton Depression Rating Scale, SAD version (SIGH‐SAD, 29 items). Fourteen females and 6 males, average age 41 years. Recruited at the Medical University of Vienna, Austria. | |
| Interventions | Open‐label escitalopram, flexible dose 10 to 20 mg per day for eight weeks. | |
| Outcomes | The only outcome of interest for the purposes of this review is adverse effects (this study did not fulfil our inclusion criteria for efficacy as it is not a randomised trial). Adverse effects were monitored at week one, week two, week four, week six and week eight using the Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale. | |
| Notes | Only the data for adverse effects are reported in this review. This study was supported by an unrestricted grant from H. Lundbeck A / S. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is an observational study with only one group. |
| Allocation concealment (selection bias) | High risk | This is an open‐label study. |
| Incomplete outcome data (attrition bias) | Low risk | Two participants out of 20 withdrew from the study at week six (10%). This level af attrition is unlikely to result in a high risk of bias for the adverse effects results of this study. |
| Other bias | High risk | |
| Blinding of participants and personnel (performance bias) | High risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Open‐label observational study (drug surveillance). | |
| Participants | Twenty‐six participants with SAD (DSM‐IV‐TR criteria). Twenty‐two females and four males, average age 41 years. Recruited at the Medical University of Vienna, Austria. | |
| Interventions | Open‐label duloxetine, flexible dose 60 to 120 mg per day for eight weeks. | |
| Outcomes | The only outcome of interest for the purposes of this review is adverse effects (this study did not fulfil our inclusion criteria for efficacy as it is not a randomised trial). Adverse effects were monitored at week one, week two, week four, week six and week eight using the Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale. | |
| Notes | Only the data for adverse effects are reported in this review. Dr Kasper has received research grants, consultancy fees and lecture fees from a number of pharmaceutical companies in the area of CNS development. Dr Konstantinidis and Dr Winkler have received lecture fees from several pharmaceutical companies in the field. The authors of this study have been supported by various travel grants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is an observational study with only one group. |
| Allocation concealment (selection bias) | High risk | This is an open‐label study. |
| Incomplete outcome data (attrition bias) | High risk | Six participants out of 26 withdrew from the study (23%). This level af attrition is likely to result in a high risk of bias for the adverse effects results of this study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Additional risk of bias assessment as per Deeks 2003, see Table 2. Authors do not report how allocation to duloxetine occurred. Participants were enrolled and data collected prospectively. Inclusion and exclusion criteria are clearly described. This study contains a small number of participants and this is a likely source of bias. Both intervention and outcomes are clearly specified. Interpretation of the results is accurate. |
| Blinding of participants and personnel (performance bias) | High risk | This is an open‐label study. |
| Blinding of outcome assessment (detection bias) | High risk | This is an open‐label study. |
| Methods | Open‐label observational study (drug surveillance). Pooled results of escitalopram (from Pjrek 2007) and reboxetine. We present the data here for the reboxetine group. | |
| Participants | Fifteen participants with SAD (DSM‐IV‐TR criteria). Thirteen females and two males, average age 42 years. Recruited at the Medical University of Vienna, Austria. | |
| Interventions | Open‐label reboxetine, 8 mg per day for six weeks. | |
| Outcomes | The only outcome of interest for the purposes of this review is adverse effects (this study did not fulfil our inclusion criteria for efficacy as it is not a randomised trial). Adverse effects were monitored at week one, week two, week four, and week six using the Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale. | |
| Notes | Only the data for adverse effects are reported in this review. The authors report no funding source for this study, although authors have received multiple grants and research support as well as consultancy and advisory board payments. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Incomplete outcome data (attrition bias) | High risk | |
| Other bias | High risk | |
| Blinding of participants and personnel (performance bias) | High risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Methods | Randomised controlled trial. One week of placebo followed by five weeks of treatment. | |
| Participants | N=40. Participants with major depression with a seasonal pattern (DSM‐III‐R). Average age 41 years and approximately 79% females. Recruited in Germany and Austria. | |
| Interventions | Fluoxetine 20 mg per day with dim light versus light therapy 3000 Lux, 2 hours per day (2 hours in the morning, 1 hour each in the morning and evening, or 2 hours in the evening) at a distance of 55 cm and a placebo capsule. | |
| Outcomes | 21‐item HDRS (item 17 omitted) and the seven‐item supplement (SUPP). Other outcome measures included the Hypomania scale, the Profile of Mood States scale, and the Adjective Mood Scale of von Zerssen. | |
| Notes | This study was supported by a grant from Eli Lilly, Germany. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | High risk | Overall 12% attrition, unequal between groups: 20% in the bright light group and 5% in the fluoxetine group. |
| Selective reporting (reporting bias) | Unclear risk | No protocol is available. All outcomes described in the methods section are reported in the results. |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) | Low risk | "rater and patients blind to treatment conditions" and "treatment conditions were unknown to the patients". |
| Blinding of outcome assessment (detection bias) | Low risk | "rater and patients blind to treatment conditions" and "all objective observer rating were done by one rater blind to study design and treatment conditions". |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This is a randomised trials of 204 participants conducted in 29 centres in Austria, Canada, Finland, France and the UK. Particpants with a seasonal pattern of major depression or bipolar disorder were randomised to sertraline or placebo. We excluded this study because the population did not meet our inclusion criteria. | |
| This is an eight‐week trial of participants with a history of SAD, but who were non‐depressed (score seven or less on the HAMD‐17) at the beginning of the study. Participants were randomised to bupropion XL or placebo for the prevention of SAD, therefore this trial did not meet our inclusion criteria. | |
| This is an eight‐week trial of participants with a history of SAD, but who were non‐depressed (score seven or less on the HAMD‐17) at the beginning of the study. Participants were randomised to bupropion XL or placebo for the prevention of SAD, therefore this trial did not meet our inclusion criteria. | |
| This is an eight‐week trial of participants with a history of SAD, but who were non‐depressed (score seven or less on the HAMD‐17) at the beginning of the study. Participants were randomised to bupropion XL or placebo for the prevention of SAD, therefore this trial did not meet our inclusion criteria. | |
| We excluded this trial because the population did not meet our inclusion criteria. The trial included 187 participants with a DSM‐III‐R diagnosis of major depression or bipolar disorder with a seasonal pattern. | |
| This is a six‐week study of 183 participants with major depression randomised to 20 to 40 mg/day of fluoxetine or 300 to 450 mg/day of moclobemide. Results were presented separately for the 34 SAD participants in the trial. We excluded this study because the comparator, moclobemide, did not meet our inclusion criteria. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Response (> 50% improvement on 29‐item HAM‐D SIGH‐SAD) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Fluoxetine versus Placebo, Outcome 1 Response (> 50% improvement on 29‐item HAM‐D SIGH‐SAD). | ||||
| 2 Harm: Overall adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Fluoxetine versus Placebo, Outcome 2 Harm: Overall adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Response (> 50% improvement on 24‐item HAM‐D SIGH‐SAD) Show forest plot | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| Analysis 2.1  Comparison 2 Fluoxetine versus Light Therapy, Outcome 1 Response (> 50% improvement on 24‐item HAM‐D SIGH‐SAD). | ||||
| 2 Remission (response plus end score eight or less) Show forest plot | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.30] |
| Analysis 2.2  Comparison 2 Fluoxetine versus Light Therapy, Outcome 2 Remission (response plus end score eight or less). | ||||
| 3 Harm: At least one treatment‐emergent adverse effect Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Fluoxetine versus Light Therapy, Outcome 3 Harm: At least one treatment‐emergent adverse effect. | ||||
| 4 Harm: Agitation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Fluoxetine versus Light Therapy, Outcome 4 Harm: Agitation. | ||||
| 5 Harm: Sleep disturbance Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Fluoxetine versus Light Therapy, Outcome 5 Harm: Sleep disturbance. | ||||
| 6 Harm: Palpitations Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Fluoxetine versus Light Therapy, Outcome 6 Harm: Palpitations. | ||||
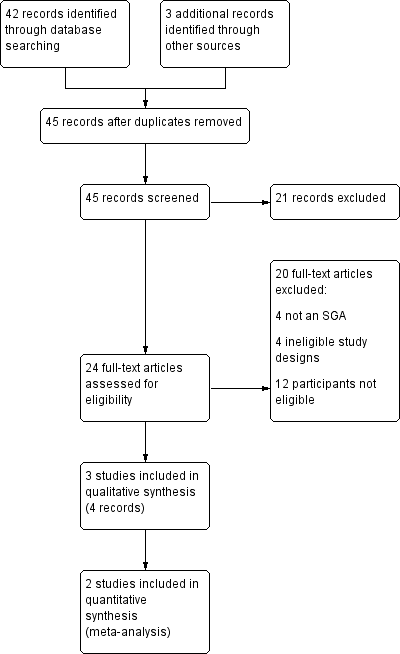
Study flow diagram for efficacy.
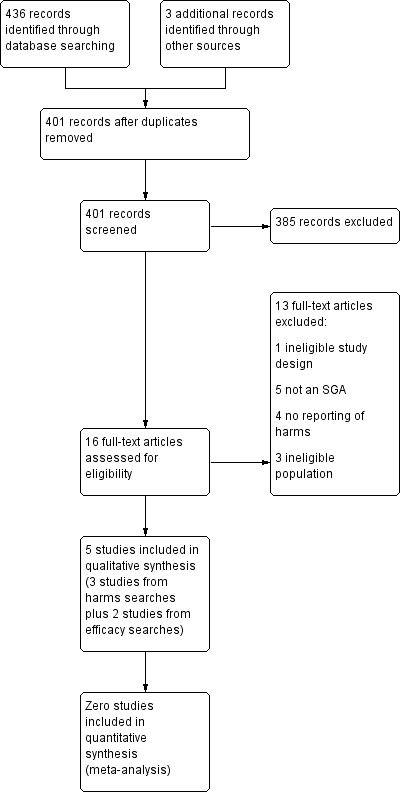
Study flow diagram for adverse effects.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: Fluoxetine versus Light Therapy, outcome: 1.1 Response (> 50% improvement on 24‐item HAM‐D SIGH‐SAD).
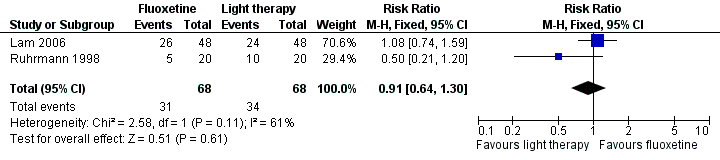
Forest plot of comparison: Fluoxetine versus Light Therapy, outcome: 1.2 Remission (response plus end score eight or less).
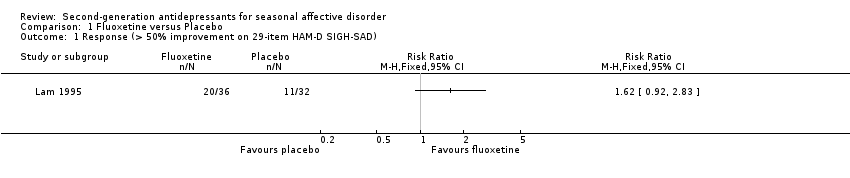
Comparison 1 Fluoxetine versus Placebo, Outcome 1 Response (> 50% improvement on 29‐item HAM‐D SIGH‐SAD).
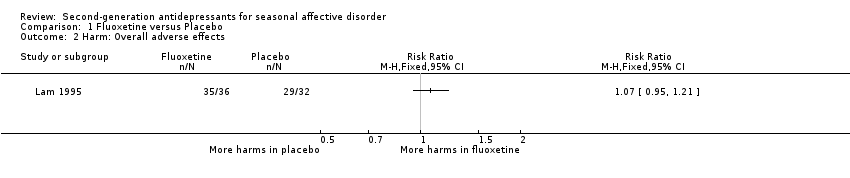
Comparison 1 Fluoxetine versus Placebo, Outcome 2 Harm: Overall adverse effects.

Comparison 2 Fluoxetine versus Light Therapy, Outcome 1 Response (> 50% improvement on 24‐item HAM‐D SIGH‐SAD).

Comparison 2 Fluoxetine versus Light Therapy, Outcome 2 Remission (response plus end score eight or less).

Comparison 2 Fluoxetine versus Light Therapy, Outcome 3 Harm: At least one treatment‐emergent adverse effect.
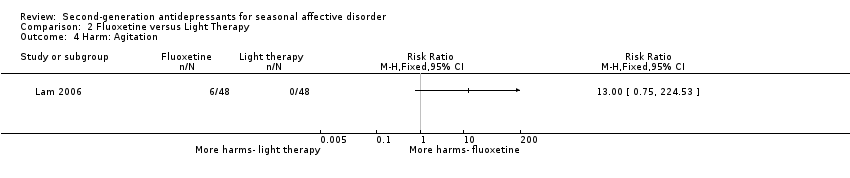
Comparison 2 Fluoxetine versus Light Therapy, Outcome 4 Harm: Agitation.
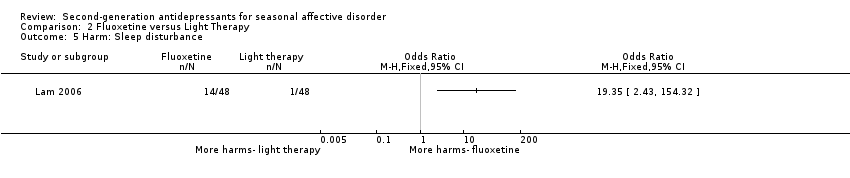
Comparison 2 Fluoxetine versus Light Therapy, Outcome 5 Harm: Sleep disturbance.

Comparison 2 Fluoxetine versus Light Therapy, Outcome 6 Harm: Palpitations.
| Fluoxetine compared with placebo for seasonal affective disorder | ||||||
| Patient or population: Adults with seasonal affective disorder Settings: Outpatients Intervention: Fluoxetine Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | fluoxetine | |||||
| Response (> 50% improvement on 29‐item HAM‐D SIGH‐SAD) Five weeks | Medium risk population | RR 1.62 (0.92 to 2.83) | 68 | ⊕⊝⊝⊝ | ||
| 34 per 100 | 56 per 100 | |||||
| Remission | NR | NR | NA | NA | NA | |
| Quality of life | NR | NR | NA | NA | NA | |
| Overall adverse effects Five weeks | Medium risk population | RR 1.07 (0.95 to 1.21) | 68 | ⊕⊝⊝⊝ | ||
| 91 per 100 | 97 per 100 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the rate in the placebo group. The corresponding risk (and its 95% CI) is based on the assumed risk in the fluoxetine group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HAM‐D SIGH‐SAD: Hamilton depression scale, seasonal affective disorder sub scale; NA: not applicable; NR: not reported; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The trial has an unclear risk of bias. The effect is very imprecise because there are few participants in the trials. | ||||||
| Fluoxetine compared with light therapy for seasonal affective disorder | ||||||
| Patient or population: Adults with seasonal affective disorder Settings: Outpatients Intervention: Fluoxetine Comparison: Light therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Light therapy | Fluoxetine | |||||
| Response (> 50% improvement on 24‐item HAM‐D SIGH‐SAD) Five to eight weeks | Medium risk population | RR 0.98 (0.77 to 1.24) | 146 | ⊕⊕⊝⊝ | ||
| 68 per 100 | 67 per 100 | |||||
| Remission (response plus end score eight or less) Five to eight weeks | Medium risk population | RR 0.81 (0.39 to 1.71) | 146 | ⊕⊝⊝⊝ | ||
| 68 per 100 | 55 per 100 | |||||
| Quality of life (SF‐20 & Q‐LES‐Q) Eight weeks | NR | NR | No significant differences | 96 (1) | NA | No relative effect was calculated |
| Adverse effects (at least one treatment‐emergent adverse effect) Eight weeks | Medium risk population | RR 0.97 (0.78 to 1.22) | 96 (1) | ⊕⊝⊝⊝ | ||
| 77 per 100 | 75 per 100 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is the response rate in the light therapy groups in the two included trials. The corresponding risk (and its 95% CI) is based on the assumed risk in the fluoxetine group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HAM‐D SIGH‐SAD: Hamilton depression scale, seasonal affective disorder sub scale; NA: not applicable; NR: not reported; Q‐LES‐Q: Quality of life enjoyment and satisfaction questionnaire; RR: Risk Ratio; SF‐20: medical outcome study | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The trials provide direct evidence for average patients with SAD and use recommended doses of fluoxetine (directness). There was no inconsistency. The risk of bias in the two trials was low to moderate. Finally, the calculated effect is imprecise because there are few participants in the trials. 2The trials provide direct evidence for average patients with SAD and use recommended doses of fluoxetine (directness). There was serious inconsistency. The risk of bias in the two trials was low to moderate. Finally, the calculated effect is imprecise because there are few participants in the trials. 3The data are from only one trial with a low risk of bias, however, the outcome encompasses many adverse effects and therefore the data are indirect. Furthermore, the effect is imprecise because there are few participants in the trial. | ||||||
| Domain | Authors' judgement | Support for judgement |
| How allocation occured? Concealment of allocation? | High risk | All patients received active therapy with escitalopram. No concealment of allocation |
| Blinding? (Participants) | High risk | Open‐label |
| Follow‐up equal and complete? | Low risk | 10% attrition |
| Baseline comparability of groups? | Unclear | Only one group |
| ITT‐analysis | Low risk | ITT analysis employed |
| Intervention clearly specified? | Low risk | Intervention clearly described |
| Outcomes clearly specified? | Low risk | Outcomes clearly specified including prospective adverse events recording with Udvalg for Kliniske Undersogelser |
| Interpretation based on results? | Low risk | Yes |
| Domain | Authors' judgement | Support for judgement |
| How allocation occured? Concealment of allocation? | High risk | All patients received active therapy with duloxetine. No concealment of allocation |
| Blinding? (Participants) | High risk | Open‐label |
| Follow‐up equal and complete? | High risk | 23% attrition |
| Baseline comparability of groups? | Unclear | Only one group |
| ITT‐analysis | HIgh risk | LOCF analysis employed |
| Intervention clearly specified? | Low risk | Intervention clearly described |
| Outcomes clearly specified? | Low risk | Outcomes clearly specified including prospective adverse events recording with Udvalg for Kliniske Undersogelser |
| Interpretation based on results? | High risk | No, due to high attrition and use of LOCF approach for missing data |
| Domain | Authors' judgement | Support for judgement |
| How allocation occured? Concealment of allocation? | High risk | All patients received active therapy with reboxetine. No concealment of allocation |
| Blinding? (Participants) | High risk | Open‐label |
| Follow‐up equal and complete? | High risk | 27% attrition |
| Baseline comparability of groups? | Unclear | Only one group |
| ITT‐analysis | HIgh risk | LOCF analysis employed |
| Intervention clearly specified? | Low risk | Intervention clearly described |
| Outcomes clearly specified? | Low risk | Outcomes clearly specified including prospective adverse events recording with Udvalg for Kliniske Undersogelser |
| Interpretation based on results? | High risk | No, due to high attrition and use of LOCF approach for missing data |
| Study ID | Lam 2006 | Lam 1995 | Prjek 2007 | Pjrek 2008 | Pjrek 2009 | ||
| Study design | RCT | RCT | Open‐label observational study | Open‐label observational study | Open‐label observational study | ||
| SGA | Fluoxetine | Light therapy | Fluoxetine | Placebo | Escitalopram | Duloxetine | Reboxetine |
| Number of participants (N) | N=48 | N= 48 | N=36 | N=32 | N = 20 | N=26 | N= 15 |
| Overall AEs (%) | 75 | 77 | 97 | 91 | 55 |
| 100 |
| Withdrawal due to AEs (%) | 25 | 23 | 6 | 3 | 15.4 | 26.6 | |
| Gastrointestinal |
|
|
|
|
| ||
| Abdominal Pain (%) | 8.3 | 6.3 |
|
|
| ||
| Nausea (%) | 10.4 | 4.2 | 40 | 53.8 | 20 | ||
| Vomiting (%) |
|
|
|
|
| ||
| Diarrhoea (%) | 10.4 | 4.2 | 5 | 23.1 | 0.0 | ||
| Constipation (%) | 6.3 | 8.3 | 5 | 11.5 | 26.7 | ||
| Decreased appetite (%) | 14.6 | 14.6 | 5 |
| 6.7 | ||
| Increased appetite (%) | 14.6 | 8.3 |
| 3.8 |
| ||
| Weight loss (%) | 6.3 | 2.1 |
| 3.8 |
| ||
| Dyspepsia (%) |
|
|
|
|
| ||
| Central nervous system |
|
|
|
|
| ||
| Anxiety (%) | 25.0 | 12.5 |
|
|
| ||
| Nervousness (%) | 10.4 | 12.5 |
|
|
| ||
| Agitation (%) | 12.5 | 0 |
|
|
| ||
| Dizziness (%) |
|
| 5 |
| 0.0 | ||
| Tremor (%) | 6.3 | 2.1 |
| 3.8 | 33.3 | ||
| Irritability (%) | 8.3 | 4.2 |
|
|
| ||
| Sleepiness (%) | 12.5 | 8.3 |
| 3.8 | 26.7 | ||
| Increased sleep (%) | 18.8 | 12.5 |
|
| 6.7 | ||
| Decreased sleep (%) | 20.8 | 22.9 |
|
|
| ||
| Sleep disturbance (%) | 29.2 | 2.1 |
| 7.7 |
| ||
| Headache (%) | 10.4 | 16.7 | 5 | 7.7 | 13.3 | ||
| Paraesthesia (%) |
|
|
|
| 20.0 | ||
| Sexual Dysfunction |
|
|
|
|
| ||
| Decreased sex drive (%) | 16.7 | 14.6 | 20 |
| 0 | ||
| Male erection problems (%) | 6.3 | 4.7 |
|
|
| ||
| Female delayed orgasm (%) | 6.3 | 0 |
|
|
| ||
| Ejaculation* (%) |
|
|
|
|
| ||
| Other |
|
|
|
|
| ||
| Feeling faint (%) | 0 | 6.3 |
|
|
| ||
| Palpitations (%) | 10.4 | 0 |
| 3.8 | 60.0 | ||
| Sweating (%) | 10.4 | 6.3 |
| 11.5 | 73.3 | ||
| Flushing (%) | 4.2 | 6.3 |
|
|
| ||
| Muscle pain (%) | 12.5 | 12.5 |
|
|
| ||
| Weakness/fatigue (%) | 16.7 | 16.5 |
|
|
| ||
| Rash (%) | 6.3 | 0 |
|
|
| ||
| Dry mouth (%) | 14.6 | 18.8 |
| 11.5 | 86.7 | ||
| Hypersalivation (%) |
|
|
|
| 6.7 | ||
| Dry eyes (%) |
|
|
|
| 6.7 | ||
| Urinary retention (%) |
|
|
|
| 6.7 | ||
| Concentration problems (%) |
|
|
| 3.8 |
| ||
| Dilated pupils (%) |
|
|
| 3.8 |
| ||
| Emotional Indifference (%) |
|
|
| 3.8 |
| ||
| Photosensitivity (%) |
|
|
| 3.8 |
| ||
| Vivid dreams (%) |
|
|
| 3.8 |
| ||
| Psychomotor agitation (%) |
|
|
|
| 73.3 | ||
| Orthostatic dysregulation (%) |
|
|
|
| 60.0 | ||
| Accomodative dysfunction (%) |
|
|
|
| 26.7 | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Response (> 50% improvement on 29‐item HAM‐D SIGH‐SAD) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Harm: Overall adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Response (> 50% improvement on 24‐item HAM‐D SIGH‐SAD) Show forest plot | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| 2 Remission (response plus end score eight or less) Show forest plot | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.30] |
| 3 Harm: At least one treatment‐emergent adverse effect Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Harm: Agitation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Harm: Sleep disturbance Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Harm: Palpitations Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

