Citologija naspram HPV testiranja za probir na rak vrata maternice u općoj populaciji
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Clinical features and settings | Women >17 years old attending the outpatient clinics of six hospitals in Northern Greece for routine cervical screening. No history of hysterectomy or treatment for CIN. | |
| Participants | 1296 women (mean age 43) from Greece | |
| Study design | Cross‐sectional study of women receiving both CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies. When colposcopy was normal biopsies were not taken. Colposcopy was performed in all screen‐positives and in a random 5% of screen‐negatives | |
| Index and comparator tests | IT: HPV testing by PCR (PGMY09/PGMY11) for the detection of 27 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, 73, 82, 83, 84). Referred for colposcopy if positive CT: CC, referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women >17 years attending for routine screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | 5% of screen‐negatives also underwent colposcopy |
| Differential verification avoided? | Yes | Same RS was applied in all cases |
| Incorporation avoided? | Yes | Screening tests did not form part of the RS |
| Reference standard results blinded? | Yes | Pathologists were blinded to the screening tests |
| Index test results blinded? | Yes | RS performed after the screening tests' interpretation |
| Relevant clinical information? | Unclear | It is not clear what information was available to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women aged 25–55 attending routine cervical screening at the outpatient clinics of 9 Gynaecology Departments (2 in Athens, 4 in Thessaloniki, 1 in Larissa, 1 in Patras and 1 in Alexandroupolis) were asked to be enrolled in the study. Exclusion criteria were current pregnancy, current or previous history of CIN in the past 5 years, follow‐up for cytological abnormalities and hysterectomy | |
| Participants | 4009 women attending for cervical screening in Greece. The mean age was 39.9 years | |
| Study design | Cross‐sectional study of women receiving both LBC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies. When colposcopy was normal biopsies were not taken. Colposcopy was performed in all screen‐positives and in a random 3% of screen‐negatives | |
| Index and comparator tests | IT: HPV testing by Cobas HPV test (Roche). Referred for colposcopy if positive CT: LBC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 25‐55 years old |
| Acceptable reference standard? | Yes | Colposcopy and colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | 3% of screen‐negatives also underwent colposcopy |
| Differential verification avoided? | Yes | Same RS was applied in all cases |
| Incorporation avoided? | Yes | Screening tests did not form part of the RS |
| Reference standard results blinded? | No | Pathologists were aware of the cytology and colposcopy result, but not of the HPV DNA test result. |
| Index test results blinded? | Yes | RS performed after the screening tests' interpretation |
| Relevant clinical information? | Unclear | It is not clear what information was available to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Non‐pregnant women 35‐50 years old with no history of pelvic radiation or hysterectomy from villages in the Shanxi Province in China, were invited to participate | |
| Participants | 8497 women (mean age 40.9) from rural China | |
| Study design | Cross‐sectional study of women receiving both cytology, direct and self‐HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy, if colposcopy was normal random biopsies were taken. Only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by HC2 direct cervical sampling (Digene), positivity threshold at 1 pg/mL. Referred for colposcopy + biopsies if positive IT: HPV testing by HC2 self‐vaginal sampling (Digene), positivity threshold at 1 pg/mL. Referred for colposcopy + biopsies if positive CT: LBC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 35‐50 years old |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | 3 months between self‐sampling and direct‐sampling |
| Partial verification avoided? | No | Colposcopy only performed when the tests were positive |
| Differential verification avoided? | Yes | The same RS was performed on all occasions |
| Incorporation avoided? | Yes | The RS did not consist of cytology or HPV testing |
| Reference standard results blinded? | Yes | Pathologists were blinded to the test results |
| Index test results blinded? | Yes | The RS was applied after the screening tests |
| Relevant clinical information? | Unclear | It is not clear what information was given to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | 1000 women were recruited by the Renmin Hospital in the Buyi‐Miao Autonomous District (BMAD) of Guizhou Province, China. Women were excluded if they were pregnant, younger than 30, did not have an intact uterus, or had a history of pelvic irradiation or cervical cancer | |
| Participants | 979 women aged 30‐54 examined in a colposcopy clinic in Guizhou, China | |
| Study design | Cross‐sectional study of women receiving both LBC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy, if colposcopy was normal random biopsies were taken. Only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by SNIPER (Genetel Pharmaceuticals). Referred for colposcopy + biopsies if positive CT: LBC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women aged 30‐54 years |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Both tests performed at the same visit |
| Partial verification avoided? | No | Only women with positive results were invited for colposcopy |
| Differential verification avoided? | Yes | The same RS applied for all occasions |
| Incorporation avoided? | Yes | The tests were not part of the RS |
| Reference standard results blinded? | Unclear | It is not clear whether the colposcopists had knowledge of the test results |
| Index test results blinded? | Yes | Colposcopy was performed after the test results were reported |
| Relevant clinical information? | Unclear | It is not clear what information was given to the cytologists |
| Uninterpretable results reported? | No | There is no mention of un interpretable results |
| Withdrawals explained? | Yes | Of the 211 women asked to return, all but 21 or 90% of the women returned for colposcopy |
| Clinical features and settings | Women mainly 30 or older attending mainly private gynaecologists in Switzerland | |
| Participants | 13,842 women (mean age 44.4 years, range 17‐93) from Switzerland | |
| Study design | Cross‐sectional study of women receiving both LBC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies, if colposcopy was normal random biopsies were taken. Screen‐positives and a random 5% sample of screen‐negatives were referred | |
| Index and comparator tests | IT: HPV testing by HC2, positivity threshold 1 pg/mL, referred for colposcopy if positive CT: LBC (Surepath), referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women > 17 years attending for routine screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | A random 5% sample of screen‐negatives was also verified |
| Differential verification avoided? | Yes | The same RS was used in all cases |
| Incorporation avoided? | Yes | The screening tests did not form part of the RS |
| Reference standard results blinded? | Unclear | It is unclear whether the pathologists had knowledge of the test results |
| Index test results blinded? | Yes | The RS was applied after the tests were reported |
| Relevant clinical information? | Unclear | It is unclear whether cytologists had knowledge of any clinical information |
| Uninterpretable results reported? | No | Unsatifactory smears were not reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women 25‐55 years old attending primary care clinics in Zimbabwe were invited. No hysterectomy or previous diagnosis of cervical cancer | |
| Participants | 2073 women from Chitungwiza and the greater Harare area in Zimbabwe | |
| Study design | Cross‐sectional study of women receiving both conventional cytology, VIA and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopy with or without biopsies was performed on all women. If colposcopy was normal, biopsies were not taken | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold 1 pg/mL IT: VIA CT: CC. For the calculation of the accuracy indices the threshold of LSIL+ was used | |
| Follow‐up | ||
| Notes | Some of the data were extracted from the publication by Womack 2000 | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 25‐55 years old attending primary care clinics |
| Acceptable reference standard? | Yes | Colposcopy with biopsy (no biopsy if colposcopy was normal) |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | All women underwent colposcopy |
| Differential verification avoided? | Yes | The same RS was applied to all women |
| Incorporation avoided? | Yes | The screening tests were not part of the RS |
| Reference standard results blinded? | Yes | Colposcopists were not aware of the screening test results |
| Index test results blinded? | Unclear | It is not clear whether cytologists were aware of the colposcopic diagnosis |
| Relevant clinical information? | Unclear | It is not clear whether cytologists had the routine clinical information |
| Uninterpretable results reported? | No | The numbers of inadequate smears and HPV tests are not given |
| Withdrawals explained? | Yes | Withdrawals are explained |
| Clinical features and settings | Women > 30 years old without prior cervical abnormalities, attending two cancer centres and one general hospital | |
| Participants | 835 women (mean age 46.7 years) undergoing routine screening in the USA and Canada | |
| Study design | Cross‐sectional study of women receiving both cytology and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy, if colposcopy was normal random biopsies were taken. All women were referred for colposcopy | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL CT: CC For the calculation of the accuracy indices an abnormal result was considered any smear showing ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women > 30 years without prior cervical abnormalities |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies and random biopsies if colposcopy was negative |
| Acceptable delay between tests? | Yes | Both tests were serially performed at the same visit |
| Partial verification avoided? | Yes | All women were referred for colposcopy |
| Differential verification avoided? | Yes | The same RS was applied in the case of positive cytology and persistent type‐specific positive HPV test |
| Incorporation avoided? | Yes | The RS was not composed of the index and comparator tests |
| Reference standard results blinded? | Yes | The pathologists were not aware of the screening tests results |
| Index test results blinded? | Yes | The personnel reporting the screening test results were not aware of the RS results |
| Relevant clinical information? | Unclear | It is not clear whether clinical information was given to the cytologists |
| Uninterpretable results reported? | Yes | All results including inadequate specimens were reported |
| Withdrawals explained? | Yes | Withdrawals were explained |
| Clinical features and settings | Women presenting for routine cervical cancer screening were enrolled into the ATHENA study at 61 clinical centres in 23 US states. Eligible women were aged 21 years or older and were not pregnant. Eligible women had an intact uterus, had not received treatment for CIN with 12 months of enrolment, and had no present or planned participation in a clinical trial for HPV treatment. For this sub‐analysis, the population was restricted to | |
| Participants | 41,955 women aged 25 years or older (mean age 41.9) in the USA | |
| Study design | Cross‐sectional study of women receiving both cytology and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: Colposcopy with or without biopsies was performed on all women with a positive screening test and a random sample of women with negative tests | |
| Index and comparator tests | IT: HPV testing by Cobas HPV test (Roche). Positivity was not a criterion for referral. Referred for colposcopy + biopsies if a first‐generation HPV test (Amplicor or Linear Array) was positive. That left only 48/4275 Cobas‐positive women without referral for colposcopy CT: LBC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women aged ≥ 25 years attending routine screening |
| Acceptable reference standard? | Yes | Colposcopy with or without biopsies |
| Acceptable delay between tests? | Yes | All tests performed at the same visit |
| Partial verification avoided? | Yes | A random sample of 1041 screen‐negative women had colposcopy |
| Differential verification avoided? | Yes | The same RS applied to all women |
| Incorporation avoided? | Yes | The screening tests did not form part of the RS |
| Reference standard results blinded? | Yes | Colposcopists and pathologists were masked to cytology and HPV test results |
| Index test results blinded? | Yes | Colposcopy was performed after the tests were reported |
| Relevant clinical information? | Unclear | It is not clear what information was available to the cytologists |
| Uninterpretable results reported? | Yes | 1054 women had missing or invalid test results |
| Withdrawals explained? | Yes | Withdrawals were explained |
| Clinical features and settings | Asymptomatic non‐pregnant women 15‐76 years old without recent cervical cytological abnormalities, or untreated cervical lesion in the last 2 years, or AIDS, attending a central urban hospital for routine screening | |
| Participants | 7932 women (median age 34) undergoing biennial or triennial routine screening, Rheims, France | |
| Study design | Longitudinal study of women receiving both cytology and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐proven HSIL RS: colposcopically‐directed biopsy or LEEP. Colposcopy only if no lesion was seen. Only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by HC2 (DIgene), positivity threshold at 1 pg/mL. If cytology was negative, women with a positive HPV test were referred for the RS 6 months later if a second HPV test was also positive CT: CC in 2281 women, LBC (Thinprep) in 5651 women. Referred for RS if the result was ASCUS or worse | |
| Follow‐up | 368/773 women with positive HPV test but negative cytology did not return for a second HPV test | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 15‐76 years old undergoing routine screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsy or LEEP |
| Acceptable delay between tests? | Yes | Test serially performed at the same examination |
| Partial verification avoided? | No | The RS was applied only if one of the test results were positive |
| Differential verification avoided? | Yes | The RS was the same in all situations |
| Incorporation avoided? | Yes | The RS was not composed of the index and comparator tests |
| Reference standard results blinded? | No | Colposcopists were aware of the test results |
| Index test results blinded? | Yes | The RS was applied after the screening tests were reported |
| Relevant clinical information? | Unclear | There is not sufficient information |
| Uninterpretable results reported? | No | The numbers of uninterpretable results that would be expected to have occurred were not given |
| Withdrawals explained? | No | The withdrawals were not completely explained |
| Clinical features and settings | Women attending a family planning clinic in London for routine smear. No history of CIN or abnormal smear in the last 3 years | |
| Participants | 1985 women (median age 29 years, 93% between 20 and 45) in London, UK | |
| Study design | Cross‐sectional study of women receiving both CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy. If colposcopy was normal a biopsy might have not been taken. RS applied only to screen‐positives | |
| Index and comparator tests | IT: HPV testing by PCR for 4 high‐risk types (16, 18, 31, 33). Referred for colposcopy if positive CT: CC referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women of the appropriate age spectrum attending for routine cervical screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | No | Only screen‐positives had colposcopy |
| Differential verification avoided? | Yes | The same RS in all cases |
| Incorporation avoided? | Yes | Screening tests not part of the RS |
| Reference standard results blinded? | Yes | Pathologists were blinded to the results of the screening tests |
| Index test results blinded? | Yes | RS performed after the interpretation of the screening tests |
| Relevant clinical information? | Unclear | It is unclear what information was given to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women aged ≥ 35 years attending for a routine smear in general practitioner practices in the UK , no previous treatment, no cytologic abnormality in the last 3 years. | |
| Participants | 2988 women (mean age 46) in the UK | |
| Study design | Cross‐sectional study of women receiving both CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy, if colposcopy was normal a biopsy was not taken. Only screen‐positives were referred for colposcopy | |
| Index and comparator tests | IT: HPV testing by PCR (MY09/11) for the detection of 10 high‐risk types (16, 18, 31, 33, 35, 45, 51, 52, 56, 58). Referred for colposcopy if positive CT: CC, referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women > 34 years attending for routine screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsy |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | No | Only screen‐positives underwent colposcopy |
| Differential verification avoided? | Yes | The same RS was applied to all screen‐positives |
| Incorporation avoided? | Yes | The screening tests were not part of the RS |
| Reference standard results blinded? | Yes | Pathologists were blinded to the results of the screening tests |
| Index test results blinded? | Yes | The RS was performed after the screening tests were reported |
| Relevant clinical information? | Unclear | It is not clear whether cytologists were given the routine clinical information |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women 30‐60 years old attending for routine cervical screening were recruited from 161 family practices in the UK. No treatment for CIN, no abnormal smear in the last 3 years | |
| Participants | 10,358 women (mean age 42 years) from the UK | |
| Study design | Women received both CC and HPV testing. Then an RCT on the management of women with minor abnormalities (borderline smears and HPV positives with negative smears) was conducted. Women were randomised to either surveillance at 6‐12 months or immediate colposcopy | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies, if colposcopy was negative biopsies were not taken. All screen‐positives eventually underwent colposcopy although some with a 12‐month delay (the ones with minor abnormalities randomised to surveillance). A random 5% sample of screen‐negatives also underwent colposcopy | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold 1 pg/mL, referred for colposcopy if positive CT: CC, referred for colposcopy if ASCUS+ | |
| Follow‐up | Women with ASCUS or HPV‐positive with negative smears were randomised either to immediate colposcopy or to surveillance at 6 and 12 months with colposcopy performed at the end of the 12 months | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 30‐60 years old attending for routine screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | No | In 296 women colposcopy was performed 12 months after the screening tests |
| Partial verification avoided? | Yes | Colposcopy was also performed in 5% of screen‐negatives |
| Differential verification avoided? | Yes | Same RS in all cases |
| Incorporation avoided? | Yes | Screening tests not part of the RS |
| Reference standard results blinded? | No | Colposcopists and pathologists were aware of the results |
| Index test results blinded? | Yes | Screening tests done before the RS |
| Relevant clinical information? | Yes | Cytologists received routine clinical information |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women older than 18, not pregnant, without a recent (< 1 year) history of surgery or laser treatment of the cervix, whose cervix was visible by the physician, attending for cervical smear in a French university hospital or private practices | |
| Participants | 1757 women (mean age 33.3) in France | |
| Study design | Cross‐sectional study of women receiving CC LBC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy, if colposcopy was normal, biopsies were not taken. Colposcopy was performed in all women | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold 1 pg/mL CT: CC, positivity threshold ASCUS+ CT: LBC, positivity threshold ASCUS+ | |
| Follow‐up | ||
| Notes | Some cytological data are taken from the article Coste 2003 | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women > 18 years attending for routine screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsy |
| Acceptable delay between tests? | Yes | Performed at the same time |
| Partial verification avoided? | Yes | RS applied to all women |
| Differential verification avoided? | Yes | The same RS in all cases |
| Incorporation avoided? | Yes | Screening tests not part of the RS |
| Reference standard results blinded? | Yes | Pathologists were blinded to the screening tests |
| Index test results blinded? | Yes | Cytologist were blinded to other results |
| Relevant clinical information? | Unclear | It is not clear what information was available to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Unclear | It is not clear whether there were any withdrawals |
| Clinical features and settings | Women undergoing routine screening in 9 gynaecological practices in Flanders (Belgium). Exclusion criteria included pregnancy and history of cervical disease | |
| Participants | 3126 women with a median age of 42.7 years (range 18.0–84.3) in Flanders, Belgium | |
| Study design | Cross‐sectional study of women receiving LBC, HPV testing and BD ProExC ICC staining | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ and CIN 3+ RS: colposcopically‐directed biopsy, if colposcopy was normal, biopsies were not taken. Colposcopy was performed in all women | |
| Index and comparator tests | IT: HPV testing by PCR for the detection of 13 high‐risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) IT: BD ProExC immunocytochemistry CT: LBC, positivity threshold ASCUS+ | |
| Follow‐up | Women were followed up for the detection of CIN 2+ for a further period of 24 months | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women attending for routine screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | Colposcopy performed in all women |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The tests did not form part of the RS |
| Reference standard results blinded? | No | In the initial colposcopy yes but in the subsequent one the colposcopist was aware of the results |
| Index test results blinded? | Unclear | Unclear whether cytologists were aware of the colposcopy results |
| Relevant clinical information? | Unclear | It is unclear what information was available to the cytologists |
| Uninterpretable results reported? | Yes | None had an inadequate smear |
| Withdrawals explained? | Unclear | It does not seem as if there have been any withdrawals from follow‐up |
| Clinical features and settings | Women residing in Santiago, Chile, were invited to participate through an outreach campaign, excluding women who were pregnant, hysterectomised or virgins | |
| Participants | 8407 women from Santiago, Chile (mean age 42.2 years) | |
| Study design | Cross‐sectional study of women receiving CC, HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ and CIN 3+ RS: colposcopically‐directed biopsy. Colposcopy was also performed in a random sample of screen‐negatives | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold 1 pg/mL CT: CC, positivity threshold ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women aged 25–64 years |
| Acceptable reference standard? | Yes | Colposcopy and colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | Colposcopy was also performed in sample of high‐risk (VIA positive) screen‐negatives |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The tests did not form part of the RS |
| Reference standard results blinded? | No | Pathologists were blind to the HPV test result, but not necessarily to the Pap test result |
| Index test results blinded? | Yes | Screening tests done before the RS |
| Relevant clinical information? | Unclear | It is unclear what information was available to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women > 25 years old mentally competent with an intact uterus were invited from 42 villages in a peri‐urban rural community | |
| Participants | 2331 women (mean age 37) from Andhra Pradesh, India | |
| Study design | Cross‐sectional study of women receiving CC, VIA and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ or CIN 3+ RS: colposcopically‐directed biopsy, colposcopy only if no lesion was seen. Only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL. Referred for colposcopy if positive IT: VIA, referred for colposcopy if positive CT: CC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | A random 20% sample of screen‐negative women also underwent colposcopy. For the calculation of the accuracy indices in this meta‐analysis only the adjusted‐for verification bias estimates are used | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women > 25 years |
| Acceptable reference standard? | Yes | Colposcopy with biopsies if indicated |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | A random sample of screen‐negative women also underwent colposcopy |
| Differential verification avoided? | Yes | The same RS applied in all occasions |
| Incorporation avoided? | Yes | The RS did not include the screening tests |
| Reference standard results blinded? | Yes | Colposcopists were not aware of screening test results |
| Index test results blinded? | Yes | Screening tests were performed before the RS |
| Relevant clinical information? | Unclear | It is not clear whether cytologists had the routine information |
| Uninterpretable results reported? | No | Inadequate or unsatisfactory specimens were not reported |
| Withdrawals explained? | Yes | Withdrawals were explained |
| Clinical features and settings | Women attending 3 gynaecological clinics in Bukavu, Democratic Republic of Congo, during November and December 2003 were recruited for the study. Exclusion criteria were pregnancy, severe gynaecological bleeding, previous hysterectomy and age < 25 or > 60 years | |
| Participants | 343 women between 25 and 60 years of age (median: 37 years) in DR Congo | |
| Study design | Cross‐sectional study of women receiving conventional and LBC, HPV DNA testing and HPV E6/E7 mRNA testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy, if no lesion was seen a random biopsy was taken. Colposcopy was performed in all women | |
| Index and comparator tests | IT: HPV testing by PCR (GP5+/6+),for the detection of 14 high‐risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) IT: E6/E7 mRNA testing (NASBA) for the detection of 5 high‐risk types (16, 18, 31, 33, 45) or 9 high‐risk types (16, 18, 31, 33, 45, 35, 51, 52, 58) CT: CC. CT: LBC | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Unclear | Women aged 25‐60 years attending gynaecological clinics. The reason was not specified |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | Colposcopy performed in all women |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The tests did not form part of the RS |
| Reference standard results blinded? | Unclear | It is not clear whether the pathologist had knowledge of the test results |
| Index test results blinded? | Unclear | It is not clear whether cytologists had knowledge of the pathology results |
| Relevant clinical information? | Unclear | It is not clear what information was given to the cytologists |
| Uninterpretable results reported? | Yes | Nine Pap (2.6%) and 14 liquid‐based smears (4.1%) were assessed as unsatisfactory |
| Withdrawals explained? | Yes | Histology was unsatisfactory in 30 cases (8.7%), and these cases were left out of the overall statistical calculations |
| Clinical features and settings | Women 30‐60 years of age who were undergoing routine cervical screening at 3 German centres, in Tübingen, Saarbrücken, and Freiburg, were invited to participate in the study. Exclusion criteria were hysterectomy or destructive therapy of the cervix, pregnancy, an abnormal cytological result within the past 6 months, HIV infection, and organ transplantation | |
| Participants | 9451 women attending routine screening | |
| Study design | Cross‐sectional study of women receiving conventional and LBC, HPV DNA testing and HPV E6/E7 mRNA testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ or CIN 3+ RS: colposcopy with colposcopically‐directed biopsy (if required) for screen‐positives and 3.6% of screen‐negatives | |
| Index and comparator tests | IT: HPV testing by HC2 (1 pg/mL), referred for colposcopy if positive IT: E6/E7 mRNA testing by Aptima HPV assay, referred for colposcopy if positive CT: LBC, referred for colposcopy if LSIL+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 30‐60 years of age who were undergoing routine cervical screening |
| Acceptable reference standard? | Yes | Colposcopy with biopsies if indicated |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | A random sample of screen negative women also underwent colposcopy |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The tests did not form part of the RS |
| Reference standard results blinded? | Yes | All LBC‐positive samples and samples with abnormal histological findings were collected by the respective clinical departments, and a blinded review was performed by independent external experts, for quality control |
| Index test results blinded? | Yes | Screening tests were performed before the RS |
| Relevant clinical information? | Unclear | It is not clear what information was given to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women aged 18‐50 years without hysterectomy, chronic immune suppression or treatment for CIN, presenting for annual examinations at planned parenthood clinics | |
| Participants | 4075 women (mean age 25) in Washington State, USA | |
| Study design | Cross‐sectional study of women receiving LBC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 3+ RS: colposcopically‐directed biopsy, if colposcopy was normal random biopsies were taken. Only for screen‐positives and 7% of screen‐negatives. | |
| Index and comparator tests | IT: HPV testing by PCR (MY09, MY11, HMB01) for the detection of 18 high‐risk HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 55, 56, 58, 59, 68, 73, 82, 84). Referred for colposcopy and biopsies if positive IT: HPV testing by HC2 (Digene) only for the last 1150 women. Positivity threshold at 1 pg/mL. Referred for colposcopy + biopsies if positive CT: LBC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | 7% of women with negative results also underwent colposcopy. For the calculation of the accuracy indices in this meta‐analysis only the corrected estimates (adjusted for verification bias and loss to follow‐up) are used | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 18 to 50 years old attending for annual routine examinations |
| Acceptable reference standard? | Yes | Colposcopy with biopsies |
| Acceptable delay between tests? | Yes | Both tests performed at the same visit |
| Partial verification avoided? | Yes | A random sample of women (7%) with negative tests also received colposcopy |
| Differential verification avoided? | Yes | The RS was the same for all women |
| Incorporation avoided? | Yes | Cytology and HPV testing were not included in the RS |
| Reference standard results blinded? | Yes | Pathologists had no knowledge of clinical data |
| Index test results blinded? | Yes | The RS was applied after the screening tests were reported |
| Relevant clinical information? | No | Cytologists had no clinical information |
| Uninterpretable results reported? | Yes | Inadequate or unsatisfactory results were reported |
| Withdrawals explained? | Yes | Withdrawals were explained |
| Clinical features and settings | All ever‐married women aged 30 to 59 years were targeted for screening. Women who had undergone a total hysterectomy, or who had been diagnosed with cancer or precancer, were excluded from the study. Menstruating women were excluded temporarily. Pregnant women were eligible to participate in the study 12 weeks after the end of their pregnancy | |
| Participants | 5032 women from Uttar Pradesh, India. The mean age of all women screened was 37.9 (SD 7.5) years | |
| Study design | Cross‐sectional study of women receiving CC, clinician‐collected HPV testing, self‐collected HPV testing and VIA | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ or CIN 3+ RS: colposcopically‐directed biopsy, colposcopy only if no lesion was seen. Only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by care HPV, positivity threshold at 1 pg/mL. Referred for colposcopy if positive IT: HPV self‐testing by vaginal care HPV, positivity threshold at 1 pg/mL. Referred for colposcopy if positive IT: VIA, referred for colposcopy if positive CT: CC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women aged 30‐59 |
| Acceptable reference standard? | Yes | Colposcopy with or without directed biopsy |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | No | Only screen‐positives had colposcopy |
| Differential verification avoided? | Yes | The same RS applied in all cases |
| Incorporation avoided? | Yes | Cytology and HPV testing were not included in the RS |
| Reference standard results blinded? | Unclear | Relevant information was not given |
| Index test results blinded? | Unclear | Relevant information was not given |
| Relevant clinical information? | Unclear | Unclear what information was given to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women aged 15‐69, mentally and physically competent, married, non‐pregnant without a hysterectomy were contacted at home by village doctors | |
| Participants | 2562 women from three provinces (Shanxi, Lianoning, Guangdong) in China | |
| Study design | Cross‐sectional study of women receiving LBC, HPV testing, VIA, screening colposcopy and fluorescence spectroscopy | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy, if colposcopy was normal random biopsies were taken. Only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL. Referred for colposcopy + biopsies if positive IT: VIA, Rreferred for colposcopy + biopsies if positive IT: fluorescence spectroscopy, referred for colposcopy + biopsies if positive IT: screening colposcopy, referred for colposcopy + biopsies if positive CT: LBC (AutoCyte). Referred for colposcopy + biopsies if LSIL+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 15‐59 years old |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies and random biopsies if colposcopy was normal |
| Acceptable delay between tests? | Yes | Both tests taken at the same visit |
| Partial verification avoided? | Yes | Women received the RS not only if they had positive cytology or HPV test, but also if they had positive VIA, spectroscopy or screening colposcopy |
| Differential verification avoided? | Yes | The same RS was applied in the case of positive cytology and positive HPV test |
| Incorporation avoided? | Yes | The RS was not composed of the LBC and HPV tests |
| Reference standard results blinded? | No | Doctors performing the final colposcopy were aware of screening results |
| Index test results blinded? | Yes | The RS was applied after the screening tests were reported |
| Relevant clinical information? | Unclear | It is not clear whether clinical information was given to the cytologists |
| Uninterpretable results reported? | Yes | All results including inadequate specimens were reported |
| Withdrawals explained? | Yes | Withdrawals were explained |
| Clinical features and settings | Women residing in a suburb of Kinshasa, Democratic Republic of Congo. Women were eligible if they were ≥ 30 years and had an intact uterus but were not pregnant | |
| Participants | 1528 women in DR Congo | |
| Study design | Cross‐sectional study of women receiving CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy, in 20% of women where colposcopy was normal random biopsies were taken. Colposcopy was performed in all women | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL. IT: HPV testing by HC2+4 (Digene), positivity threshold at 1 pg/mL. CT: CC | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Unscreened women 30 or older |
| Acceptable reference standard? | Yes | Colposcopy and directed biopsy |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | Colposcopy was performed in all women |
| Differential verification avoided? | Yes | The same RS was used in all situations |
| Incorporation avoided? | Yes | The screening tests did not form part of the RS |
| Reference standard results blinded? | Yes | Pathologists and colposcopists were not aware of the screening test results |
| Index test results blinded? | Unclear | Cytopathologists were blinded to the results of the colposcopy and the HPV tests, but unclear if blinded to results of the pathology |
| Relevant clinical information? | Unclear | It is not clear what information was given to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | A pilot study recruited women aged 30‐50 years in 2006 by poster and flier advertisement, radio publicity, and nurse ‘‘awareness’’ visits to villages round Port Vila, Efate Island, Vanuatu. Women with a history of gynaecological surgery were excluded | |
| Participants | 499 apparently healthy Ni‐Vanuatu women (mean age 39.3 years) | |
| Study design | Cross‐sectional study of women receiving CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy. Colposcopy was performed in all women | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL IT: VIA IT: VILI CT: LBC | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Apparently healthy women 30‐50 years old |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsy |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | Colposcopy was performed in all women |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The screening tests did not form part of the RS |
| Reference standard results blinded? | Yes | The colposcopists and pathologists were not aware of the screening test results |
| Index test results blinded? | Yes | All cytology and histology examinations were blinded to the clinical and HPV findings |
| Relevant clinical information? | Unclear | It is not clear what information was given to the cytologists |
| Uninterpretable results reported? | Yes | Rates of unsatisfactory results were given |
| Withdrawals explained? | Unclear | Not clear whether all women with an indication for LLETZ had the procedure |
| Clinical features and settings | A pilot study recruited women aged 30‐50 years in 2006 by poster and flier advertisement, radio publicity, and nurse ‘‘awareness’’ visits to villages round Port Vila, Efate Island, Vanuatu. Women with a history of gynaecological surgery were excluded | |
| Participants | 512 apparently healthy Ni‐Vanuatu women (mean age 38.36 SD 5.6) | |
| Study design | Cross‐sectional study of women receiving CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: LLETZ only in screen‐positive women | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL CT: LBC | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Apparently healthy women 30‐50 years old |
| Acceptable reference standard? | Yes | LLETZ |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | No | LLETZ was performed only in screen‐positives |
| Differential verification avoided? | Unclear | The same RS applied in all situations |
| Incorporation avoided? | Unclear | The screening tests did not form part of the RS |
| Reference standard results blinded? | Unclear | The colposcopists and pathologists were not aware of the screening test results |
| Index test results blinded? | Unclear | All cytology and histology examinations were blinded to the clinical and HPV findings |
| Relevant clinical information? | Unclear | It is not clear what information was given to the cytologists |
| Uninterpretable results reported? | Unclear | Rates of unsatisfactory results were given |
| Withdrawals explained? | Yes | Number of women who did not consent to LLEZT was given |
| Clinical features and settings | From April 2008‐February 2009, women aged 20–65 years who were seen for their annual exam in 17 private gynaecology practices in Paris, France, were invited to participate in this voluntary screening. Women were not eligible if they had undergone total hysterectomy, were pregnant or had an abnormal cytology in the past 6 months | |
| Participants | 4429 women in Paris, France | |
| Study design | Cross‐sectional study of women receiving CC, HPV DNA testing and HPV mRNA testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies, for screen‐positives and a random sample 14% of screen‐negatives. If colposcopy was negative random biopsies were taken | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL. Referred for colposcopy if positive IT: HPV mRNA testing by Aptima (Gen‐Probe). Referred for colposcopy if positive CT: LBC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 20‐65 years old attending routine screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | Colposcopy was performed on a random sample of screen‐negatives |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The screening tests did not form part of the RS |
| Reference standard results blinded? | No | Histopathologists were not blinded to cytology results |
| Index test results blinded? | Yes | Colposcopy was performed after the tests were reported |
| Relevant clinical information? | Unclear | It is unclear what information was given to the pathologists |
| Uninterpretable results reported? | Yes | Reported |
| Withdrawals explained? | Yes | Explained |
| Clinical features and settings | Screening activities were conducted in 4 women’s and children’s hospitals in 3 provinces (Shanxi, Jiangxi, and Gansu) in China. From 2003‐2005, women who were 30‐49 years of age were eligible. In 2006, women who were 30‐54 years of age were eligible. For all screening years, women were eligible if they were married or reported previous sexual activity; had no clinical suspicion of pregnancy (last menstrual period began < 5 weeks previously in non‐menopausal women); were able to give informed consent; had no reported history of CIN, cancer of cervix, or hysterectomy; had no debilitating disease (physically unable to undergo study procedures); and had no reported history of cervical cancer screening | |
| Participants | 9057 women (mean age 39) in China | |
| Study design | Cross‐sectional study of women receiving LBC, HPV testing, VIA and VILI | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 3+ RS: colposcopically‐directed biopsy. The criteria for referral varied by year. In 2003 and 2005, if a woman was VIA‐ or VILI‐positive, she was referred for colposcopy. In 2004 and 2006, all women had colposcopy regardless of the results of VIA and VILI. Directed biopsy was performed on any visible lesion. If a woman was VIA‐ or VILI‐negative, but with either a Pap test of ASC‐H, AGUS, LSIL or higher, or positive for HR‐HPV DNA by HC2 testing, she was recalled after 2 weeks for colposcopy and received four‐quadrant biopsy | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL. IT: VIA IT: VILI CT: LBC. Positivity threshold LSIL+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 30‐54 without prior screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | Colposcopy was not limited to screen‐positives |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The tests did not form part of the RS |
| Reference standard results blinded? | Yes | The pathologists had no knowledge of the test results |
| Index test results blinded? | Yes | Colposcopy was performed after the test results were reported |
| Relevant clinical information? | Unclear | It is not clear what information was given to the cytologists |
| Uninterpretable results reported? | Yes | Reported |
| Withdrawals explained? | Unclear | It is not clear whether all women that should have had colposcopy attended |
| Clinical features and settings | Women aged 32‐38 attending the Swedish Cervical Cancer Screening Programme | |
| Participants | 6257 women attending cervical screening in 5 Swedish cities (Stockholm, Uppsala, Malmo, Umea, Gothenburg) | |
| Study design | RCT of HPV testing and CC versus CC alone. Only the cross‐sectional results of the first screening round, from the experimental arm only were included in this meta‐analysis | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy, if colposcopy was normal, random biopsies were taken | |
| Index and comparator tests | IT: HPV testing by PCR (GP5+, GP6+) for the detection of 14 high‐risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68). If cytology was negative, women with a positive HPV test were referred for the RS if a second HPV test 12 months later was also type‐specific positive CT: CC. For the calculation of the accuracy indices an abnormal result was considered any smear showing ASCUS+. However in 4 cities the option of a repeat smear was given after a result of ASCUS | |
| Follow‐up | 73 of 328 women who were HPV‐positive and CC‐negative in the first exam did not return for a second exam one year later | |
| Notes | Apart from the histology specimens taken inside the protocol colposcopy, the study had access to histology specimen taken outside the protocol through the national pathology registry | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | No | Only women aged 32‐38 years were included |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies and random biopsies if colposcopy was normal |
| Acceptable delay between tests? | Yes | Both tests taken at the same visit |
| Partial verification avoided? | No | Only women with positive tests were referred for colposcopy |
| Differential verification avoided? | Yes | The same RS was applied in the case of positive cytology and persistent type‐specific positive HPV test |
| Incorporation avoided? | Yes | The RS was not composed of the index and comparator tests |
| Reference standard results blinded? | Yes | The women and the clinical personnel were not aware of the screening test results |
| Index test results blinded? | Yes | The RS was applied after the screening tests were reported |
| Relevant clinical information? | Unclear | It is not clear whether clinical information was given to the cytologists |
| Uninterpretable results reported? | Yes | All results including inadequate specimens were reported |
| Withdrawals explained? | Yes | Withdrawals were explained |
| Clinical features and settings | The study was conducted in rural Mexico. Women aged 30‐50 years, non‐pregnant, with no history of hysterectomy or pelvic irradiation and varied histories of screening, participated | |
| Participants | 2049 women in rural Mexico, median age 39.2 years (range, 30‐50 years) | |
| Study design | Cross‐sectional study of women receiving LBC, HPV DNA testing, HPV mRNA testing, self‐HPV DNA testing, self‐HPV mRNA testing and VIA | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 3+ RS: colposcopically‐directed biopsy for all women, if colposcopy was normal random biopsies were taken. Colposcopy was performed to screen‐positives only | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL. Referred for colposcopy if positive IT: HPV mRNA testing by Aptima (Gen‐Probe). Referred for colposcopy if positive IT: self‐HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL. Referred for colposcopy if positive IT: self‐HPV mRNA testing by Aptima (Gen‐Probe). Referred for colposcopy if positive IT: VIA CT: LBC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 30‐50 years |
| Acceptable reference standard? | Yes | Colposcopy and biopsies |
| Acceptable delay between tests? | Yes | Performed on the same visit |
| Partial verification avoided? | No | Only screen‐positives had colposcopy |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The tests were not part of the RS |
| Reference standard results blinded? | Unclear | Not clear whether pathologists had access to test results |
| Index test results blinded? | Yes | Technicians and cytologists were not aware of the other test results |
| Relevant clinical information? | Unclear | Not clear what information was given to cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Previously unscreened women 35‐45 years old, with no history of pelvic radiation or hysterectomy residing in the Shanxi Province in China | |
| Participants | 1993 women (mean age 39.1) from rural China | |
| Study design | Cross‐sectional study of women receiving self‐HPV testing, LBC, direct HPV testing and VIA | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: Colposcopically‐directed biopsy for all women, if colposcopy was normal random biopsies were taken. Colposcopy was performed on all women | |
| Index and comparator tests | IT: HPV testing by HC2 direct sampling (Digene), positivity threshold at 1 pg/mL. IT: HPV testing by HC2 self sampling (Digene), positivity threshold at 1 pg/mL. CT: LBC. For the calculation of the accuracy indices an abnormal result was considered any smear showing ASCUS+ | |
| Follow‐up | ||
| Notes | Some data were obtained from the publication by Belinson 2001 | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | No | Very limited age spectrum of previously unscreened women |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | The RS was applied to all women |
| Differential verification avoided? | Yes | The same RS was performed on all occasions |
| Incorporation avoided? | Yes | The screening tests were not part of the RS |
| Reference standard results blinded? | Yes | Pathologists had no knowledge of the screening tests |
| Index test results blinded? | Yes | Cytologists were not aware of the final diagnosis |
| Relevant clinical information? | Unclear | It is not clear whether cytologists had the routine clinical information |
| Uninterpretable results reported? | Yes | The numbers of inadequate smears were given |
| Withdrawals explained? | Yes | It does not seem as if there were any withdrawals |
| Clinical features and settings | Women 17‐79 years old without prior history of cervical pathology attending the outpatient clinics of a university hospital for routine screening | |
| Participants | 977 women (mean age 38) in Ioannina, Greece | |
| Study design | Cross‐sectional study of women receiving both CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy. Colposcopy only if no lesion was seen. Only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by PCR (MY09, MY11) for the detection of 11 high‐risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58). Referred for colposcopy if positive CT: CC. Referred for colposcopy if reactive cellular changes+ | |
| Follow‐up | ||
| Notes | For the calculation of CC accuracy indices the thresholds of ASCUS+ and LSIL+ were used in this meta‐analysis. Since women with reactive cellular changes also underwent colposcopy, this limits verification bias | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 17‐79 years old attending routine screening |
| Acceptable reference standard? | Yes | Colposcopy with biopsies if necessary |
| Acceptable delay between tests? | Yes | Both tests performed at the same visit |
| Partial verification avoided? | Yes | Women with less than ASCUS were also referred for the RS |
| Differential verification avoided? | Yes | The same RS was used in all occasions of positive screening test |
| Incorporation avoided? | Yes | The RS was not composed of the screening tests |
| Reference standard results blinded? | No | Colposcopists were aware of the results |
| Index test results blinded? | Yes | RS performed after the screening tests were reported |
| Relevant clinical information? | Unclear | It is not clear whether relevant clinical information was revealed to the cytologists |
| Uninterpretable results reported? | No | Inadequate and unsatisfactory results were not reported |
| Withdrawals explained? | Yes | Withdrawals were explained |
| Clinical features and settings | Women > 30 years old attending urban, suburban or rural office‐based gynaecology practices in Hannover and Tuebingen for routine screening. No hysterectomy, not pregnant, no history of atypical cytology or CIN in the last year | |
| Participants | 8101 women (mean age 42.7) from Germany | |
| Study design | Cross‐sectional study of women receiving both CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies. If colposcopy was negative a biopsy might have not been taken. Screen‐positives and a random 3.4% sample of screen‐negatives underwent colposcopy | |
| Index and comparator tests | IT: HPV testing by HC2 (DIgene), positivity threshold 1 pg/mL, referred for colposcopy if positive CT: CC, referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women > 30 years old attending for routine screening |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | 3.4% of screen‐negatives were also verified |
| Differential verification avoided? | Yes | The same RS was applied in each case |
| Incorporation avoided? | Yes | Screening tests were not part of the RS |
| Reference standard results blinded? | Unclear | It is not clear whether colposcopists and pathologists had knowledge of the screening test results |
| Index test results blinded? | Yes | RS was performed after the screening tests were reported |
| Relevant clinical information? | Yes | Cytologists were given routine clinical information |
| Uninterpretable results reported? | Unclear | Unsatisfactory smears were reported as Pap IIw, which also included ASCUS. There was no mention of unsatisfactory HPV testing specimens |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women aged 30‐54 living in rural villages in Shanxi Province, China. Non pregnant, no history of CIN, pelvic radiation or hysterectomy | |
| Participants | 2530 women (mean age 43.4) from rural China | |
| Study design | Cross‐sectional study of women receiving self‐HPV testing, direct‐HPV testing, LBC and VIA | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy for all women. Colposcopy only if no lesion was seen | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene). For the calculation of the accuracy indices the test was considered positive at the threshold of 1 pg/mL IT: HPV testing by Care HPV test (self‐sampling). For the calculation of the accuracy indices the test was considered positive at the threshold of 1 pg/mL. IT: HPV testing by Care HPV test (directed sampling). For the calculation of the accuracy indices the test was considered positive at the threshold of 1 pg/mL. CT: LBC (Surepath). For the calculation of the accuracy indices the test was considered positive at the threshold of LSIL+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women aged 30‐54 |
| Acceptable reference standard? | Yes | Colposcopy with directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | All women underwent colposcopy and biopsies |
| Differential verification avoided? | Yes | The same RS was applied in all circumstances |
| Incorporation avoided? | Yes | The screening tests were not part of the RS |
| Reference standard results blinded? | Unclear | It is not clear whether pathologists were aware of the screening test results |
| Index test results blinded? | Yes | Cytologists were not aware of the histology results |
| Relevant clinical information? | Unclear | It is not clear whether cytologists had the routine clinical information |
| Uninterpretable results reported? | Yes | Unsatisfactory results were reported |
| Withdrawals explained? | Yes | Withdrawals were explained |
| Clinical features and settings | Women > 35 years attending routine cervical screening without hysterectomy, without treatment for CIN in the last 5 years, non pregnant | |
| Participants | 16,706 women (median age 45) in Italy | |
| Study design | RCT of HPV testing and LBC versus CC. Only the cross‐sectional results of the first screening round, from the experimental arm only were included in this meta‐analysis | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy only for screen‐positives. Colposcopy only if no lesion was seen | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL or 2 pg/mL. Referred for colposcopy if positive CT: LBC (Thinprep). Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women >35 years attending routine screening |
| Acceptable reference standard? | Yes | Colposcopy with biopsy if required |
| Acceptable delay between tests? | Yes | At the same visit |
| Partial verification avoided? | No | RS not applied if both tests were negative |
| Differential verification avoided? | Yes | The RS was the same in all situations |
| Incorporation avoided? | Yes | The RS was not composed of the index and comparator tests |
| Reference standard results blinded? | No | Colposcopists were aware of the test results |
| Index test results blinded? | Yes | The RS was applied after the screening tests were reported |
| Relevant clinical information? | Unclear | There was not sufficient information |
| Uninterpretable results reported? | Yes | All results were reported |
| Withdrawals explained? | Yes | Withdrawals were explained |
| Clinical features and settings | Women attending the cervical cancer screening services in Morelos state, Mexico. Non pregnant, no hysterectomy, without history of CIN 2+ | |
| Participants | 7732 women (mean age 42.5) in Morelos, Mexico | |
| Study design | Cross‐sectional study of women receiving both CC, self‐collected HPV testing and direct HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsy only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene) direct sampling, positivity threshold 1 pg/mL, referred for colposcopy if positive IT: HPV testing by HC2 (Digene) self‐sampling, positivity threshold 1 pg/mL, referred for colposcopy if positive CT: CC, referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women attending a cervical cancer screening programme |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | No | Only screen‐positives received the RS |
| Differential verification avoided? | Yes | Same RS in all cases |
| Incorporation avoided? | Yes | Screening tests not part of the RS |
| Reference standard results blinded? | No | Colposcopists were aware of screening tests |
| Index test results blinded? | Yes | RS performed after screening tests were reported |
| Relevant clinical information? | Unclear | It is not clear what information was available to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Opportunistic recruitment of healthy asymptomatic women aged 25‐65, with an intact uterus and no previous history of cervical neoplasia from three different locations in India. None had been previously screened | |
| Participants | 11,518 women from Kolkata, Muumbai and Trivandum, India | |
| Study design | Cross‐sectional study of women receiving both CC, HPV testing, VIA and VILI | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies for all women, if colposcopy was negative biopsies were not taken | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold 1 pg/mL IT: VIA IT: VILI CT: CC, for the calculation of the accuracy indices the threshold of LSIL+ was used | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women aged 25‐65 without history of cervical neoplasia |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsy |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | Yes | All women received the RS |
| Differential verification avoided? | Yes | The same RS was applied to all cases |
| Incorporation avoided? | Yes | The screening tests were not part of the RS |
| Reference standard results blinded? | Yes | Colposcopists were not aware of screening test results |
| Index test results blinded? | Yes | Laboratory personnel were not aware of RS results |
| Relevant clinical information? | Unclear | It is not clear what information was given to the cytologists |
| Uninterpretable results reported? | Yes | Were reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | Women aged 18‐60, with an intact uterus, no history of abnormal Pap test in the last year, not under treatment for genital warts, not immunosuppressed, were invited by the local health units to attend for screening in Brazil and Argentina | |
| Participants | 10,138 women (mean age 37.9) from the cities of Campinas, Sao Paolo, Porto Alegre (Brazil) and Buenos Aires (Argentina) | |
| Study design | Cross‐sectional study of women receiving CC, HPV testing, VIA and VILI | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies. If colposcopy was negative biopsies were not taken unless the smear was HSIL. Colposcopy was performed in screen‐positives and in a random 5% of screen‐negatives | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), referred for colposcopy if > 1 pg/mL IT: VIA, referred for colposcopy if positive IT: VILI, referred for colposcopy if positive CT: CC, referred for colposcopy if LSIL+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 18‐60 years old |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Screening tests performed at the same visit, colposcopy 45 days later |
| Partial verification avoided? | Yes | 5% random sample of screen‐negatives had colposcopy plus the VIA and VILI positives |
| Differential verification avoided? | Yes | The same RS in all occasions |
| Incorporation avoided? | Yes | Screening tests not part of the RS |
| Reference standard results blinded? | No | Colposcopists and pathologists were aware of test results |
| Index test results blinded? | Yes | RS performed later |
| Relevant clinical information? | Unclear | It is not clear what information was available to the cytologists |
| Uninterpretable results reported? | No | Not reported |
| Withdrawals explained? | No | Not explained |
| Clinical features and settings | Women 18‐70 years old visiting the offices of 10 private gynaecologists in East Thirungia, Germany for screening. Non‐pregnant, no history of cervical conisation, no hysterectomy or CIN, no atypical cytology in the last year | |
| Participants | 4761 women (median age 35) from Germany | |
| Study design | Cross‐sectional study of women receiving both CC, HPV testing and screening colposcopy | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies. If colposcopy was negative random biopsies were taken. Only for screen‐positive women | |
| Index and comparator tests | IT: HPV testing by PCR (GP) for the detection of 14 high‐risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68). Referred for colposcopy if positive CT: CC. Referred for colposcopy if LSIL+ | |
| Follow‐up | A second screening round was done for women negative for all three tests 4‐8 months later | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 18‐70 years old |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | No | 8 months elapsed between screening tests and verification |
| Partial verification avoided? | Yes | The presence of a third screening test (colposcopy) and the referral of the screen‐positives for verification limits the problem of partial verification |
| Differential verification avoided? | Yes | The same RS was used for all tests |
| Incorporation avoided? | Yes | The screening tests did not form part of the RS |
| Reference standard results blinded? | Yes | Pathologists were not aware of the screening test results |
| Index test results blinded? | Yes | The RS was performed later |
| Relevant clinical information? | Unclear | It is not clear whether cytologists were given the routine clinical information |
| Uninterpretable results reported? | Yes | The numbers were given |
| Withdrawals explained? | Yes | The reasons for withdrawals were explained |
| Clinical features and settings | Consecutive women aged 30–65 years, not pregnant and with no history of treatment for CIN grade 2 and higher (CIN 2+), receiving routine gynaecological care at the outpatient department of the D.O. Ott Research Institute of Obstetrics and Gynaecology, St. Petersburg, Russia from June 2008‐April 2009 were enrolled | |
| Participants | 823 women (mean age 39.5 + 8.4 years ) in St. Petersburg, Russia | |
| Study design | Cross‐sectional study of women receiving CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), referred for colposcopy if > 1 pg/mL CT: CC, referred for colposcopy if LSIL+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women 30‐65 years old |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | No | Only screen‐positives were verified |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The screening tests did not form part of the RS |
| Reference standard results blinded? | Unclear | It is not clear what information was available to the pathologists and colposcopists |
| Index test results blinded? | Yes | Colposcopy was performed after the tests were reported |
| Relevant clinical information? | Unclear | It is not clear what information was available to the cytologists |
| Uninterpretable results reported? | Yes | In six (0.7%) women, samples were initially graded unsatisfactory for cytological assessment. Those women were called for repeated cytology and were tested negative |
| Withdrawals explained? | Yes | 44 women did not return for colposcopy |
| Clinical features and settings | Consecutive women attending 6 different outpatient clinics in 3 New Independent States of the former Soviet Union. The study focused on 3 target populations 1. women participating locally organised screening programmes for cervical cancer, 2. those attending regular gynaecology clinics for various indications, 3. those attending STD clinics | |
| Participants | 3175 women (mean age 32.7) from Belarus, Russia and Latvia | |
| Study design | Cross‐sectional study of women receiving both CC and HPV testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 3+ RS: colposcopically‐directed biopsies, if colposcopy was normal a biopsy might have not been taken. Only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by HC2, positivity threshold 1 pg/mL, referred for colposcopy if positive CT: CC, referred for colposcopy if LSIL+ | |
| Follow‐up | ||
| Notes | For the calculation of the accuracy indices the results from primary screening and not from re‐screening were used | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Most of the women were attending a local cervical screening programme |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | No | Only screen‐positives were verified |
| Differential verification avoided? | Yes | The same RS was performed in all cases |
| Incorporation avoided? | Yes | Screening tests were not part of the RS |
| Reference standard results blinded? | Unclear | It is not clear what information was available to the colposcopists and pathologists |
| Index test results blinded? | Yes | The RS was performed after the screening tests were reported |
| Relevant clinical information? | Unclear | It is not clear what information was available to the cytologists |
| Uninterpretable results reported? | No | Not reported |
| Withdrawals explained? | Yes | Were explained |
| Clinical features and settings | The Shenzhen Cervical Cancer Screening Trial I (SHENCCAST I) took place in the city of Shenzhen, Guangdong Province, in southern China. Women were eligible if they were 25 to 59 years of age, were non pregnant, had had no cervical cancer screening for at least 3 years, had no prior hysterectomy, and had no prior pelvic radiation | |
| Participants | 2098 women in southern China (mean age 35) | |
| Study design | Cross‐sectional study of women receiving CC, HPV DNA testing and HPV mRNA testing | |
| Target condition and reference standard(s) | TC: histologically‐confirmed CIN 2+ RS: colposcopically‐directed biopsies. If colposcopy was negative random biopsies were taken. Only for screen‐positives | |
| Index and comparator tests | IT: HPV testing by HC2 (Digene), positivity threshold at 1 pg/mL. Referred for colposcopy if positive IT: HPV mRNA testing by Aptima (Gen‐Probe). Referred for colposcopy if positive CT: LBC. Referred for colposcopy if ASCUS+ | |
| Follow‐up | ||
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Women aged 25‐59 |
| Acceptable reference standard? | Yes | Colposcopically‐directed biopsies |
| Acceptable delay between tests? | Yes | Performed at the same visit |
| Partial verification avoided? | No | Only screen‐positives were referred |
| Differential verification avoided? | Yes | The same RS applied in all situations |
| Incorporation avoided? | Yes | The screening tests did not form part of the RS |
| Reference standard results blinded? | Yes | Pathologists were blinded to HPV test results |
| Index test results blinded? | Yes | Cytologists were blinded to the pathology and the HPV tests |
| Relevant clinical information? | Unclear | It is not clear what information was available to the pathologists |
| Uninterpretable results reported? | No | Were there any invalid results? |
| Withdrawals explained? | Yes | 95 women who were requested to return for colposcopy based on their test results did not return |
ASCUS: atypical squamous cells of undetermined significance; CC: conventional cytology; CIN: cervical intraepithelial neoplasia; CT: comparator test; HC2: hybrid capture 2; HPV: human papillomavirus; HSIL: high‐grade squamous intraepithelial lesion; IT: index test; LBC: liquid‐based cytology; LEEP: loop electro‐excision procedure; LLETZ – large loop excision of the transformation zone; LSIL: low‐grade squamous intraepithelial lesion; Pap: Papanicolaou; PCR: polymerase chain reactions; RS: reference standard; RCT: randomised controlled trial; TC: target condition; VIA: visual inspection with acetic acid; VILI: visual inspection with Lugol's iodine
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The reference standard was biopsy without colposcopy and the criteria for the reference standard application were undetermined | |
| Presents data on the same participants as the study by Pan 2003 | |
| Data on cytology were not given | |
| Data on cytology were not given | |
| Data on cytology were not given | |
| Retrospective study on selected population with a positive HC2 test | |
| Retrospective study on selected population consisting mainly of abnormal smears | |
| The outcome was CIN 2+ detection within 24 months of the screening round | |
| The population consisted of women with abnormal (ASCUS) smears | |
| A longitudinal study of baseline HPV and cytology‐negative women | |
| The study sample was a mix of low and high risk populations | |
| HC1 was used for HPV testing which is no longer used in clinical practice | |
| Contained only data on cytology. Referred to the included study de Cremoux 2003 | |
| HPV testing not a criterion for reference standard application | |
| Population consisted of HIV‐infected women | |
| 105 of 653 women included in this study were being investigated because of an abnormal Pap smear | |
| HC1 was used for HPV testing which is no longer used in clinical practice | |
| Accuracy given for cumulative diagnosis of CIN 2+ over the follow‐up period | |
| No appropriate reference standard | |
| Refers to the study by Schiffman 2000, which was also excluded because the HPV testing was not a criterion for the gold standard application | |
| Selected population with high rate of CIN2+ | |
| Selected population with high rates of CIN2+ | |
| No appropriate reference standard | |
| A retrospective study. Has as outcome measure the cumulative incidence of CIN2+ over 5 years. Data to calculate cross‐sectional accuracy immediately after screening were not given. Data on how many women had colposcopy were not given | |
| The sample was not representative of the population attending routine screening (archived cytological samples of women with archived histological samples) | |
| Histology given as normal or abnormal (CIN1+) | |
| HC2 was not a criterion for immediate colposcopy referral. A second positive test one year later was required | |
| HC1 was used for HPV testing which is no longer used in clinical practice | |
| HPV testing was not a criterion for colposcopy referral | |
| Not general population (30% had CIN 2+) | |
| Refers to the study Sarian 2005 | |
| Cytology results were not given | |
| HPV testing was performed only on 477/3000 women. The population included women under investigation for abnormal smears | |
| Provides the longitudinal data of the Cuzick 2003 study | |
| Same population as in the Monsonego 2011 study | |
| A positive HPV test was not a criterion for the application of the reference standard. The sample was not representative of the general population | |
| Reference standard (biopsy) not applied in all screen positives. The criteria for the application of the reference standard were unclear. Colposcopy was not a part of the reference standard | |
| Unclear what the referral criteria for colposcopy were | |
| Not appropriate gold standard | |
| 69% of women received HCI as HPV testing and 31% HCII. HPV testing results were not presented separately for the two methods | |
| The target condition was not CIN but HPV infection. CIN rates were not reported. 130/596 women were referred because of an abnormal smear | |
| Not all screen‐positives would be offered verification (ie HPV‐positive/cyto‐negative and low‐grade cyto/HPV neg) | |
| Presented data on the same population as the study Sankaranarayanan 2004a, which is included in the analysis | |
| RCT where women received either VIA or cytology or HPV testing | |
| A positive HPV test was not a criterion for the application of the reference standard | |
| Presented data on a subgroup of the population of the study Sankaranarayanan 2004a which is included in the analysis | |
| It is a longitudinal study of the risk of CIN 3 10 years after a baseline Pap smear and HPV test. It is not a cross‐sectional comparison of diagnostic accuracy. HPV testing was not a criterion for reference standard application | |
| HPV test positivity was not a criterion for reference standard application | |
| HPV testing was only done on a selected subgroup of the population | |
| Severe selection bias | |
| Presented data on the same population as the study by Blumenthal 2001 | |
| A pooled analysis of individual participant data. The published studies that have contributed their participants to this paper are already included in our meta‐analysis. However this paper also included data from unpublished studies |
ASCUS: atypical squamous cells of undetermined significance; CIN: cervical intraepithelial neoplasia; HC2: hybrid capture 2; HPV: human papillomavirus;Pap: Papanicolaou
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 CC (ASCUS+) for CIN2+ Show forest plot | 16 | 61099 |
| Test 1  CC (ASCUS+) for CIN2+. | ||
| 2 CC (ASCUS+) for CIN3+ Show forest plot | 9 | 51857 |
| Test 2  CC (ASCUS+) for CIN3+. | ||
| 3 CC (LSIL+) for CIN2+ Show forest plot | 9 | 41494 |
| Test 3  CC (LSIL+) for CIN2+. | ||
| 4 CC (LSIL+) for CIN3+ Show forest plot | 5 | 35648 |
| Test 4  CC (LSIL+) for CIN3+. | ||
| 5 LBC (ASCUS+) for CIN2+ Show forest plot | 15 | 82003 |
| Test 5  LBC (ASCUS+) for CIN2+. | ||
| 6 LBC (ASCUS+) for CIN3+ Show forest plot | 13 | 71919 |
| Test 6  LBC (ASCUS+) for CIN3+. | ||
| 7 LBC (LSIL+) for CIN2+ Show forest plot | 10 | 33519 |
| Test 7  LBC (LSIL+) for CIN2+. | ||
| 8 LBC (LSIL+) for CIN3+ Show forest plot | 5 | 21166 |
| Test 8  LBC (LSIL+) for CIN3+. | ||
| 9 HC2 (1pg/mL) for CIN2+ Show forest plot | 25 | 138230 |
| Test 9  HC2 (1pg/mL) for CIN2+. | ||
| 10 HC2 (1 pg/mL) for CIN3+ Show forest plot | 19 | 120380 |
| Test 10  HC2 (1 pg/mL) for CIN3+. | ||
| 11 HC2 (2 pg/mL) for CIN2+ Show forest plot | 2 | 26768 |
| Test 11  HC2 (2 pg/mL) for CIN2+. | ||
| 12 HC2 (2 pg/mL) for CIN3+ Show forest plot | 2 | 26768 |
| Test 12  HC2 (2 pg/mL) for CIN3+. | ||
| 13 PCR (13 hr types or more) for CIN2+ Show forest plot | 6 | 16343 |
| Test 13  PCR (13 hr types or more) for CIN2+. | ||
| 14 PCR (13 hr types or more) for CIN3+ Show forest plot | 4 | 14048 |
| Test 14  PCR (13 hr types or more) for CIN3+. | ||
| 15 PCR (10‐11 hr types) for CIN2+ Show forest plot | 2 | 3965 |
| Test 15  PCR (10‐11 hr types) for CIN2+. | ||
| 16 PCR (10‐11 hr types) for CIN3+ Show forest plot | 1 | 2988 |
| Test 16  PCR (10‐11 hr types) for CIN3+. | ||
| 17 Aptima for CIN2+ Show forest plot | 3 | 15895 |
| Test 17  Aptima for CIN2+. | ||
| 18 Aptima for CIN3+ Show forest plot | 4 | 17944 |
| Test 18  Aptima for CIN3+. | ||
| 19 PCR (4 hr types) for CIN2+ Show forest plot | 1 | 1985 |
| Test 19  PCR (4 hr types) for CIN2+. | ||
| 20 Care HPV test (0.5 pg/ml) for CIN2+ Show forest plot | 2 | 7044 |
| Test 20  Care HPV test (0.5 pg/ml) for CIN2+. | ||
| 21 Care HPV test (0.5 pg/ml) for CIN3+ Show forest plot | 2 | 7046 |
| Test 21  Care HPV test (0.5 pg/ml) for CIN3+. | ||
| 22 Cobas for CIN2+ Show forest plot | 2 | 11666 |
| Test 22  Cobas for CIN2+. | ||
| 23 Cobas for CIN3+ Show forest plot | 2 | 11666 |
| Test 23  Cobas for CIN3+. | ||
| 24 NASBA (5 types) for CIN2+ Show forest plot | 1 | 313 |
| Test 24  NASBA (5 types) for CIN2+. | ||
| 25 NASBA (9 types) for CIN2+ Show forest plot | 1 | 313 |
| Test 25  NASBA (9 types) for CIN2+. | ||
| 26 HC2+4 (1 pg/ml) for CIN2+ Show forest plot | 1 | 1352 |
| Test 26  HC2+4 (1 pg/ml) for CIN2+. | ||
| 27 HC2+4 (1 pg/ml) for CIN3+ Show forest plot | 1 | 1352 |
| Test 27  HC2+4 (1 pg/ml) for CIN3+. | ||
| 28 HC2 (1pg/mL) for CIN2+ no verification bias Show forest plot | 12 | 53013 |
| Test 28  HC2 (1pg/mL) for CIN2+ no verification bias. | ||
| 29 CC or LBC (ASCUS+) for CIN2+ no verification bias Show forest plot | 8 | 31341 |
| Test 29  CC or LBC (ASCUS+) for CIN2+ no verification bias. | ||
| 30 HC2 (1pg/mL) for CIN2+ women >30 Show forest plot | 13 | 69334 |
| Test 30  HC2 (1pg/mL) for CIN2+ women >30. | ||
| 31 self HPV test for CIN2+ Show forest plot | 4 | 23474 |
| Test 31  self HPV test for CIN2+. | ||

Study flow diagram detailing the number of the initially retrieved articles and consequent exclusions
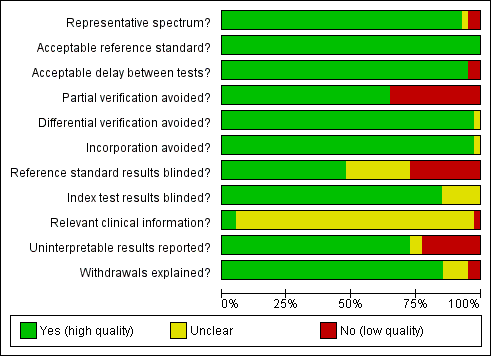
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
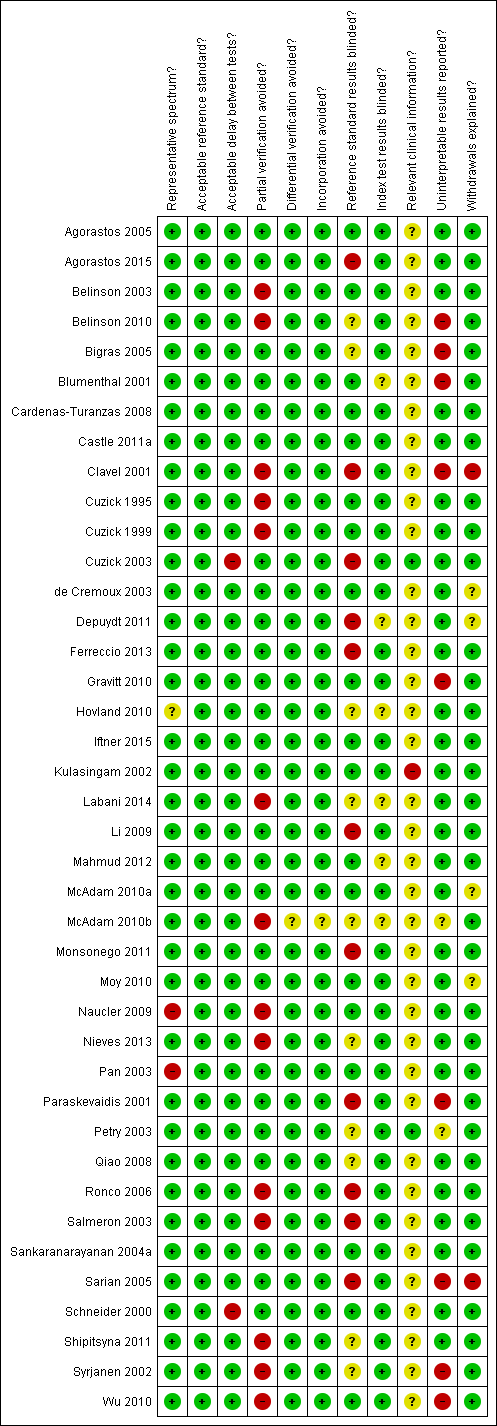
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
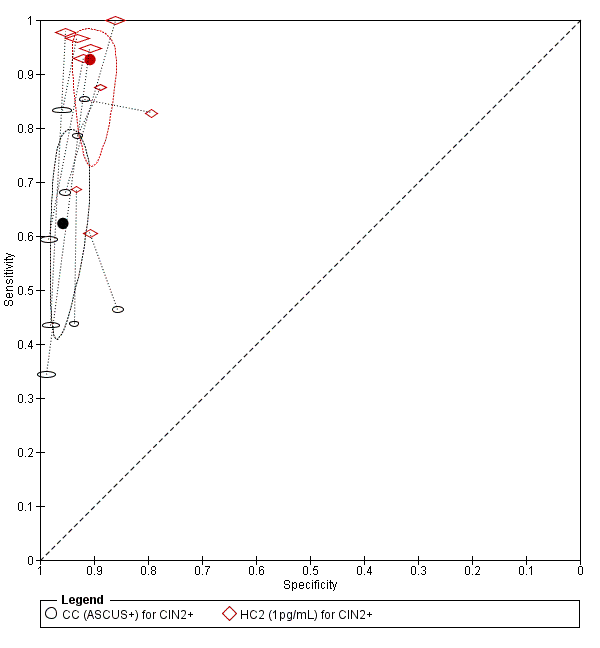
Summary ROC plot of 2 tests for detection of CIN 2+ (verified with histology): Conventional Cytology (ASCUS+) and HPV testing with hybrid capture 2 (1pg/mL). The black and red solid circles correspond to the summary estimates of sensitivity and specificity, and are shown with a 95% confidence region.

Summary ROC plot of 2 tests for detection of CIN 3+ (verified with histology): Conventional Cytology (CC) (ASCUS+) and HPV testing with hybrid capture (HC) 2 (1pg/mL). The black and red solid circles correspond to the summary estimates of sensitivity and specificity, and are shown with a 95% confidence region.

Summary ROC plot of 2 tests for detection of CIN 2+ (verified with histology): Conventional Cytology (CC) (LSIL+) and HPV testing with hybrid capture (HC) 2 (1pg/mL). The black and red solid circles correspond to the summary estimates of sensitivity and specificity, and are shown with a 95% confidence region.
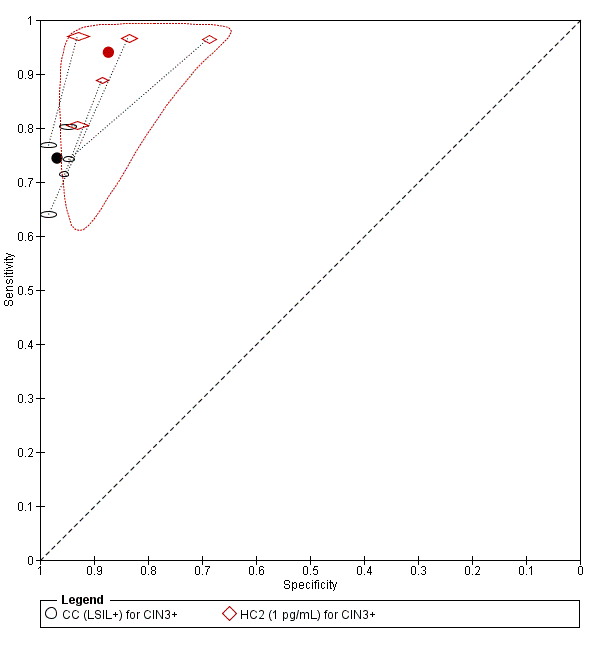
Summary ROC plot of 2 tests for detection of CIN 3+ (verified with histology): Conventional Cytology (CC) (LSIL+) and HPV testing with hybrid capture (HC) (1pg/mL). The black and red solid circles correspond to the summary estimates of sensitivity and specificity, and are shown with a 95% confidence region.

Summary ROC plot of 2 tests for detection of CIN 2+ (verified with histology): Liquid Based Cytology (LBC) (ASCUS+) and HPV testing with hybrid capture (HC) 2 (1pg/mL). The black and red solid circles correspond to the summary estimates of sensitivity and specificity, and are shown with a 95% confidence region.

Summary ROC plot of 2 tests for detection of CIN 3+ (verified with histology): Liquid Based Cytology (LBC) (ASCUS+) and HPV testing with hybrid capture (HC) 2 (1pg/mL). The black and red solid circles correspond to the summary estimates of sensitivity and specificity, and are shown with a 95% confidence region.

Summary ROC plot of 2 tests for detection of CIN 2+ (verified with histology): Liquid Based Cytology (LBC) (LSIL+) and HPV testing by hybrid capture (HC) 2 (1pg/mL). The black and red solid circles correspond to the summary estimates of sensitivity and specificity, and are shown with a 95% confidence region.

CC (ASCUS+) for CIN2+.
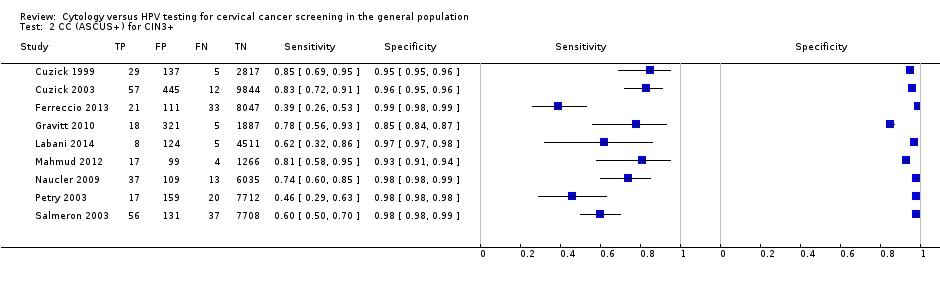
CC (ASCUS+) for CIN3+.
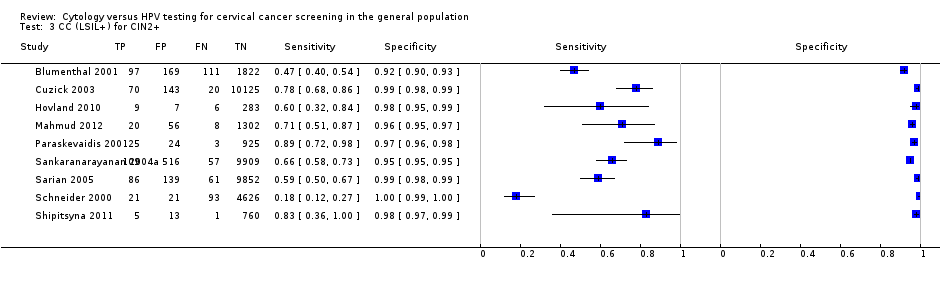
CC (LSIL+) for CIN2+.
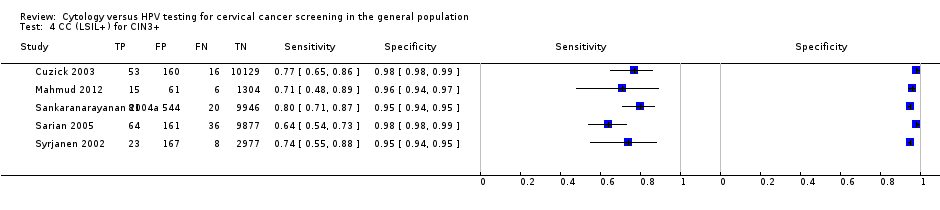
CC (LSIL+) for CIN3+.
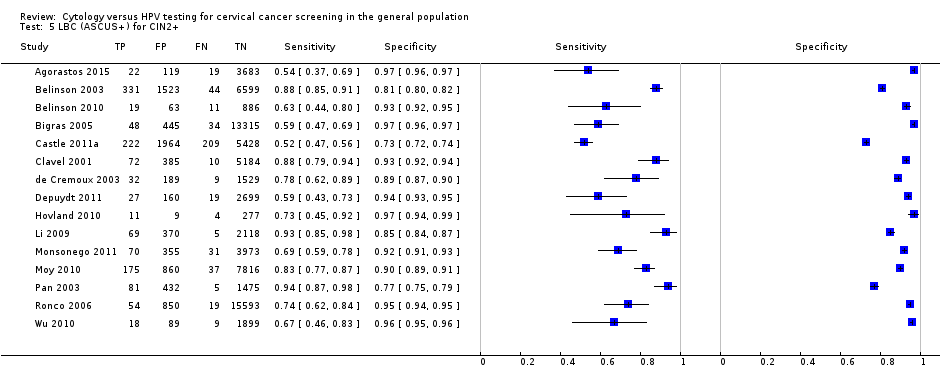
LBC (ASCUS+) for CIN2+.

LBC (ASCUS+) for CIN3+.
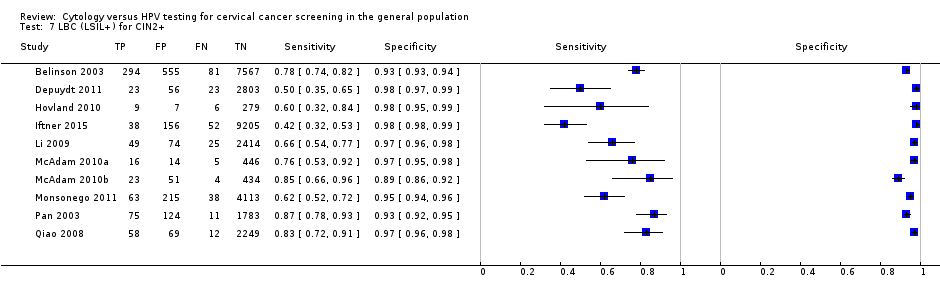
LBC (LSIL+) for CIN2+.
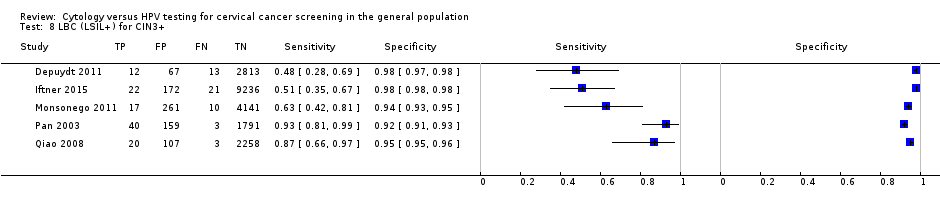
LBC (LSIL+) for CIN3+.

HC2 (1pg/mL) for CIN2+.

HC2 (1 pg/mL) for CIN3+.

HC2 (2 pg/mL) for CIN2+.

HC2 (2 pg/mL) for CIN3+.
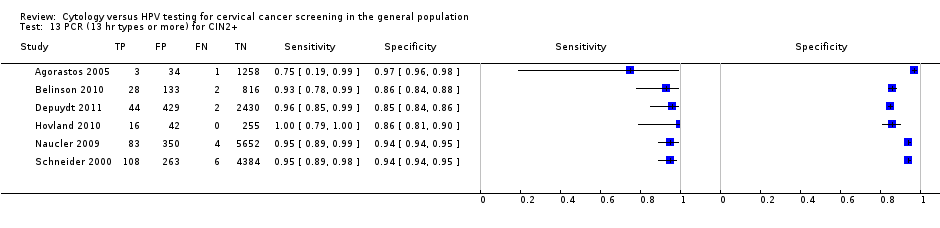
PCR (13 hr types or more) for CIN2+.
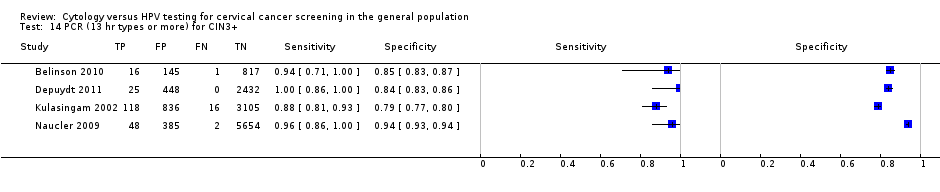
PCR (13 hr types or more) for CIN3+.

PCR (10‐11 hr types) for CIN2+.

PCR (10‐11 hr types) for CIN3+.

Aptima for CIN2+.
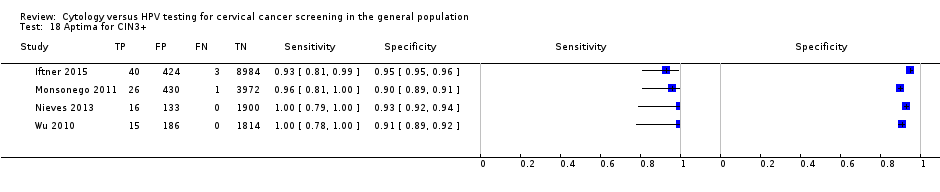
Aptima for CIN3+.

PCR (4 hr types) for CIN2+.
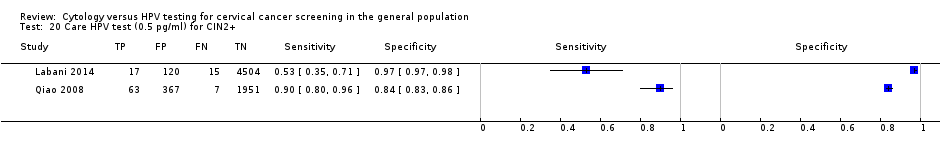
Care HPV test (0.5 pg/ml) for CIN2+.
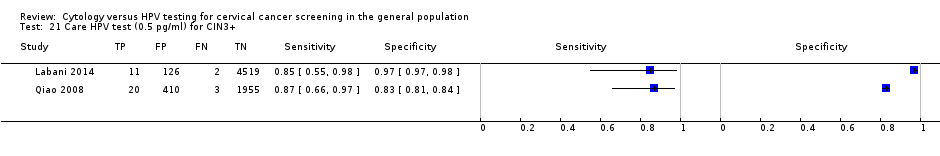
Care HPV test (0.5 pg/ml) for CIN3+.

NASBA (5 types) for CIN2+.

NASBA (9 types) for CIN2+.

HC2+4 (1 pg/ml) for CIN2+.

HC2+4 (1 pg/ml) for CIN3+.

HC2 (1pg/mL) for CIN2+ no verification bias.

CC or LBC (ASCUS+) for CIN2+ no verification bias.

HC2 (1pg/mL) for CIN2+ women >30.
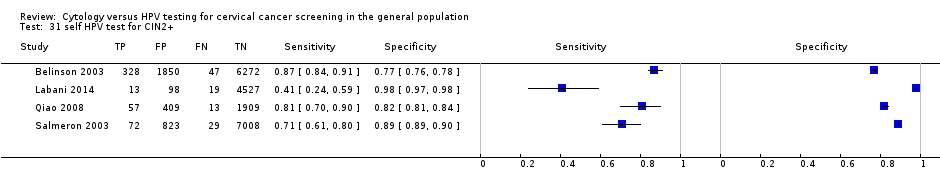
self HPV test for CIN2+.
| Human papillomavirus (HPV) compared to Papanicolaou (Pap) test for detection of cervical intraepithelial neoplasia (CIN 2+) in asymptomatic women | |||||
| Patient or population: adult asymptomatic women Settings: outpatient screening programmes New Test: HPV, HC2 test Cut‐off value: 1 pg/mL Comparison Test: Pap, liquid‐based cytology (LBC) test Cut‐off value: atypical squamous cells of undetermined significance (ASCUS) Reference Test: a colposcopy exam with or without biopsy as clinically indicated | |||||
| HPV | 138,230 women | Pooled sensitivity | 89.9% (88.6 to 91.1%) | Pooled specificity | 89.9% (89.7 to 90.0%) |
| Pap | 82,003 women | Pooled sensitivity | 72.9% (70.7 to 75%) | Pooled specificity | 90.3% (90.1 to 90.5%) |
| Test results | Number of results per 1000 women tested | Quality of the evidence | Comments | ||
| Prevalence of CIN 2+, 2%1 | |||||
| HPV | Pap | ||||
| True positives (TP) | 18 (18 to 18) | 15 (14 to 15) | ⊕⊕⊕⊝ | Women will be correctly classified and will receive further confirmatory testing or treatment | |
| TP absolute difference | 3 more | ||||
| False negatives (FN) | 2 (2 to 2) | 5 (5 to 6) | Women will be falsely reassured that they do not have CIN 2+, and the potentially beneficial treatment may be missed or will be delayed | ||
| FN absolute difference | 3 fewer | ||||
| True negatives (TN) | 881 (879 to 882) | 885 (883 to 887) | ⊕⊕⊕⊕ | Women will be correctly reassured that they do not have CIN 2+ | |
| TN absolute difference | 4 fewer | ||||
| False positives (FP) | 99 (98 to 101) | 95 (93 to 97) | Women will likely receive unnecessary further testing and possibly also unnecessary treatment; additionally further testing and unnecessary treatment may lead to adverse effects and use of resources without any health benefits | ||
| FP absolute difference | 4 more | ||||
| CI: Confidence interval; HPV human papillomavirus; Pap: Papanicolaou test, CIN: cervical intraepithelial neoplasia | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Prevalence of 2% (20 women out of 1000) was assumed to be the average prevalence of cervical intraepithelial neoplasia 2+ in non HIV asymptomatic women. | |||||
| Test | Disease threshold | studies | Pooled sensitivity (95% CI) | Pooled specificity (95% CI) |
| CC (ASCUS+) | CIN 2+ | 16 | 65.87% (54.94 to 75.33) | 96.28% (94.72 to 97.39) |
| LBC (ASCUS+) | CIN 2+ | 15 | 75.51% (66.57 to 82.68) | 91.85% (88.43 to 94.32) |
| CC (LSIL+) | CIN 2+ | 9 | 62.84% (46.79‐76.50) | 97.73% (96.09‐98.70) |
| LBC (LSIL+) | CIN 2+ | 10 | 70.33% (59.73 to 79.11) | 96.20% (94.57 to 97.36) |
| HC2 (1 pg/mL) | CIN 2+ | 25 | 92.60% (99.45 to 95.30) | 89.30% (87.03 to 91.20) |
| PCR (> 12 types) | CIN 2+ | 6 | 95.13% (89.50 to 97.84) | 91.89% (83.79 to 96.13) |
| APTIMA | CIN 2+ | 3 | 92.66% (31.77 to 99.71) | 93.31% (47.30 to 99.54) |
| CC (ASCUS+) | CIN 3+ | 9 | 70.27% (57.87 to 80.30) | 96.67% (94.56 to 98.00) |
| LBC (ASCUS+) | CIN 3+ | 13 | 75.97% (64.72 to 84.49) | 91.19% (87.21 to 94.01) |
| CC (LSIL+) | CIN 3+ | 5 | 74.43% (67.81 to 80.10) | 96.86% (94.87 to 98.10) |
| LBC (LSIL+) | CIN 3+ | 5 | 71.91% (51.68 to 86.00) | 96.05% (93.53 to 97.60) |
| HC2 (1 pg/mL) | CIN 3+ | 19 | 96.50% (94.00 to 97.90) | 89.20% (86.70 to 91.30) |
| PCR (> 12 types) | CIN 3+ | 4 | 93.57% (69.90 to 98.91) | 86.49% (68.16 to 95.04) |
| APTIMA | CIN 3+ | 4 | 96.04% (72.91 to 99.54) | 92.80% (86.15 to 96.39) |
| Tests with fewer than three studies are not included in the table. | ||||
| Comparison | Disease threshold | Relative sensitivity (95% CI) | Relative specificity (95% CI) | Studies | Analysis number |
| HC2 vs CC (ASCUS+) | CIN 2+ | 1.52 (1.24 to 1.86) | 0.94 (0.92 to 0.96) | 9 | 1 |
| HC2 vs CC (ASCUS+) | CIN 3+ | 1.46 (1.12 to 1.91) | 0.95 (0.93 to 0.9) | 6 | 2 |
| PCR (> 12 types) vs CC (ASCUS+) | CIN 2+ | 1.37 (0.58 to 3.21) | 0.95 (0.76 to 1.19) | 3 | 5 |
| HC2 vs CC (LSIL+) | CIN 2+ | 1.28 (1.15 to 1.41) | 0.91 (0.87 to 0.95) | 6 | 7 |
| HC2 vs CC (LSIL+) | CIN 3+ | 1.22 (1.12 to 1.32) | 0.91 (0.87 to 0.95) | 5 | 8 |
| HC2 vs LBC (ASCUS+) | CIN 2+ | 1.18 (1.10 to 1.26) | 0.96 (0.95 to 0.97) | 10 | 11 |
| HC2 vs LBC (ASCUS+) | CIN 3+ | 1.17 (1.05 to 1.30) | 0.96 (0.95 to 0.98) | 8 | 12 |
| PCR (> 12 types) vs LBC (ASCUS+) | CIN 2+ | 1.53 (0.53 to 4.44) | 0.90 (0.89 to 0.92) | 3 | 15 |
| PCR (> 12 types) vs LBC (ASCUS+) | CIN 3+ | 1.47 (0.64 to 3.35) | 0.94 (0.8 to 1.09) | 3 | 16 |
| HC2 vs LBC (LSIL+) | CIN 2+ | 1.35 (1.19 to 1.53) | 0.92 (0.89 to 0.95) | 8 | 17 |
| HC2 vs LBC (LSIL+) | CIN 3+ | 1.30 (0.86 to 1.96) | 0.92 (0.8 to 1.00) | 4 | 18 |
| APTIMA vs LBC (ASCUS+) | CIN 3+ | 1.30 (0.49 to 3.41) | 0.98 (0.93 to 1.04) | 3 | 22 |
| Comparisons with fewer than three studies are not included in the table | |||||
| Comparison | Studies | Disease threshold | Relative sensitivity (95% CI) | Relative specificity (95% CI) |
| Age > 30 vs any age | 17 vs 20 | CIN 2+ | 1.13 (1.03 to 1.25) | 1.01 (0.98 to 1.04) |
| 13 vs 14 | CIN 3+ | 1.10 (1.02 to 1.19) | 1.04 (1.00 to 1.08) | |
| Increased vs low risk of verification bias | 17 vs 20 | CIN 2+ | 1.05 (0.95 to 1.16) | 1.00 (0.97 to 1.04) |
| 12 vs 15 | CIN 3+ | 1.09 (1.01 to 1.18) | 1.00 (0.96 to 1.05) | |
| High‐income vs middle‐/low‐income countries | 21 vs 16 | CIN 2+ | 1.01 (0.91 to 1.12) | 1.03 (1.00 to 1.07) |
| 13 vs 14 | CIN 3+ | 0.94 (0.87 to 1.02) | 1.01 (0.96 to 1.05) | |
| Assessed by bivariate random‐effects meta‐analysis including one covariate each time. | ||||
| Test | No. of studies | No. of participants |
| 1 CC (ASCUS+) for CIN2+ Show forest plot | 16 | 61099 |
| 2 CC (ASCUS+) for CIN3+ Show forest plot | 9 | 51857 |
| 3 CC (LSIL+) for CIN2+ Show forest plot | 9 | 41494 |
| 4 CC (LSIL+) for CIN3+ Show forest plot | 5 | 35648 |
| 5 LBC (ASCUS+) for CIN2+ Show forest plot | 15 | 82003 |
| 6 LBC (ASCUS+) for CIN3+ Show forest plot | 13 | 71919 |
| 7 LBC (LSIL+) for CIN2+ Show forest plot | 10 | 33519 |
| 8 LBC (LSIL+) for CIN3+ Show forest plot | 5 | 21166 |
| 9 HC2 (1pg/mL) for CIN2+ Show forest plot | 25 | 138230 |
| 10 HC2 (1 pg/mL) for CIN3+ Show forest plot | 19 | 120380 |
| 11 HC2 (2 pg/mL) for CIN2+ Show forest plot | 2 | 26768 |
| 12 HC2 (2 pg/mL) for CIN3+ Show forest plot | 2 | 26768 |
| 13 PCR (13 hr types or more) for CIN2+ Show forest plot | 6 | 16343 |
| 14 PCR (13 hr types or more) for CIN3+ Show forest plot | 4 | 14048 |
| 15 PCR (10‐11 hr types) for CIN2+ Show forest plot | 2 | 3965 |
| 16 PCR (10‐11 hr types) for CIN3+ Show forest plot | 1 | 2988 |
| 17 Aptima for CIN2+ Show forest plot | 3 | 15895 |
| 18 Aptima for CIN3+ Show forest plot | 4 | 17944 |
| 19 PCR (4 hr types) for CIN2+ Show forest plot | 1 | 1985 |
| 20 Care HPV test (0.5 pg/ml) for CIN2+ Show forest plot | 2 | 7044 |
| 21 Care HPV test (0.5 pg/ml) for CIN3+ Show forest plot | 2 | 7046 |
| 22 Cobas for CIN2+ Show forest plot | 2 | 11666 |
| 23 Cobas for CIN3+ Show forest plot | 2 | 11666 |
| 24 NASBA (5 types) for CIN2+ Show forest plot | 1 | 313 |
| 25 NASBA (9 types) for CIN2+ Show forest plot | 1 | 313 |
| 26 HC2+4 (1 pg/ml) for CIN2+ Show forest plot | 1 | 1352 |
| 27 HC2+4 (1 pg/ml) for CIN3+ Show forest plot | 1 | 1352 |
| 28 HC2 (1pg/mL) for CIN2+ no verification bias Show forest plot | 12 | 53013 |
| 29 CC or LBC (ASCUS+) for CIN2+ no verification bias Show forest plot | 8 | 31341 |
| 30 HC2 (1pg/mL) for CIN2+ women >30 Show forest plot | 13 | 69334 |
| 31 self HPV test for CIN2+ Show forest plot | 4 | 23474 |



