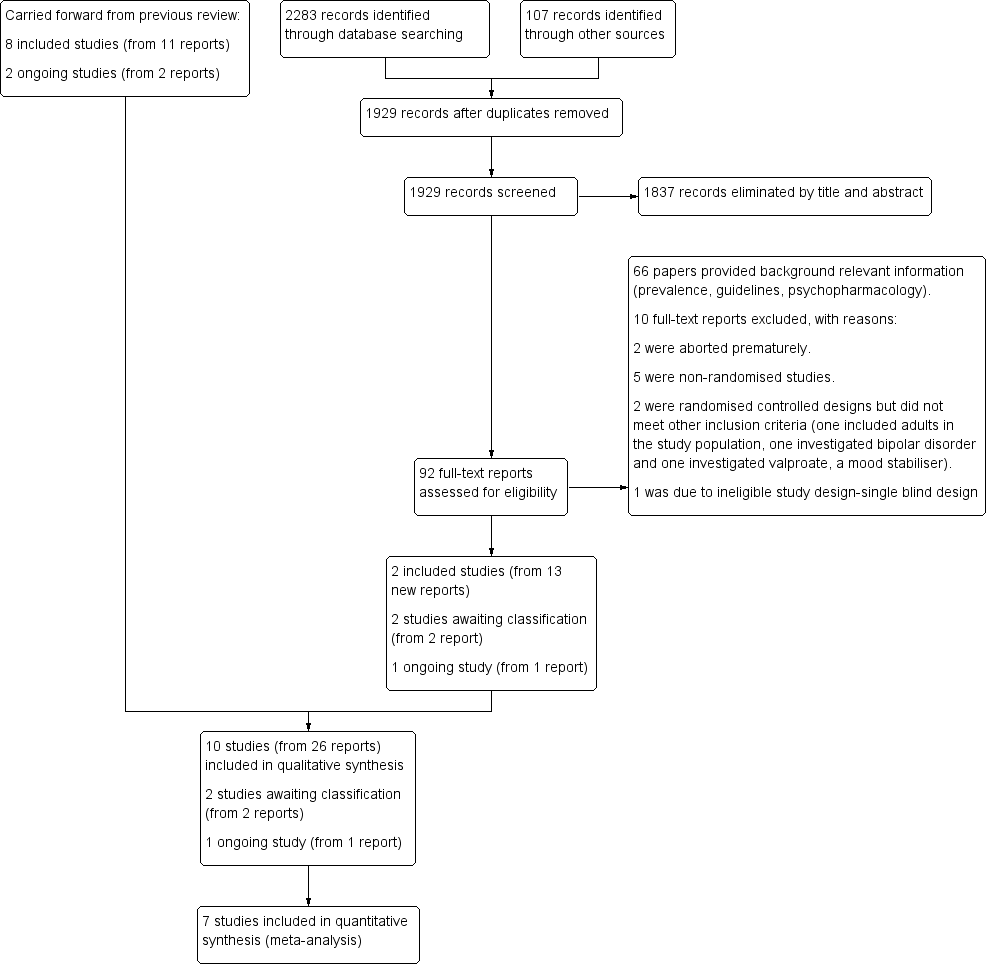
| Study ID | General | Neurological | Gastrointestinal | Respiratory | Cardiovascular/Metabolic | Serious adverse event (unspecified) | Other |
| Armenteros 2007 (risperidone = 12, placebo = 13) | -
Sedation (risperidone = 1, placebo = 2) | -
Agitation (risperidone = 1, placebo = 0) | -
Abdominal pain (risperidone = 3, placebo = 1) -
Vomiting (risperidone = 2, placebo = 3) -
Increased appetite (risperidone = 1, placebo = 0) | ‐ | Not reported | ‐ | ‐ |
| Buitelaar 2001 (risperidone = 19, placebo = 19) | -
Sedation (risperidone = 2, placebo = 0) -
Headache (risperidone = 4, placebo = 2) -
Dizziness (risperidone = 2, placebo = 1) -
Decreased energy/fatigue (risperidone = 2, placebo = 0) -
Tiredness (risperidone = 2, placebo = 5) | -
Akathisia/restless leg syndrome (risperidone = 3, placebo = 5) -
Tremor (risperidone = 4, placebo = 2) -
Muscle stiffness (risperidone = 3, placebo = 2) -
Difficulty swallowing (risperidone = 4, placebo = 0) -
Tardive dyskinesia (risperidone = 0, placebo = 1) | -
Nausea (risperidone = 3, placebo = 0) -
Sialorrhoea (risperidone = 4, placebo = 0) | -
Rhinitis/rhinorrhoea (risperidone = 11, placebo = 1) | Not reported | ‐ | ‐ |
| Connor 2008 (quetiapine = 9, placebo = 10) | -
Sedation (quetiapine = 6, placebo = 9) -
Decreased energy/fatigue (quetiapine = 3, placebo = 5) | -
Akathisia/restless leg syndrome (quetiapine = 1, placebo = 0) -
Agitation (quetiapine = 6, placebo = 9 -
Muscle stiffness (quetiapine = 1, placebo = 2) -
Decreased facial expression (quetiapine = 1, placebo = 6) | ‐ | ‐ | No differences across groups found on ECG QRS or QTc intervals. | ‐ | ‐ |
| Findling 2000 (risperidone = 10, placebo = 10) | -
Sedation (risperidone = 3, placebo = 2) -
Headache (risperidone = 3, placebo = 2) | ‐ | -
Nausea (risperidone = 1, placebo = 1) -
Increased appetite (risperidone = 3, placebo = 0) | ‐ | No clinically significant changes in ECG. | ‐ | -
Enuresis/urinary incontinence (risperidone = 0, placebo = 1) -
Restlessness (risperidone = 0, placebo = 1) -
Irritability (risperidone = 0, placebo = 1) -
Sleeping problems (risperidone = 0, placebo = 1) |
| Van Bellinghen 2001 (risperidone = 6, placebo = 7) | No side effects reported in any category. | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Aman 2002 (risperidone = 55, placebo = 63) | -
Sedation (risperidone = 28, placebo = 6) -
Headache (risperidone = 16, placebo = 9) | -
Hyperprolactinaemia (risperidone = 7, placebo = 1) -
EPSE (unspecified; risperidone = 2, placebo = 0) | -
Abdominal pain/dyspepsia (risperidone = 3, placebo = 1) -
Vomiting (risperidone = 2, placebo = 3) -
Increased appetite (risperidone = 1, placebo = 0) | -
Rhinitis/rhinorrhoea (risperidone = 6, placebo = 3 | -
No QTc abnormalities. | ‐ | ‐ |
| Reyes 2006a (risperidone = 172, placebo = 163) | -
Sedation (risperidone = 3, placebo = 2) -
Headache (risperidone = 8, placebo = 11) -
Decreased energy/fatigue (risperidone = 3, placebo = 0) | -
Hyperprolactinaemia (risperidone = 5, placebo = 0) -
EPSE (unspecified; risperidone = 3, placebo = 1) | -
Abdominal pain/dyspepsia (risperidone = 6, placebo = 3) -
Increased appetite (risperidone = 4, placebo = 0) | -
Pharyngitis (risperidone = 10, placebo = 4 -
URTI (risperidone = 13, placebo = 9) | -
No significant changes in QTc intervals. | -
Serious adverse event (unspecified; risperidone = 6, placebo = 5) | ‐ |
| Snyder 2002 (risperidone = 53, placebo = 57) | -
Sedation (risperidone = 22, placebo = 8) -
Headache (risperidone = 9, placebo = 4) -
Decreased energy/fatigue (risperidone = 4, placebo = 0) | -
Hyperprolactinaemia (risperidone = 4, placebo = 0) -
EPSE (unspecified; risperidone = 7, placebo = 3) -
Tardive dyskinesia (risperidone = 0, placebo = 1) | -
Abdominal pain/dyspepsia (risperidone = 8, placebo = 4) -
Vomiting (risperidone = 6, placebo = 4) -
Increased appetite (risperidone = 8, placebo = 2) -
Anorexia (risperidone = 4, placebo = 2) -
Sialorrhoea (risperidone = 6, placebo = 1) | -
Pharyngitis (risperidone = 5, placebo = 3) -
Nose bleeds (risperidone = 5, placebo = 0) -
Rhinitis/rhinorrhoea (risperidone = 7, placebo = 5) | -
No abnormal QTc intervals. | -
Adverse events (unspecified; risperidone = 5, placebo = 10) | -
Rash (risperidone = 4, placebo = 1) -
Abnormal crying (risperidone = 4, placebo = 0) -
Enuresis/urinary incontinence (risperidone = 7, placebo = 3) |
| TOSCA study (risperidone = 73, placebo = 80) | -
Sedation (risperidone = 16, placebo = 20) -
Headache (risperidone = 16, placebo =17) | -
Hyperprolactinaemia (risperidone = 2, placebo = 0) | -
Abdominal pain/dyspepsia (risperidone = 12, placebo = 4) -
Vomiting (risperidone = 10, placebo = 6) -
Increased appetite (risperidone = 10, placebo = 7) -
Anorexia (risperidone = 9, placebo = 19) -
Diarrhoea (risperidone = 5, placebo = 9) | -
Cough (risperidone = 14, placebo = 20) -
Rhinitis/rhinorrhoea (risperidone = 11, placebo = 14) | -
Hyperlipidaemia (risperidone = 2, placebo = 0) -
Elevated fasting glucose and insulin (risperidone = 0, placebo = 2) | ‐ | -
Sleeping problems (risperidone = 14, placebo = 29) |
| Fleischhaker 2011 (ziprasidone = 25, placebo = 25) | -
Headache (ziprasidone = 8, placebo = 10) -
Decreased energy/fatigue (ziprasidone = 12, placebo = 7) | -
Hyperprolactineamia (ziprasidone = 3, placebo = 1) -
Hypopolactinaemia (ziprasidone = 1, placebo = 3) -
Akathisa/restless leg syndrome (ziprasidone = 5, placebo = 2) -
EPSE (unspecified; ziprasidone = 3, placebo = 1) -
Tremor (ziprasidone = 11, placebo = 8) -
Muscle stiffness (ziprasidone = 5, placebo = 1) | -
Dyspepsia/abdominal pain (ziprasidone = 5, placebo = 4) -
Vomiting (ziprasidone = 7, placebo = 2) -
Nausea (ziprasidone = 1, placebo = 4) -
Increased appetite (ziprasidone = 3, placebo = 1) -
Anorexia (ziprasidone = 3, placebo = 2) -
Diarrhoea (ziprasidone = 5, placebo = 3) | -
Pharyngitis (ziprasidone = 12, placebo = 10) -
Cough (ziprasidone = 9, placebo = 11) -
Rhinitis/rhinorrhoea (ziprasidone = 3, placebo = 0) | -
No increases in QTc levels were observed in either group. | -
Adverse events (unspecified; ziprasidone = 3, placebo = 2) | -
Fever (ziprasidone = 5, placebo = 3) -
Oropharyngeal pain (ziprasidone = 3, placebo = 0) -
Excessive blinking (ziprasidone = 2, placebo = 3) -
Aggression (ziprasidone = 3, placebo = 7) |