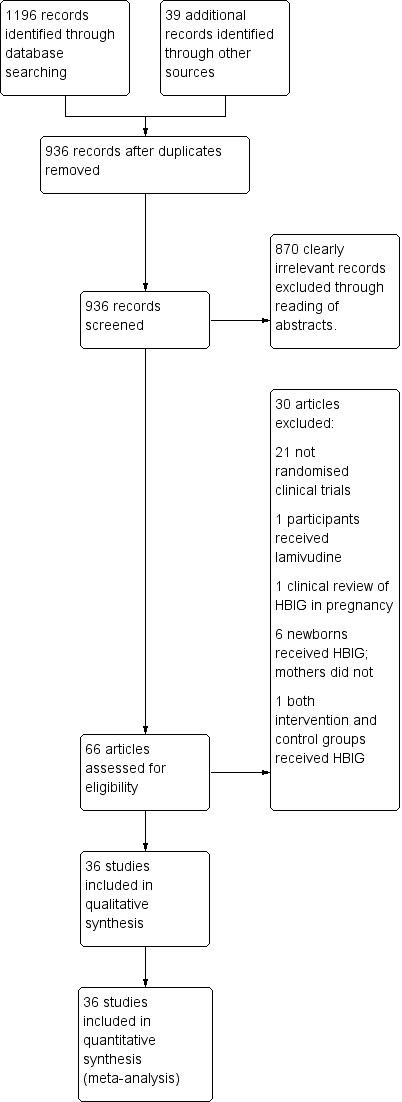
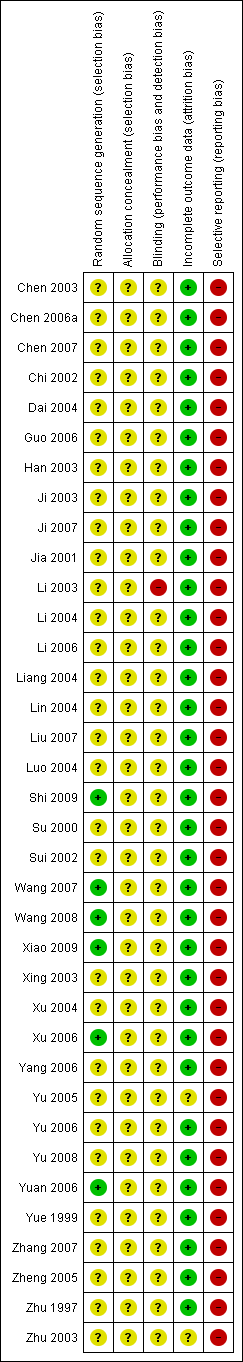
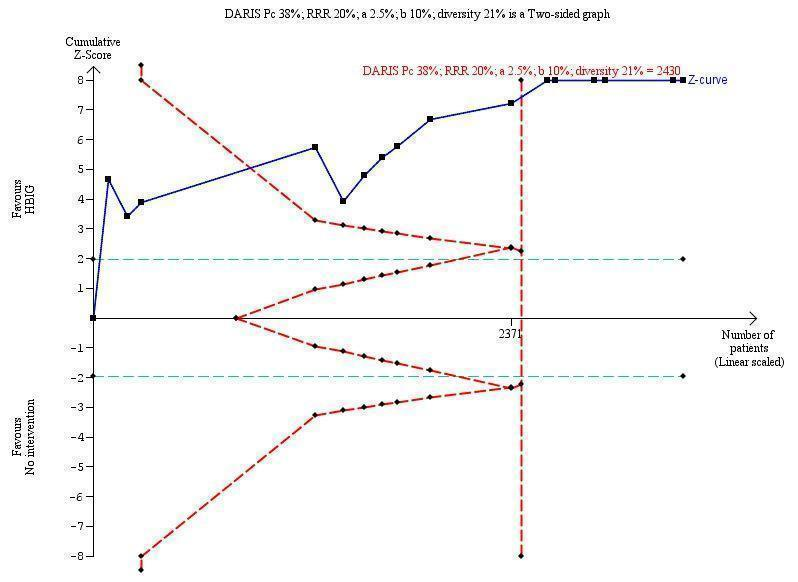
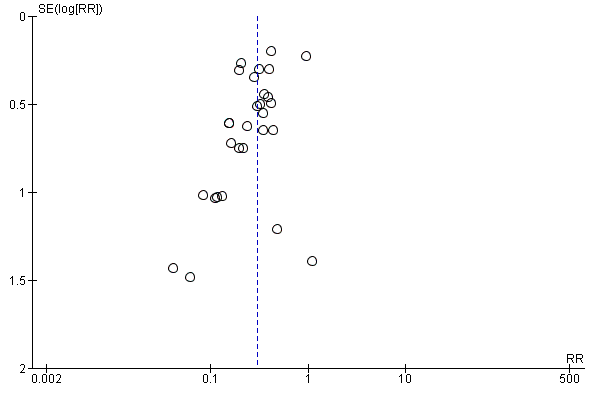
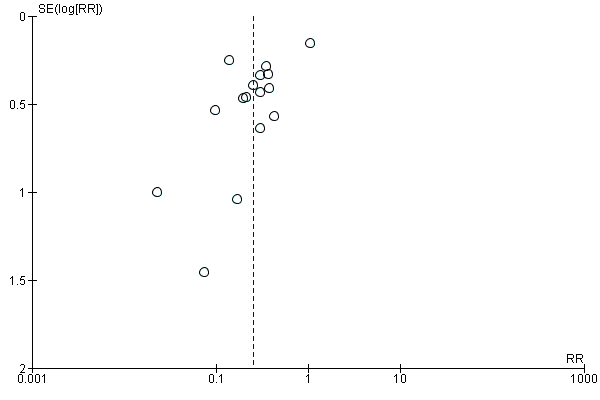
| Study ID | Study location | Participants | Interventions | Outcomes | Funding |
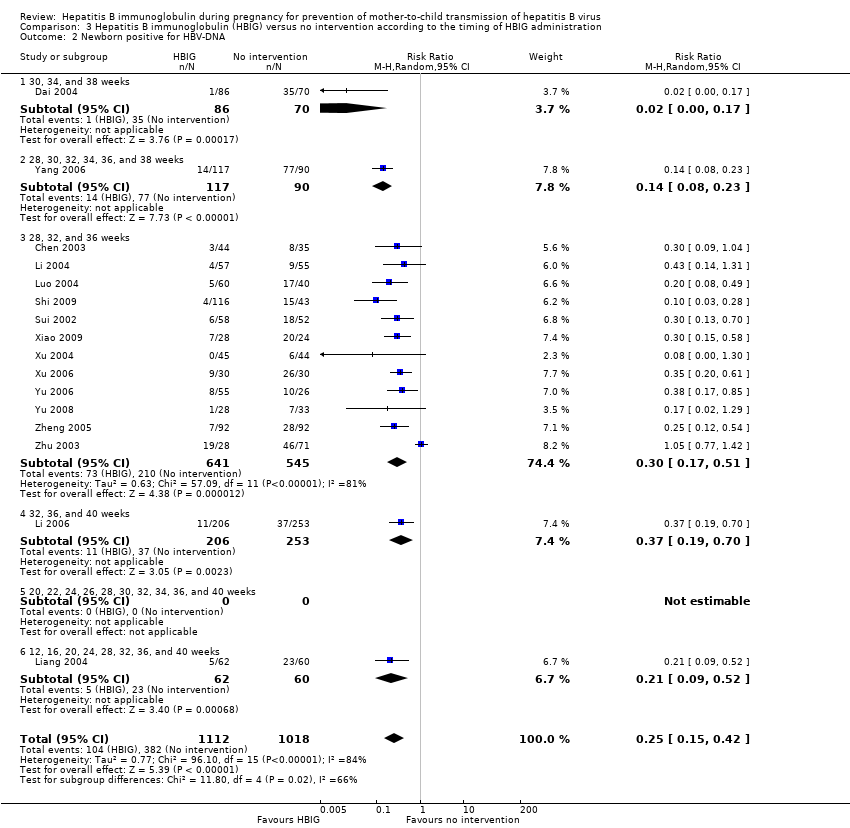
| Chen 2003 | Zhejiang | 79 participants (44 intervention, 35 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. |
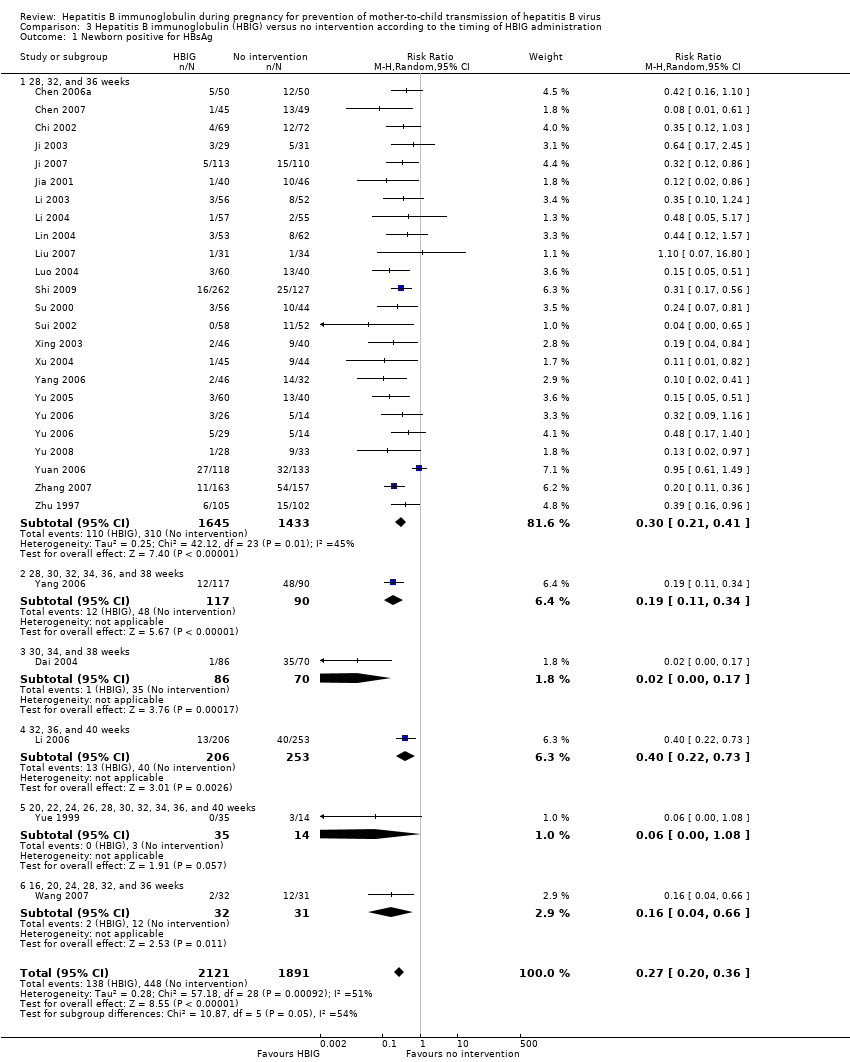
| Chen 2006a | Guangdong | 100 participants (50 intervention, 50 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. |
| Chen 2007 | Shenzhen | 94 participants (45 intervention, 49 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. |
| Chi 2002 | Zhejiang | 141 participants (69 intervention, 72 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. |
| Dai 2004 | Zhejiang | 156 participants (86 intervention, 70 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, anti‐HBs | Not stated. |
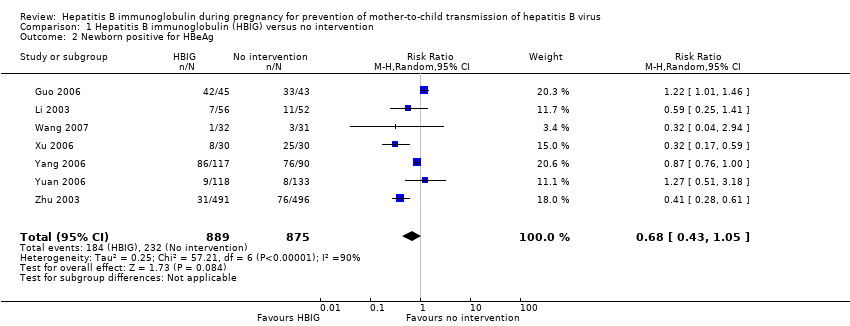
| Guo 2006 | Henan | 88 participants (45 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg anti‐HBs. | Not stated |
| Han 2003 | Guangdong | 216 participants (126 intervention, 90 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. |
| Ji 2003 | Zhejiang | 60 participants (29 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs, adverse events. | Not stated |
| Ji 2007 | Shanghai | 223 participants (113 intervention, 110 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. |
| Jia 2001 | Jiangsu | 86 participants (40 intervention, 46 control) aged 22 to 32 years. | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. |
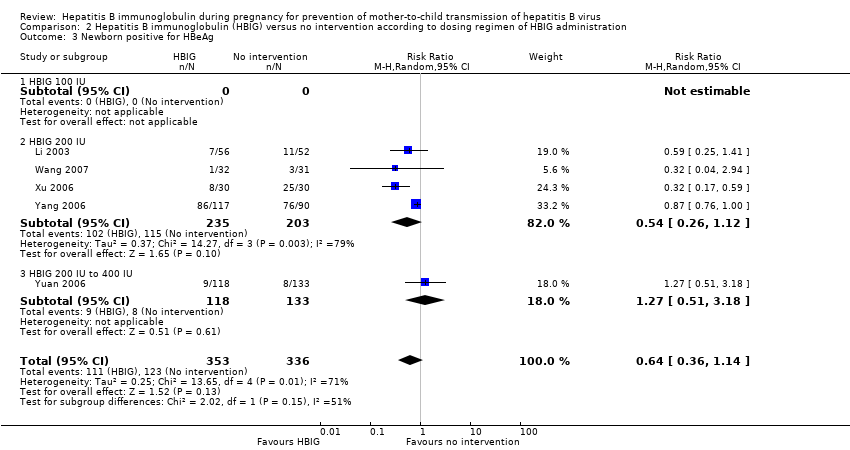
| Li 2003 | Guangdong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBeAg, or both. | Not stated. |
| Li 2004 | Guangdong | 112 participants (57 intervention, 55 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both. | Not stated. |
| Li 2006 | Hubei | 448 participants (202 intervention, 246 control) aged 18 to 38 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. |
| Liang 2004 | Guangdong | 122 participants (62 intervention, 60 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA. | Not stated. |
| Lin 2004 | Shanghai | 117 participants (55 intervention, 62 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. |
| Liu 2007 | Henan | 86 participants (43 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. |
| Luo 2004 | Jiangxi | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. |
| Shi 2009 | Guangzhou | 389 participants (262 intervention, 127 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both | Research supported by GlaxoSmithKline Research and Development Grant NUC30914; Science and Research Foundations of Sun Yat‐Sen University and Guangzhou Science Committee, No 1999‐J‐005‐01. |
| Su 2000 | Henan | 98 participants (55 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. |
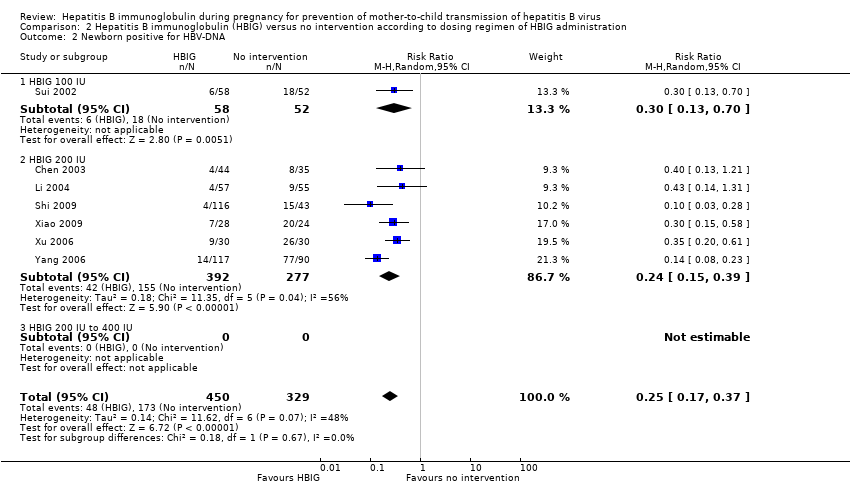
| Sui 2002 | Shandong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA, and anti‐HBs. | Not stated. |
| Wang 2007 | Shandong | 63 participants (32 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg and HBeAg | Not stated. |
| Wang 2008 | Taizhou | 279 participants (159 intervention, 120 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. |
| Xiao 2009 | Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBV‐DNA. | Not stated. |
| Xing 2003 | Henan | 86 participants (46 intervention, 40 control) aged 22 to 28 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Supported by Technology Research Fund Committee of Henan province (No. 981170112). |
| Xu 2004 | Shandong | 88 participants (44 intervention, 44 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA and anti‐HBs. 8‐month‐old babies positive for HBsAg, HBV‐DNA, and anti‐HBs. Maximum duration of surveillance: 8 months. Follow‐up time point: 8 months after birth. | Not stated. |
| Xu 2006 | Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBeAg and HBV‐DNA. | Not stated. |
| Yang 2006 | Jiangsu | 285 participants (163 intervention, 162 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg and HBV‐DNA | Not stated. |
| Yu 2005 | Guangdong | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBeAg, anti‐HBs | Not stated. |
| Yu 2006 | Shanghai | 83 participants (26 intervention I, 29 intervention II, 28 control) aged 20 to 33 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. |
| Yu 2008 | Guangxi | 61 participants (28 intervention, 33 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg | Not stated. |
| Yuan 2006 | Huizhou | 250 participants (117 intervention, 113 control) | Intervention: HBIG 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg; adverse effects of the immunoglobulins to the neonates and mothers | Supported by Huizhou Municipal Central hospital and Huizhou Science and Technology Bureau. |
| Yue 1999 | Shanxi | 48 participants (34 intervention, 14 control) aged 20 to 33 years | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. |
| Zhang 2007 | Guangdong | 320 participants (163 intervention, 157 control) aged 19 to 36 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. |
| Zheng 2005 | Guangdong | 184 participants (92 intervention, 92 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. |
| Zhu 1997 | Shanghai | 204 participants (103 intervention, 101 control) aged 20 to 34 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg. | Not stated. |
| Zhu 2003 | Shanghai | 980 participants (487 intervention, 493 control) aged 19 to 35 years | Intervention: HBIG 200 IU or 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, and HBV‐DNA. | Supported by a grant from the Ministry of Public Health China (No. 97030223). |