Immunoglobulins secara intravena untuk epilepsi
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 MeSH DESCRIPTOR Immunoglobulins, Intravenous Explode All
#2 intravenous immun* OR IV immunoglobulin* OR IVIG OR intravenous IG
#3 intraglobi* OR intravenous antibod* OR IV antibod*
#4 #1 OR #2 OR #3
#5 INREGISTER AND >18/05/2016:CRSCREATED
#6 #4 AND #5
Appendix 2. CENTRAL via CRSO search strategy
#1 MESH DESCRIPTOR Immunoglobulins, Intravenous EXPLODE ALL TREES
#2 (intravenous immun* OR IV immunoglobulin* OR IVIG OR intravenous IG):TI,AB,KY
#3 (intraglobi* OR intravenous antibod* OR IV antibod*):TI,AB,KY
#4 #1 OR #2 OR #3
#5 (epilep* OR seizure* OR convuls*):TI,AB,KY
#6 MESH DESCRIPTOR Epilepsy EXPLODE ALL TREES
#7 MESH DESCRIPTOR Seizures EXPLODE ALL TREES
#8 #5 OR #6 OR #7
#9 * NOT INMEDLINE AND 31/05/2016 TO 28/02/2017:DL
#10 #4 AND #8 AND #9
Appendix 3. MEDLINE (Ovid) search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials published in Lefebvre 2011.
1. exp Immunoglobulins, Intravenous/
2. (intravenous immu* or IV immunoglobulin* or IVIG or intravenous IG).tw.
3. (intraglobi* or intravenous antibod* or IV antibod*).tw.
4. 1 or 2 or 3
5. exp Epilepsy/
6. exp Seizures/
7. (epilep$ or seizure$ or convuls$).tw.
8. 5 or 6 or 7
9. exp *Pre‐Eclampsia/ or exp *Eclampsia/
10. 8 not 9
11. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
12. clinical trials as topic.sh.
13. trial.ti.
14. 11 or 12 or 13
15. exp animals/ not humans.sh.
16. 14 not 15
17. 4 and 10 and 16
18. remove duplicates from 17
19. limit 18 to ed= 20160518‐20170202
Appendix 4. Web of Science search strategy
Timespan=2016‐2017
| #5 | #3 AND #2 AND #1 |
| #4 | #3 AND #2 AND #1 |
| #3 | TOPIC: (intravenous immunoglobulin* OR IV immunoglobulin* OR IVIG OR intravenous IG OR intravenous antibod*) |
| #2 | TOPIC: (epilepsy OR seizures) |
| #1 | TOPIC: (random* or placebo* or trial or study) |
Appendix 5. Search strategies for other databases
WHO ICTRP
Advanced Search
Condition: epilepsy
Intervention: immunoglobulin OR IVIG
Date of registration between 18/05/2016 and 02/02/2017
ISRCTN registry (formerly Current Controlled Trials)
Condition: epilepsy AND Interventions: immunoglobulin or IVIG
ClinicalTrials.gov
Condition: epilepsy
Intervention: immunoglobulin or IVIG
received from 05/18/2016 to 02/02/2017
Appendix 6. Sources searched and number of hits retrieved
| Source | Date range searched | Hits retrieved |
| CENTRAL (CRSO) | Up to 2 February 2017 | 44 |
| MEDLINE (Ovid) | Up to 2 February 2017 | 160 |
| Web of Science (All databases) | Up to 2 February 2017 | 158 |
| WHO ICTRP | Up to 2 February 2017 | 2 |
| ISRCTN registry | Up to 2 February 2017 | 7 |
| ClinicalTrials.gov | Up to 2 February 2017 | 6 |

Study flow diagram.

Comparison 1 IVIg versus placebo, Outcome 1 50% or greater reduction in seizure frequency for refractory epilepsy, ITT analysis.

Comparison 1 IVIg versus placebo, Outcome 2 50% or greater reduction in seizure frequency for refractory epilepsy, per‐protocol.

Comparison 1 IVIg versus placebo, Outcome 3 50% or greater reduction in seizure frequency for refractory epilepsy, best‐case.

Comparison 1 IVIg versus placebo, Outcome 4 50% or greater reduction in seizure frequency for refractory partial epilepsy, ITT analysis.

Comparison 1 IVIg versus placebo, Outcome 5 50% or greater reduction in seizure frequency for refractory partial epilepsy, per‐protocol.

Comparison 1 IVIg versus placebo, Outcome 6 50% or greater reduction in seizure frequency for refractory partial epilepsy, best‐case.
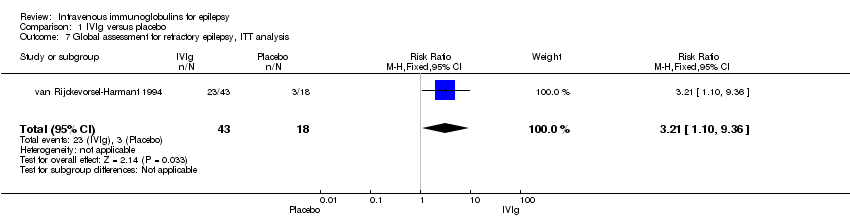
Comparison 1 IVIg versus placebo, Outcome 7 Global assessment for refractory epilepsy, ITT analysis.

Comparison 1 IVIg versus placebo, Outcome 8 Global assessment for refractory epilepsy, per‐protocol.
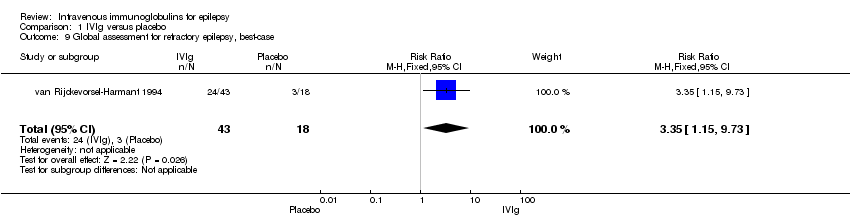
Comparison 1 IVIg versus placebo, Outcome 9 Global assessment for refractory epilepsy, best‐case.
| IVIg compared to placebo for refractory epilepsy | ||||||
| Patient or population: patients with refractory epilepsy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | IVIg | |||||
| Seizure freedom | Not reported | |||||
| Satisfactory seizure control | 278 per 1000 | 525 per 1000 | RR 1.89 | 58 | ⊕⊕⊝⊝ | |
| Incidence of adverse or harmful effects | Not reported | |||||
| Global assessment | 167 per 1000 | 548 per 1000 | RR 3.29 | 60 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unclear risk of selection bias: no information was obtained on generation of the random sequence or concealment of allocation of the randomisation. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% or greater reduction in seizure frequency for refractory epilepsy, ITT analysis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.79, 3.93] |
| 2 50% or greater reduction in seizure frequency for refractory epilepsy, per‐protocol Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.85, 4.21] |
| 3 50% or greater reduction in seizure frequency for refractory epilepsy, best‐case Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.91, 4.43] |
| 4 50% or greater reduction in seizure frequency for refractory partial epilepsy, ITT analysis Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.84, 11.34] |
| 5 50% or greater reduction in seizure frequency for refractory partial epilepsy, per‐protocol Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.91, 12.30] |
| 6 50% or greater reduction in seizure frequency for refractory partial epilepsy, best‐case Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.98, 13.00] |
| 7 Global assessment for refractory epilepsy, ITT analysis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [1.10, 9.36] |
| 8 Global assessment for refractory epilepsy, per‐protocol Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.13, 9.57] |
| 9 Global assessment for refractory epilepsy, best‐case Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [1.15, 9.73] |

