Immunoglobulins secara intravena untuk epilepsi
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized, add‐on, double‐blind, placebo‐controlled, multi‐centre trial | |
| Participants | Setting: multi‐centre (Centre Neurologique William Lennox, Ottignies, Belgium; Department of Neurology, University Hospital Zurich, Zurich, Switzerland; Epilepsy Center, Kehl‐Kork, Germany) Number of participants enrolled: 61 (43 in IVIg group, 18 in placebo group) Sex: 42 male (31 in IVIg group, 11 in placebo group), 19 female (12 in IVIg group, 7 in placebo group) Mean age (years): IVIg group (24.4 to 26.2), placebo group (18.5) Mean duration of epilepsy (years): IVIg group (15.33 to 19.44), placebo group (12.54) (data from 58 participants who were included in the seizure frequency analysis) Race: Caucasian Types of participants: refractory epilepsy Inclusion criteria: patients suffering from West syndrome, Lennox‐Gastaut syndrome or an early myoclonic encephalopathy were enrolled as soon as the diagnosis had been made. Patients with any kind of intractable epilepsy were allowed to enter the trial, provided the diagnosis had been made at least 1 year previously and that the seizure frequency was at least 1 per week during the previous 6 months before admission. Exclusion criteria: patients presenting with any kind of neoplasia, any progressive and/or expansive cerebral disorders (except for Rasmussen syndrome), symptoms of severe renal insufficiency (serum creatinine > 3 mg/100 ml), a known intolerance to blood products, a seropositivity to HIV1 and 2 or a known chromosomal abnormality. Involvement in another clinical trial during the course of the study or the previous 6 months was also excluded. | |
| Interventions | Comparison: IVIg as add‐on treatment to classic AEDs versus add‐on placebo 1. IVIg (BI61011, Behringwerke, Germany): 100 mg/kg, 250 mg/kg, 400 mg/kg 2. Placebo consisted of 2% human albumin solution Duration of treatment period: 6 weeks | |
| Outcomes | 1. 50% or greater reduction in seizure frequency 2. Incidence or severity of adverse effects 3. Global assessment | |
| Notes | Participants with any type of refractory partial or generalized epilepsy were included in the trial IVIg and placebo groups were not comparable at baseline, including age and median number of daily seizures All the withdrawals were from IVIg‐treated groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A multi‐centre, randomized trial |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinding. Investigators were blinded regarding IVIg versus placebo. External outcome assessors (neurologists) were totally blinded |
| Incomplete outcome data (attrition bias) | Low risk | 3 patients were excluded from the evaluation of seizure frequency: 1 with unaccountable seizures after baseline (100 mg/kg), 1 with continuous partial motor seizures at the beginning and rarely after the IVIg treatment (250 mg/kg) and 1 patient who dropped out (100 mg/kg). The 1 patient who dropped out (100 mg/kg) was also excluded from the global assessment. Conclusions remained unchanged for the sensitivity analysis of missing data. |
| Selective reporting (reporting bias) | Low risk | The main outcome measure for this trial was the reduction of seizure frequencies, and secondary outcome measure was the type and severity of seizures, the interictal status and neurophysiological parameters. 'Global assessment' was used to evaluate several clinical outcomes listed above. |
| Other bias | Unclear risk | IVIg and placebo groups were not comparable in terms of some baseline data including the mean duration of epilepsy and median number of daily seizures. For patients in the IVIg group, mean duration of epilepsy seemed to be longer than comparison, while median number of daily seizures were less. |
AED: antiepileptic drugs
IVIg: intravenous immunoglobulin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| A historical controlled trial; results of the trial were compared with those reported in the literature and with data from a retrospective survey of their earlier patients | |
| 16 patients with Rasmussen encephalitis (RE) were randomized to tacrolimus or IVIg. However, 4 patients were diagnosed with "no epilepsy" at baseline | |
| A self‐controlled trial; each patient served as his or her own control before or after treatment | |
| A retrospective, multi‐centre study comprised of patients treated with immunoglobulins for either epileptic encephalopathy or refractory epilepsy | |
| A self‐controlled trial; each patient served as his or her own control before or after treatment | |
| A self‐controlled trial; each patient served as his or her own control before or after treatment | |
| A self‐controlled trial; each patient served as his or her own control before or after treatment | |
| Patients were randomized to receive IVlG or 'best available therapy' for a period of 12 weeks. Patients who did not respond to BAT crossed over to receive IVIG. However, data from the totally randomized phase were not available. | |
| A self‐controlled trial; each patient served as his or her own control before or after treatment |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | IVIG in Patients With VGKC Ab Associated Autoimmune Epilepsy |
| Methods | Randomized, crossover, double blind (subject, caregiver, investigator, outcomes assessor) study |
| Participants | Cases of autoimmune epilepsy with ≥ 2 seizures per week (mean of total over 1 week) and duration of epilepsy <3 years |
| Interventions | IVIG (0.5g/kg not exceeding 80 gr) for 1 day, then IVIG (1g/kg not exceeding 80 gr) for 1 day, then once every 2 weeks for 4 weeks (0.6g/kg IVIG) with placebo crossover |
| Outcomes | Decrease in seizure frequency |
| Starting date | February 2016 |
| Contact information | Jessica Sagen, Mayo Clinic, United States, [email protected] |
| Notes | This study is currently recruiting participants (verified February 2016) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% or greater reduction in seizure frequency for refractory epilepsy, ITT analysis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.79, 3.93] |
| Analysis 1.1 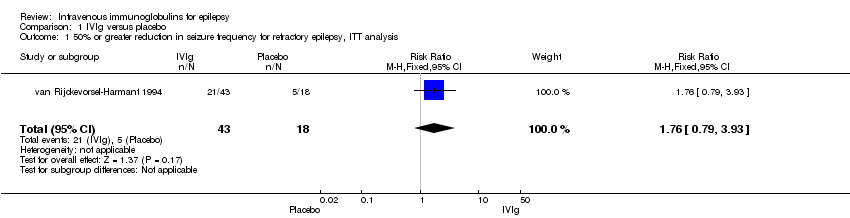 Comparison 1 IVIg versus placebo, Outcome 1 50% or greater reduction in seizure frequency for refractory epilepsy, ITT analysis. | ||||
| 2 50% or greater reduction in seizure frequency for refractory epilepsy, per‐protocol Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.85, 4.21] |
| Analysis 1.2  Comparison 1 IVIg versus placebo, Outcome 2 50% or greater reduction in seizure frequency for refractory epilepsy, per‐protocol. | ||||
| 3 50% or greater reduction in seizure frequency for refractory epilepsy, best‐case Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.91, 4.43] |
| Analysis 1.3  Comparison 1 IVIg versus placebo, Outcome 3 50% or greater reduction in seizure frequency for refractory epilepsy, best‐case. | ||||
| 4 50% or greater reduction in seizure frequency for refractory partial epilepsy, ITT analysis Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.84, 11.34] |
| Analysis 1.4  Comparison 1 IVIg versus placebo, Outcome 4 50% or greater reduction in seizure frequency for refractory partial epilepsy, ITT analysis. | ||||
| 5 50% or greater reduction in seizure frequency for refractory partial epilepsy, per‐protocol Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.91, 12.30] |
| Analysis 1.5  Comparison 1 IVIg versus placebo, Outcome 5 50% or greater reduction in seizure frequency for refractory partial epilepsy, per‐protocol. | ||||
| 6 50% or greater reduction in seizure frequency for refractory partial epilepsy, best‐case Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.98, 13.00] |
| Analysis 1.6  Comparison 1 IVIg versus placebo, Outcome 6 50% or greater reduction in seizure frequency for refractory partial epilepsy, best‐case. | ||||
| 7 Global assessment for refractory epilepsy, ITT analysis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [1.10, 9.36] |
| Analysis 1.7  Comparison 1 IVIg versus placebo, Outcome 7 Global assessment for refractory epilepsy, ITT analysis. | ||||
| 8 Global assessment for refractory epilepsy, per‐protocol Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.13, 9.57] |
| Analysis 1.8  Comparison 1 IVIg versus placebo, Outcome 8 Global assessment for refractory epilepsy, per‐protocol. | ||||
| 9 Global assessment for refractory epilepsy, best‐case Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [1.15, 9.73] |
| Analysis 1.9  Comparison 1 IVIg versus placebo, Outcome 9 Global assessment for refractory epilepsy, best‐case. | ||||

Study flow diagram.

Comparison 1 IVIg versus placebo, Outcome 1 50% or greater reduction in seizure frequency for refractory epilepsy, ITT analysis.

Comparison 1 IVIg versus placebo, Outcome 2 50% or greater reduction in seizure frequency for refractory epilepsy, per‐protocol.

Comparison 1 IVIg versus placebo, Outcome 3 50% or greater reduction in seizure frequency for refractory epilepsy, best‐case.

Comparison 1 IVIg versus placebo, Outcome 4 50% or greater reduction in seizure frequency for refractory partial epilepsy, ITT analysis.

Comparison 1 IVIg versus placebo, Outcome 5 50% or greater reduction in seizure frequency for refractory partial epilepsy, per‐protocol.

Comparison 1 IVIg versus placebo, Outcome 6 50% or greater reduction in seizure frequency for refractory partial epilepsy, best‐case.
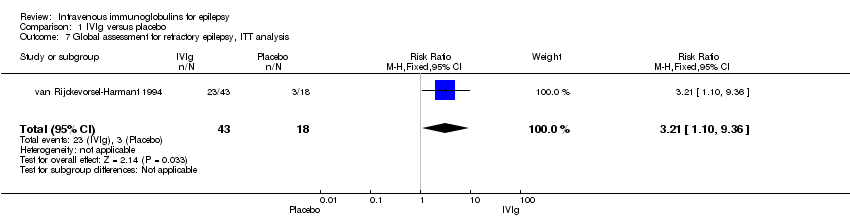
Comparison 1 IVIg versus placebo, Outcome 7 Global assessment for refractory epilepsy, ITT analysis.

Comparison 1 IVIg versus placebo, Outcome 8 Global assessment for refractory epilepsy, per‐protocol.
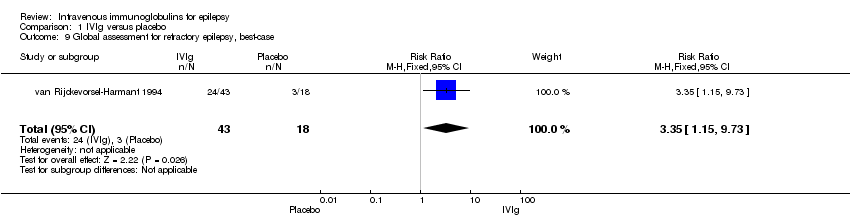
Comparison 1 IVIg versus placebo, Outcome 9 Global assessment for refractory epilepsy, best‐case.
| IVIg compared to placebo for refractory epilepsy | ||||||
| Patient or population: patients with refractory epilepsy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | IVIg | |||||
| Seizure freedom | Not reported | |||||
| Satisfactory seizure control | 278 per 1000 | 525 per 1000 | RR 1.89 | 58 | ⊕⊕⊝⊝ | |
| Incidence of adverse or harmful effects | Not reported | |||||
| Global assessment | 167 per 1000 | 548 per 1000 | RR 3.29 | 60 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unclear risk of selection bias: no information was obtained on generation of the random sequence or concealment of allocation of the randomisation. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% or greater reduction in seizure frequency for refractory epilepsy, ITT analysis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.79, 3.93] |
| 2 50% or greater reduction in seizure frequency for refractory epilepsy, per‐protocol Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.85, 4.21] |
| 3 50% or greater reduction in seizure frequency for refractory epilepsy, best‐case Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.91, 4.43] |
| 4 50% or greater reduction in seizure frequency for refractory partial epilepsy, ITT analysis Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.84, 11.34] |
| 5 50% or greater reduction in seizure frequency for refractory partial epilepsy, per‐protocol Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.91, 12.30] |
| 6 50% or greater reduction in seizure frequency for refractory partial epilepsy, best‐case Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.98, 13.00] |
| 7 Global assessment for refractory epilepsy, ITT analysis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [1.10, 9.36] |
| 8 Global assessment for refractory epilepsy, per‐protocol Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.13, 9.57] |
| 9 Global assessment for refractory epilepsy, best‐case Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [1.15, 9.73] |

