Inmunoglobulina anti‐hepatitis B durante el embarazo para la prevención de la transmisión maternoinfantil del virus de la hepatitis B
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008545.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Hepatobiliar
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
ACE and GUE wrote the background, methodology, data extraction and results section with UAE providing comments and suggestions.
YX and JL undertook the data extraction for the Chinese studies, JL inputted the data into Review Manager 5, and YX checked the data entry.
GUE and ACE performed the Trial Sequential Analysis.
ACE and GUE drafted the discussion and conclusions.
All authors signed off the final version of the review.
Sources of support
Internal sources
-
Copenhagen Trial Unit, Centre for Clinical Intervention Research, H:S Rigshospitalet, Denmark.
The trial unit helped us with the literature search. Some of the papers had to be scanned to us at some cost.
-
The Chinese Cochrane centre, China.
The Chinese papers were retrieved courtesy of the Chinese Cochrane centre
External sources
-
No external sources of support was received, Other.
Declarations of interest
ACE: no conflict of interest
GUE: no conflict of interest
UAE: no conflict of interest
YX: no conflict of interest
JL: no conflict of interest
Acknowledgements
We would like to thank the Co‐ordinating Editor of The Cochrane Hepato‐Biliary Group, Christian Gluud, for his encouragement and advice that led to the completion of this review. We also thank Dimitrinka Nikolova for her numerous corrections and words of advice.
As part of the prepublication editorial process, this review was commented on by Ronald Koretz (Contact Editor), Kurinchi Gurusamy (Deputy Co‐ordinating editor), and Janus Christian Jakobsen (Deputy Co‐ordinating editor); members of The Cochrane Hepato‐Biliary Group, and peer reviewed by two referees (Michelle Giles and Ivan Gentile) who were external to the editorial team. We thank them for their very helpful comments and suggestions.
We remain grateful to the Nigerian branch of the South African Cochrane Centre (SACC) for training us in the conduct of systematic reviews. We also remain grateful to Joy Oliver of the South African Cochrane Centre for encouraging us in the conduct of systematic reviews.
George Eleje was awarded a fellowship by the South African Cochrane Centre through a grant received from the Effective Health Care Research Consortium (www.evidence4health.org), which is funded by UK aid from the UK Government for International Development.
We want to thank especially, Jesper Brok of the Department of Neonatology and the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark and Irena Hrstic of the Division of Gastroenterology and Hepatology, University Hospital Zagreb, Zagreb, Croatia for peer reviewing the protocol for this review and sending us very constructive and helpful comments.
Finally, we thank Sarah Louise Klingenberg, Information Specialist of The Cochrane Hepato‐Biliary Group for helping us with the search strategies and the literature search.
Review:
Peer Reviewers: Emily Webb, London; Timothy Morgan, USA; Irena Hrstic, Croatia.
Contact Editors: Ronald Koretz, USA; Kurinchi S Gurusamy, UK.
Sign‐off Editors: Janus C Jakobsen and Christian Gluud, Denmark.
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of The Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 11 | Hepatitis B immunoglobulin during pregnancy for prevention of mother‐to‐child transmission of hepatitis B virus | Review | Ahizechukwu C Eke, George U Eleje, Uzoamaka A Eke, Yun Xia, Jiao Liu | |
| 2010 Jun 16 | Hepatitis B immunoglobulin during pregnancy for the prevention of mother to child transmission of hepatitis B virus | Protocol | Ahizechukwu C Eke, Uzoamaka A Eke, Eleje Uchenna | |
Differences between protocol and review
-
Three authors completed the protocol (ACE, GE, UE). Two authors (YX and JL) joined the team because many of the trials were in Chinese, and because of their expertise and interest in the topic. YX and JL extracted data from all the Chinese papers. There was rearrangement of the order of authors such that we moved GUE to second author due to his level of involvement during review development.
-
We added mortality and other serious adverse events in the mothers due to administration of HBIG and newborns with HBV‐DNA‐positive laboratory result as primary outcomes in the review stage. This was to enable the review be contemporary.
-
There was a correction in an author's name: George Uchenna Eleje's name was written as Uchenna, Eleje in the published protocol.
-
As the clinical signs of HBV infection are nearly absent in the newborn, the primary outcome: we removed "clinical signs of hepatitis B infection of the newborn" from the review. However, we added 'newborns with HBV‐DNA‐positive laboratory results' as a primary outcome as the majority of trials reported on it.
-
The third primary outcome is now 'serological signs of hepatitis B infection of the newborn'. One of the primary outcomes 'Serologic signs of hepatitis B infection of the newborn', was planned to be reported as newborns with HBsAg‐positive laboratory result; newborns with HBeAg‐positive laboratory result; newborns with HBV‐DNA‐positive laboratory result; and newborns with antibodies to hepatitis B core antigen'. This planned approach was at end of treatment (newborn with HBsAg positive laboratory result, newborn with HBeAg positive laboratory result, newborn with antibodies to hepatitis B core antigen (post hoc analyses). The 'end of treatment' is the time point of primary interest.
-
In the update of this review, we will limit the number of primary outcomes to three and the total number of outcomes to seven. Furthermore, we will remove cost‐effectiveness of treatment (methodological limitation) because it is not possible to meta‐analyse cost outcomes from different trials.
-
To keep up to date with the recommended Cochrane Hepato‐Biliary Group Domains for assessing risk of bias on the web site, we removed the following two risk of bias assessment domains, despite them being originally stated in the protocol: baseline imbalance and early stopping.
-
Assessment of significance. We have updated the choice between fixed‐ and random‐effects meta‐analysis. We used both types of analyses, but reported only the more conservative results (Jakobsen 2014).
-
We included Trial Sequential Analysis in the review in order for the review to become contemporary, in agreement with The Cochrane Hepato‐Biliary Group. This is because traditional meta‐analysis runs the risk of random errors due to sparse data and repetitive testing of accumulating data when updating reviews. Therefore, we performed Trial Sequential Analysis on the outcomes to calculate the required information size and assess the eventual breach of the cumulative Z‐curve of the relevant trial sequential monitoring boundaries for benefit, harm, or futility (Wetterslev 2008; Wetterslev 2009; Jakobsen 2014).
Notes
A protocol for this systematic review was first published in 2010, Issue 6 of the Cochrane Library with the same title. The authors, EAC, EUA, and EGU were involved with the protocol development.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Hepatitis B virus [genetics, immunology];
- DNA, Viral [blood];
- Hepatitis B [blood, *transmission];
- Hepatitis B Surface Antigens [blood];
- Hepatitis B e Antigens [blood];
- Immunization, Passive [*methods];
- Immunoglobulins [*administration & dosage];
- Infectious Disease Transmission, Vertical [prevention & control];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Adult; Female; Humans; Infant, Newborn; Pregnancy;
PICO
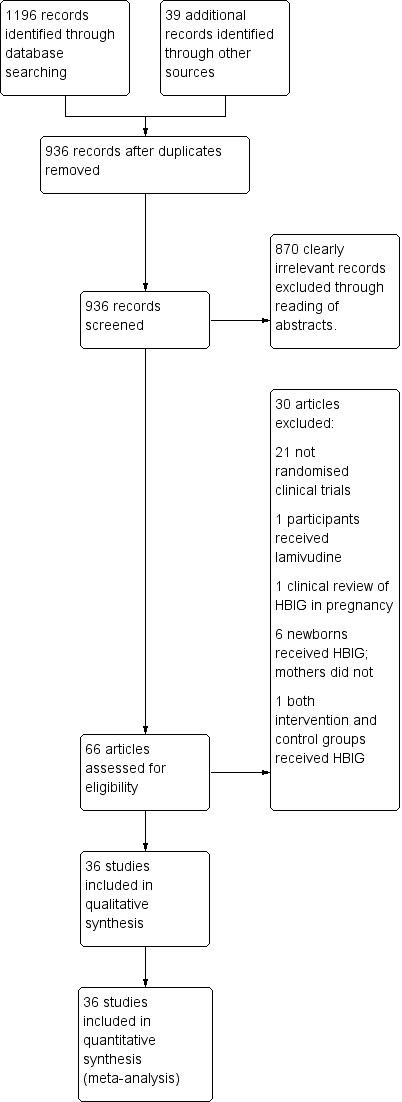
Study flow diagram for searches on hepatitis B Immunoglobulin (HBIG).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
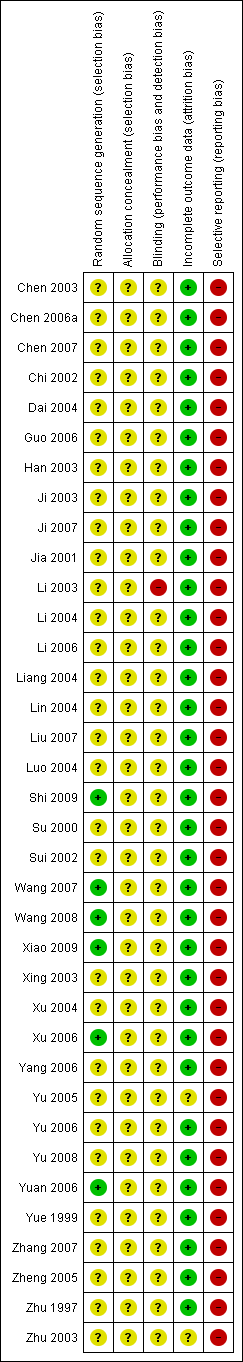
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
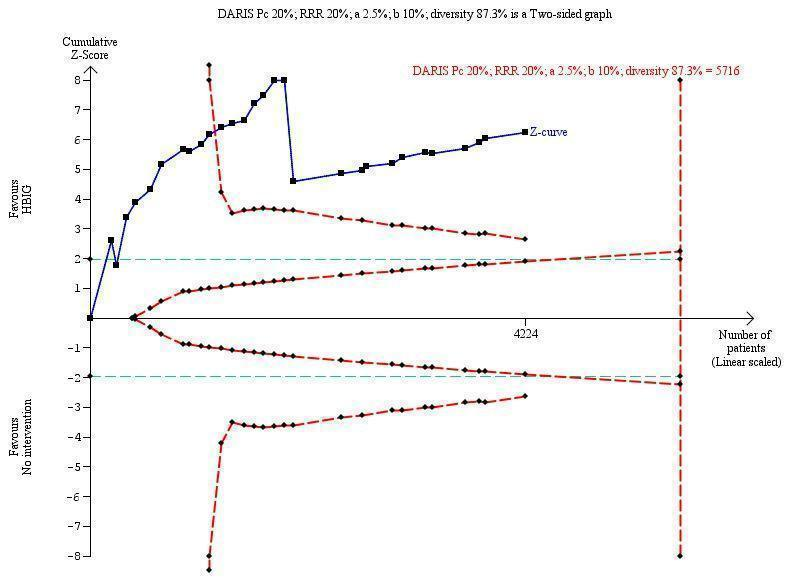
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with HBsAg‐positive results at end of follow‐up. The diversity‐adjusted required information size (DARIS) of 5716 participants was calculated based upon a proportion of 20% of babies tested positive for HBsAg in the control group, a relative risk reduction of a 20% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 87.3%. The actually accrued number of participants is 4224, which is 74% of the DARIS. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve crosses the red trial sequential monitoring boundary for benefit during the 11th trial. This implies that there is no risk of random error in the estimate of a beneficial effect of HBIG versus no intervention on the number of newborns with HBsAg‐positive results at end of follow‐up. The TSA‐adjusted confidence interval is 0.20 to 0.52.
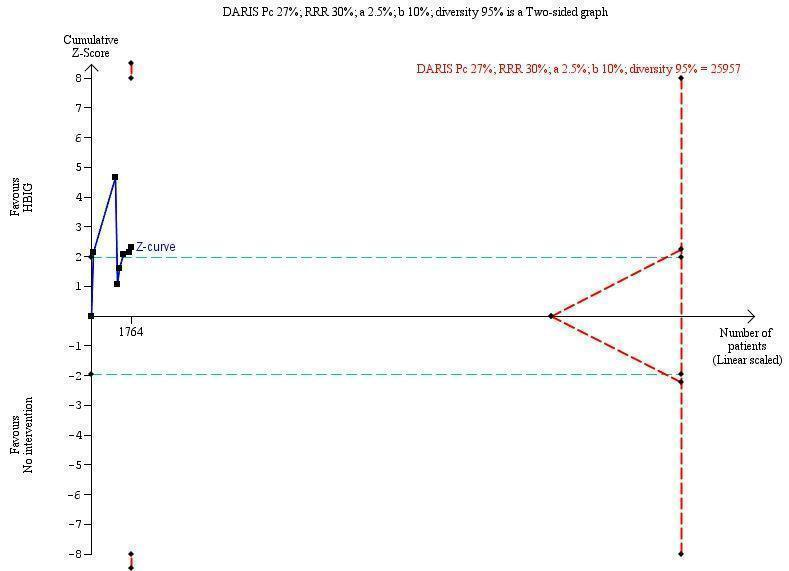
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with hepatitis B envelope antigen (HBeAg)‐positive results at end of follow‐up. The diversity‐adjusted required information size (DARIS) of 25,957 participants was calculated based upon a proportion of 27% of babies tested positive for HBeAg in the control group, a relative risk reduction of a 30% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 95%. The actually accrued number of participants is 1764, which is only 6.8% of the DARIS. (We planned to use a relative risk reduction of 20%, but this led to a DARIS of 60,715 participants and the TSA figure could not be drawn by the program; therefore, a relative risk reduction of 30% was adopted instead.) The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve does not cross the red inward sloping trial sequential monitoring boundaries for benefit or harm. Therefore, there is no evidence to support that HBIG influences number of newborns with HBeAg‐positive results at end of follow‐up. The cumulative Z‐curve does not reach the futility area, demonstrating that further trials are needed. The TSA‐adjusted confidence interval is wider than 0.04 to 6.37.
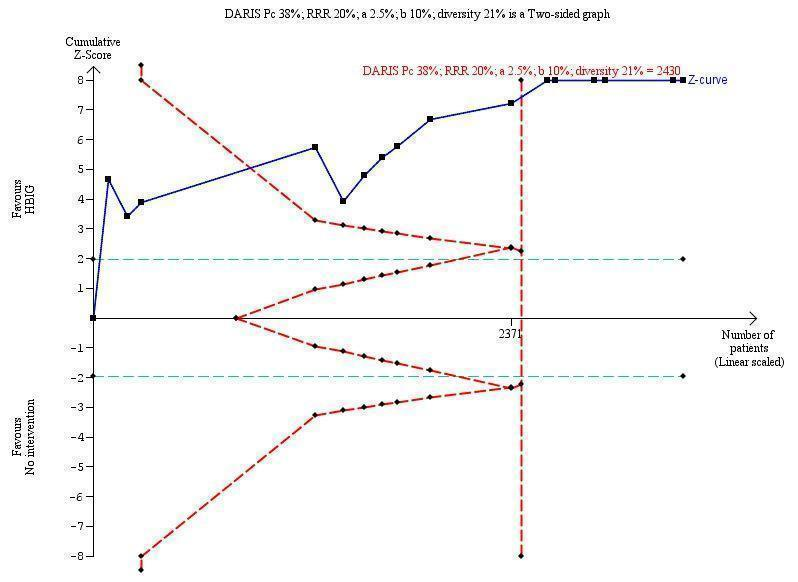
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with hepatitis B virus DNA (HBV‐DNA) positive results at end of treatment. The diversity‐adjusted required information size (DARIS) of n = 2430 participants was calculated based upon a proportion of 38% of babies tested positive for HBV‐DNA, a relative risk reduction of a 20% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 21%. The actually accrued number of participants is 2994, which is more than the DARIS of 2430 participants. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve crosses the red trial sequential monitoring boundary for benefit during the fourth trial. This implies that there is no risk of random error in the estimate of a beneficial effect of HBIG versus no intervention on the number of newborns with HBV‐DNA positive results at end of treatment. The TSA‐adjusted and 95% confidence intervals is from 0.22 to 0.37.
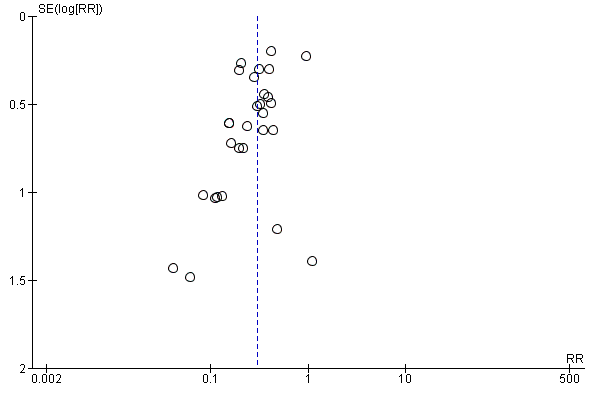
Funnel plot of comparison: 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, outcome: 1.1 Newborn positive for HBsAg.
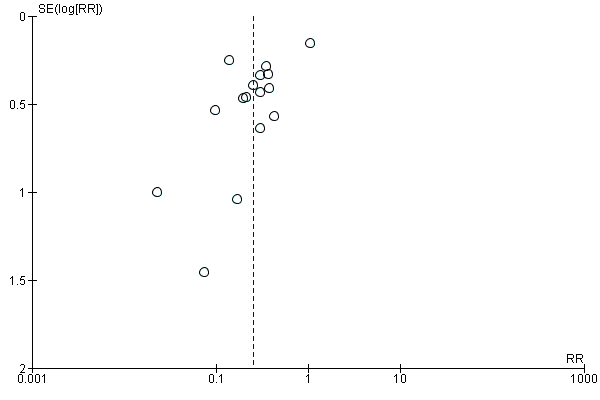
Funnel plot of comparison: 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, outcome: 1.3 Newborn positive for HBV‐DNA.

Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 1 Newborn positive for HBsAg.
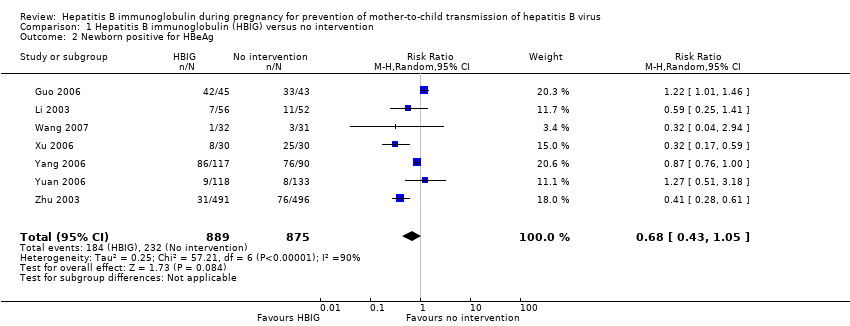
Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 2 Newborn positive for HBeAg.

Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 3 Newborn positive for HBV‐DNA.

Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 1 Newborn positive for HBsAg.
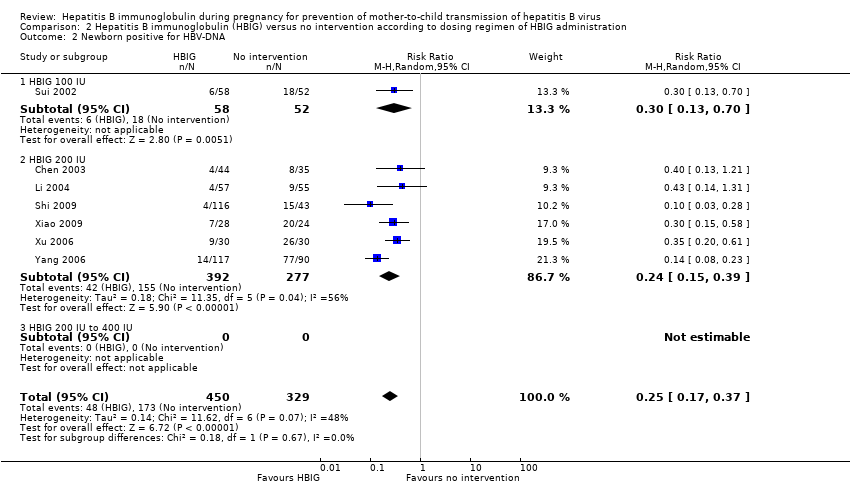
Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 2 Newborn positive for HBV‐DNA.
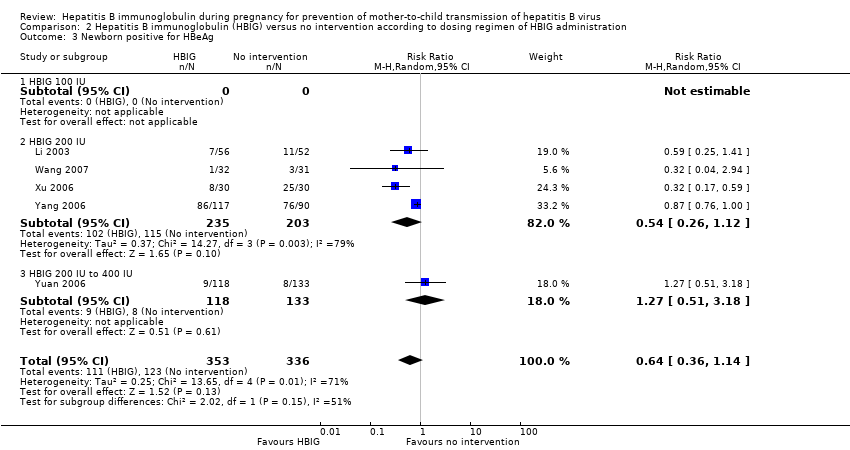
Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 3 Newborn positive for HBeAg.
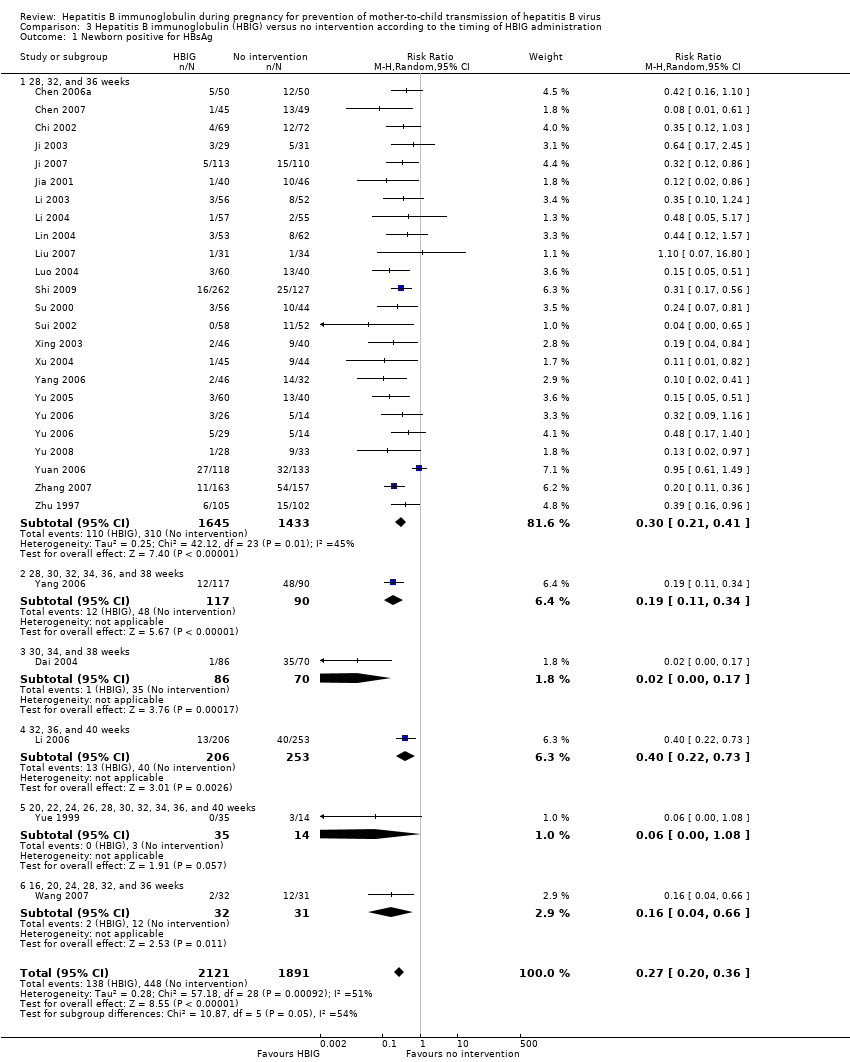
Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 1 Newborn positive for HBsAg.
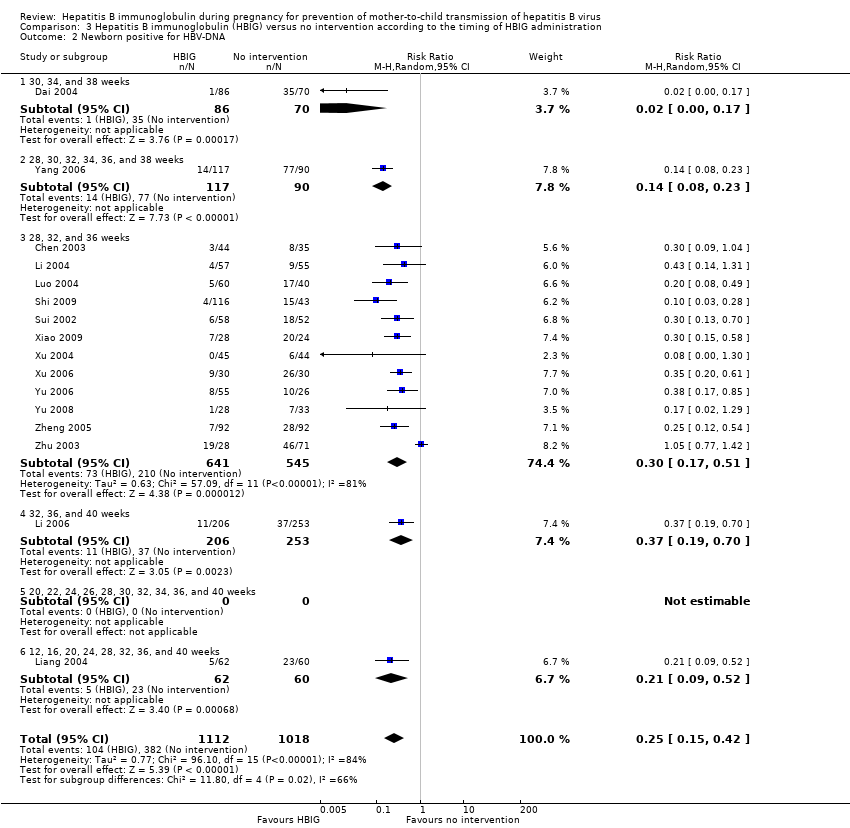
Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 2 Newborn positive for HBV‐DNA.
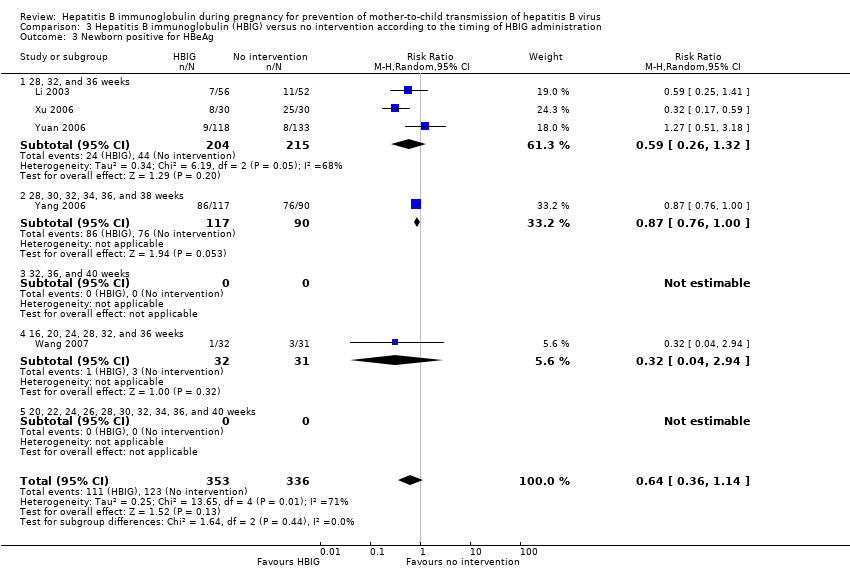
Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 3 Newborn positive for HBeAg.
| Hepatitis B immunoglobulin (HBIG) vs no intervention for prevention of mother‐to‐child transmission of hepatitis B virus | ||||||
| Participants: pregnant women positive for HBsAg or positive for HBeAg, or both. Comparison: no intervention. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | HBIG versus no intervention | |||||
| All‐cause mortality or other serous adverse events of the newborn | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| All‐cause mortality or other serous adverse events of the mothers | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Newborn with HBsAg‐positive result | Study population | RR 0.3 | 5310 | ⊕⊝⊝⊝ | ||
| 211 per 1000 | 63 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 64 per 1000 | |||||
| Newborn with HBeAg‐positive result | Study population | RR 0.68 | 1764 | ⊕⊝⊝⊝ | ||
| 265 per 1000 | 180 per 1000 | |||||
| Moderate | ||||||
| 212 per 1000 | 144 per 1000 | |||||
| Newborn with HBV‐DNA‐positive result | Study population | RR 0.25 | 2130 | ⊕⊕⊝⊝ | ||
| 375 per 1000 | 94 per 1000 | |||||
| Moderate | ||||||
| 366 per 1000 | 91 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Comment: Data for this outcome was not reported in any of the included trials. | ||||||
| Study ID | Study location | Participants | Interventions | Outcomes | Funding |
| Zhejiang | 79 participants (44 intervention, 35 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Guangdong | 100 participants (50 intervention, 50 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Shenzhen | 94 participants (45 intervention, 49 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. | |
| Zhejiang | 141 participants (69 intervention, 72 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Zhejiang | 156 participants (86 intervention, 70 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, anti‐HBs | Not stated. | |
| Henan | 88 participants (45 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg anti‐HBs. | Not stated | |
| Guangdong | 216 participants (126 intervention, 90 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Zhejiang | 60 participants (29 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs, adverse events. | Not stated | |
| Shanghai | 223 participants (113 intervention, 110 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Jiangsu | 86 participants (40 intervention, 46 control) aged 22 to 32 years. | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Guangdong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBeAg, or both. | Not stated. | |
| Guangdong | 112 participants (57 intervention, 55 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both. | Not stated. | |
| Hubei | 448 participants (202 intervention, 246 control) aged 18 to 38 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. | |
| Guangdong | 122 participants (62 intervention, 60 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA. | Not stated. | |
| Shanghai | 117 participants (55 intervention, 62 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Henan | 86 participants (43 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Jiangxi | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. | |
| Guangzhou | 389 participants (262 intervention, 127 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both | Research supported by GlaxoSmithKline Research and Development Grant NUC30914; Science and Research Foundations of Sun Yat‐Sen University and Guangzhou Science Committee, No 1999‐J‐005‐01. | |
| Henan | 98 participants (55 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Shandong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA, and anti‐HBs. | Not stated. | |
| Shandong | 63 participants (32 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg and HBeAg | Not stated. | |
| Taizhou | 279 participants (159 intervention, 120 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBV‐DNA. | Not stated. | |
| Henan | 86 participants (46 intervention, 40 control) aged 22 to 28 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Supported by Technology Research Fund Committee of Henan province (No. 981170112). | |
| Shandong | 88 participants (44 intervention, 44 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA and anti‐HBs. 8‐month‐old babies positive for HBsAg, HBV‐DNA, and anti‐HBs. Maximum duration of surveillance: 8 months. Follow‐up time point: 8 months after birth. | Not stated. | |
| Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBeAg and HBV‐DNA. | Not stated. | |
| Jiangsu | 285 participants (163 intervention, 162 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg and HBV‐DNA | Not stated. | |
| Guangdong | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBeAg, anti‐HBs | Not stated. | |
| Shanghai | 83 participants (26 intervention I, 29 intervention II, 28 control) aged 20 to 33 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Guangxi | 61 participants (28 intervention, 33 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg | Not stated. | |
| Huizhou | 250 participants (117 intervention, 113 control) | Intervention: HBIG 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg; adverse effects of the immunoglobulins to the neonates and mothers | Supported by Huizhou Municipal Central hospital and Huizhou Science and Technology Bureau. | |
| Shanxi | 48 participants (34 intervention, 14 control) aged 20 to 33 years | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. | |
| Guangdong | 320 participants (163 intervention, 157 control) aged 19 to 36 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Guangdong | 184 participants (92 intervention, 92 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Shanghai | 204 participants (103 intervention, 101 control) aged 20 to 34 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg. | Not stated. | |
| Shanghai | 980 participants (487 intervention, 493 control) aged 19 to 35 years | Intervention: HBIG 200 IU or 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, and HBV‐DNA. | Supported by a grant from the Ministry of Public Health China (No. 97030223). | |
| anti‐HBc: anti‐hepatitis core; anti‐HBe: anti‐hepatitis B envelope; anti‐HBs: anti‐hepatitis B surface; HBIG: hepatitis B immunoglobulin; HBcAg: hepatitis B core antigen; HBeAg: hepatitis B envelope antigen; HBsAg: hepatitis B surface antigen; HBV‐DNA: hepatitis B virus DNA. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 29 | 5310 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.24, 0.38] |
| 2 Newborn positive for HBeAg Show forest plot | 7 | 1764 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.05] |
| 3 Newborn positive for HBV‐DNA Show forest plot | 16 | 2130 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 28 | 4281 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.21, 0.37] |
| 1.1 HBIG 100 IU | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.36] |
| 1.2 HBIG 200 IU | 25 | 3855 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.21, 0.33] |
| 1.3 HBIG 400 IU | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.30, 1.53] |
| 2 Newborn positive for HBV‐DNA Show forest plot | 7 | 779 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.17, 0.37] |
| 2.1 HBIG 100 IU | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.70] |
| 2.2 HBIG 200 IU | 6 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.39] |
| 2.3 HBIG 200 IU to 400 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Newborn positive for HBeAg Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.14] |
| 3.1 HBIG 100 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 HBIG 200 IU | 4 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.12] |
| 3.3 HBIG 200 IU to 400 IU | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.51, 3.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 27 | 4012 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.20, 0.36] |
| 1.1 28, 32, and 36 weeks | 23 | 3078 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.21, 0.41] |
| 1.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.11, 0.34] |
| 1.3 30, 34, and 38 weeks | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.17] |
| 1.4 32, 36, and 40 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.22, 0.73] |
| 1.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.08] |
| 1.6 16, 20, 24, 28, 32, and 36 weeks | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.66] |
| 2 Newborn positive for HBV‐DNA Show forest plot | 16 | 2130 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.42] |
| 2.1 30, 34, and 38 weeks | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.17] |
| 2.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.08, 0.23] |
| 2.3 28, 32, and 36 weeks | 12 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.51] |
| 2.4 32, 36, and 40 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.19, 0.70] |
| 2.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 12, 16, 20, 24, 28, 32, 36, and 40 weeks | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.09, 0.52] |
| 3 Newborn positive for HBeAg Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.14] |
| 3.1 28, 32, and 36 weeks | 3 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.26, 1.32] |
| 3.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 3.3 32, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 16, 20, 24, 28, 32, and 36 weeks | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.94] |
| 3.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

