Иммуноглобулин против гепатита В во время беременности для предотвращения передачи вируса гепатита B от матери к ребенку.
Abstract
Background
Hepatitis is a viral infection of the liver. It is mainly transmitted between people through contact with infected blood, frequently from mother to baby in‐utero. Hepatitis B poses significant risk to the fetus and up to 85% of infants infected by their mothers at birth develop chronic hepatitis B virus (HBV) infection. Hepatitis B immunoglobulin (HBIG) is a purified solution of human immunoglobulin that could be administered to the mother, newborn, or both. HBIG offers protection against HBV infection when administered to pregnant women who test positive for hepatitis B envelope antigen (HBeAg) or hepatitis B surface antigen (HBsAg), or both. When HBIG is administered to pregnant women, the antibodies passively diffuse across the placenta to the child. This materno‐fetal diffusion is maximal during the third trimester of pregnancy. Up to 1% to 9% infants born to HBV‐carrying mothers still have HBV infection despite the newborn receiving HBIG plus active HBV vaccine in the immediate neonatal period. This suggests that additional intervention such as HBIG administration to the mother during the antenatal period could be beneficial to reduce the transmission rate in utero.
Objectives
To determine the benefits and harms of hepatitis B immunoglobulin (HBIG) administration to pregnant women during their third trimester of pregnancy for the prevention of mother‐to‐child transmission of hepatitis B virus infection.
Search methods
We searched the The Cochrane Hepato‐Biliary Group Controlled Trials Register, CENTRAL, MEDLINE Ovid, Embase Ovid, Science Citation Index Expanded (Web of Science), SCOPUS, African Journals OnLine, and INDEX MEDICUS up to June 2016. We searched ClinicalTrials.gov and portal of the WHO International Clinical Trials Registry Platform (ICTRP) in December 2016.
Selection criteria
We included randomised clinical trials comparing HBIG versus placebo or no intervention in pregnant women with HBV.
Data collection and analysis
Two authors extracted data independently. We analysed dichotomous outcome data using risk ratio (RR) and continuous outcome data using mean difference (MD) with 95% confidence intervals (CI). For meta‐analyses, we used a fixed‐effect model and a random‐effects model, along with an assessment of heterogeneity. If there were statistically significant discrepancies in the results, we reported the more conservative point estimate. If the two estimates were equal, we used the estimate with the widest CI as our main result. We assessed bias control using the Cochrane Hepato‐Biliary Group suggested bias risk domains and risk of random errors using Trial Sequential Analysis (TSA). We assessed the quality of the evidence using GRADE.
Main results
All 36 included trials originated from China and were at overall high risk of bias. The trials included 6044 pregnant women who were HBsAg, HBeAg, or hepatitis B virus DNA (HBV‐DNA) positive. Only seven trials reported inclusion of HBeAg‐positive mothers. All 36 trials compared HBIG versus no intervention. None of the trials used placebo.
Most of the trials assessed HBIG 100 IU (two trials) and HBIG 200 IU (31 trials). The timing of administration of HBIG varied; 30 trials administered three doses of HBIG 200 IU at 28, 32, and 36 weeks of pregnancy. None of the trials reported all‐cause mortality or other serious adverse events in the mothers or babies. Serological signs of hepatitis B infection of the newborns were reported as HBsAg, HBeAg, and HBV‐DNA positive results at end of follow‐up. Twenty‐nine trials reported HBsAg status in newborns (median 1.2 months of follow‐up after birth; range 0 to 12 months); seven trials reported HBeAg status (median 1.1 months of follow‐up after birth; range 0 to 12 months); and 16 trials reported HBV‐DNA status (median 1.2 months of follow‐up; range 0 to 12 months). HBIG reduced mother‐to‐child transmission (MTCT) of HBsAg when compared with no intervention (179/2769 (6%) with HBIG versus 537/2541 (21%) with no intervention; RR 0.30, TSA‐adjusted CI 0.20 to 0.52; I2 = 36%; 29 trials; 5310 participants; very low quality evidence). HBV‐DNA reduced MTCT of HBsAg (104/1112 (9%) with HBV‐DNA versus 382/1018 (38%) with no intervention; RR 0.25, TSA‐adjusted CI 0.22 to 0.27; I2 = 84%; 16 trials; 2130 participants; low quality evidence). TSA supported both results. Meta‐analysis showed that maternal HBIG did not decrease HBeAg in newborns compared with no intervention (184/889 (21%) with HBIG versus 232/875 (27%) with no intervention; RR 0.68, TSA‐adjusted CI 0.04 to 6.37; I2 = 90%; 7 trials; 1764 participants; very low quality evidence). TSA could neither support nor refute this observation as data were too sparse. None of the trials reported adverse events of the immunoglobulins on the newborns, presence of local and systemic adverse events on the mothers, or cost‐effectiveness of treatment.
Authors' conclusions
Due to very low to low quality evidence found in this review, we are uncertain of the effect of benefit of antenatal HBIG administration to the HBV‐infected mothers on newborn outcomes, such as HBsAg, HBV‐DNA, and HBeAg compared with no intervention. The results of the effects of HBIG on HBsAg and HBeAg are surrogate outcomes (raising risk of indirectness), and we need to be critical while interpreting the findings. We found no data on newborn mortality or maternal mortality or both, or other serious adverse events. Well‐designed randomised clinical trials are needed to determine the benefits and harms of HBIG versus placebo in prevention of MTCT of HBV.
PICO
Резюме на простом языке
Иммуноглобулин против гепатита В (ИГГВ) во время беременности для предотвращения передачи вируса гепатита B (ВГВ) от матери к ребенку
Вопрос обзора
Нашей целью было рассмотреть доказательства пользы и вреда инъекции ИГГВ беременным женщинам в течение последних трёх месяцев беременности в сравнении с отсутствием лечения для предотвращения передачи вируса гепатита B от матери к ребенку.
Актуальность
Вирус гепатита В является вирусом, поражающим печень. Когда инфекция продолжается в течение длительного времени, ее называют "хронической". Это может привести к поражению печени, а также стать причиной печеночной недостаточности и рака печени.
В основном, гепатит B передается при контакте с зараженной кровью, но чаще всего от матери к ребенку в утробе. Гепатит B широко распространен в Африке и Азии, и при заражении во время беременности инфекция представляет большую опасность для будущего ребенка. Как правило, на ранних стадиях инфекции не проявляются никакие симптомы. Однако у 85% детей, зараженных от своих матерей при рождении, развивается хроническая инфекция ВГВ.
ИГГВ ‐ это вещество, полученное из человеческой крови, которое используется для предотвращения передачи инфекции ВГВ от матери к ребенку. При введении ИГГВ беременным женщинам, зараженным ВГB, высокий уровень антител (белки, вырабатываемые иммунной системой) к вирусу легко проникают через плаценту к ребенку для защиты от инфекции. Это эффективнее всего в течение последней трети беременности.
Дата поиска
Мы провели поиск доказательств 22 декабря 2016 года.
Источники финансирования исследований
Четыре клинических испытания были финансированы фармацевтической компанией или группой с финансовой (или другой) заинтересованностью в результатах исследований.
Характеристика исследований
После поиска медицинской литературы на предмет соответствующих испытаний, мы выявили 36 клинических испытаний, в которых приняли участие 6044 беременные женщины с признаками инфекции ВГB. Все испытания были проведены в Китае. Все клинические испытания и их результаты были подвержены высоким рискам смещения, что делает более вероятной переоценку пользы и недооценку вреда.
Основные результаты
В исследованиях оценивали только статус поверхностного антигена гепатита В (HBsAg) (белки на поверхности ВГВ, которые провоцируют выработку антител иммунной системой организма при контакте с ВГВ), ДНК вируса гепатита B (HBV‐DNA) (саморазделяющийся материал ВГВ, который несет в себе генетическую информацию) и антигена "е" гепатита B (HBeAg) (белки крови, которые указывают на то, что вирус в печени все еще активен) у новорожденных. Не было какой‐либо информации о влиянии ИГГВ на смертность по всем причинам (новорожденного или матери), об антителах к внутреннему антигену вируса гепатита B (белки, производимые иммунной системой, которые прикрепляются к вирусу и разрушают его), об эффективности ИГГВ с точки зрения затрат и побочных эффектов.
Антенатальное (до рождения) введение ИГГВ может повлиять на профилактику передачи ВГВ от матери к ребенку, поскольку большее число младенцев, которые подверглись лечению, по сравнению с младенцами, не получившими лечение, не имели антигена HBsAg или HBV‐DNA; однако, оба результата могли оказаться под влиянием способа проведения испытаний и быть с высоким риском смещения. Авторы не смогли сформулировать какие‐либо выводы о побочных эффектах ИГГВ у беременных женщин c ВГВ инфекцией. Необходимо провести хорошо продуманные клинические испытания с низким риском смещения, чтобы выявить пользу и вред ИГГВ в сравнении с отсутствием лечения у беременных женщин с ВГВ.
Качество доказательств
Из‐за очень низкого качества доказательств в этом обзоре мы не знаем, оказывает ли антенатальное введение ИГГВ влияние на процент новорожденных с HBsAg и HBV‐DNA, по сравнению с отсутствием лечения. Мы не смогли сформулировать какие‐либо выводы о смертности новорожденных и матерей, поскольку не обнаружили данных.
Authors' conclusions
Summary of findings
| Hepatitis B immunoglobulin (HBIG) vs no intervention for prevention of mother‐to‐child transmission of hepatitis B virus | ||||||
| Participants: pregnant women positive for HBsAg or positive for HBeAg, or both. Comparison: no intervention. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | HBIG versus no intervention | |||||
| All‐cause mortality or other serous adverse events of the newborn | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| All‐cause mortality or other serous adverse events of the mothers | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Newborn with HBsAg‐positive result | Study population | RR 0.3 | 5310 | ⊕⊝⊝⊝ | ||
| 211 per 1000 | 63 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 64 per 1000 | |||||
| Newborn with HBeAg‐positive result | Study population | RR 0.68 | 1764 | ⊕⊝⊝⊝ | ||
| 265 per 1000 | 180 per 1000 | |||||
| Moderate | ||||||
| 212 per 1000 | 144 per 1000 | |||||
| Newborn with HBV‐DNA‐positive result | Study population | RR 0.25 | 2130 | ⊕⊕⊝⊝ | ||
| 375 per 1000 | 94 per 1000 | |||||
| Moderate | ||||||
| 366 per 1000 | 91 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Comment: Data for this outcome was not reported in any of the included trials. | ||||||
Background
Description of the condition
Infection with hepatitis B virus (HBV) is a serious global public health problem (CDC 2008; Visvanathan 2016; Yi 2016). Hepatitis B viral infection is the ninth most common cause of death worldwide (Rivkina 2002). In Africa and Asia, it remains a major cause of morbidity and mortality, with a prevalence higher than 8% (CDC 2008). There have been several concerted efforts in preventing the impact of the HBV on the mother and the baby since HBV was first identified in 1966 by Baruch Blumberg (Blumberg 1977).
Acute HBV infection is transmitted by HBV, a DNA‐containing virus of the Hepadnaviridae family with an incubation period of six weeks to six months (Kumar 2007). It usually presents as a subclinical, mild illness, with only up to 30% of people developing scleral icterus, nausea, vomiting, and right‐upper quadrant tenderness (Bodihar 2004). Serum alanine aminotransferase and aspartate aminotransferase levels are usually elevated, with values in the thousands. In most people, symptoms resolve within several weeks with supportive care, but 0.5% to 1.5% of people develop fulminant hepatic failure (Gambarin‐Gelwel 2007).
Chronic HBV infection is a chronic necro‐inflammatory liver disease caused by persistent HBV liver infection (Lock 2007; Ahn 2010). Chronic HBV can be divided into hepatitis B envelope antigen (HBeAg)‐positive and HBeAg‐negative chronic HBV. Host factors associated with increased risk of cirrhosis development include older age, alcohol consumption, and coinfection with hepatitis C virus, hepatitis D virus, or HIV (Lock 2007). Hepatitis B factors associated with increased risk of cirrhosis include duration of infection, HBV genotype C, and high levels of hepatitis B virus DNA (HBV‐DNA) (Lock 2007).
People with chronic HBV are at increased risk of developing cirrhosis, hepatic decompensation, and hepatocellular carcinoma. HBV can be transmitted by parenteral, sexual, and vertical routes (Seow 1999). The sources of the virus include blood, saliva, tears, breast milk, pathological effusions, vaginal secretions, and semen (Gambarin‐Gelwel 2007). The virus is present in all physiological and pathological body fluids with the exception of stool (Kumar 2007; Eke 2011). In‐utero infection of the fetus, vertical transmission, constitutes the main mode of transmission in endemic regions of Africa and Asia (Rasha 2007). In one‐third of people, the source of infection is unknown (Kumar 2007). Horizontal hepatitis B infection in adults is mostly a self‐limiting disease, but vertical transmission produces a high rate of chronic infection (Seow 1999). Perinatal transmission of HBV represents one of the efficient modes of HBV transmission and often leads to severe long‐term sequelae. Up to 85% of infants infected by their mothers at birth develop chronic HBV infection (Bodihar 2004).
The risk of vertical transmission of HBV increases with the gestational age at which the mother is infected. Vertical transmission occurs in up to 10% of neonates when the mother is infected during the first trimester, and in 60% to 90% of babies when acute infection occurs when the mother is infected during the third trimester (Bodihar 2004). Prematurity is increased if acute hepatitis B is acquired in the last trimester, and over 60% of pregnant women who acquire acute hepatitis B infection at or near term transmit HBV to their offspring (Bodihar 2004). The commonly used markers to determine chronic infection with HBV are hepatitis B surface antigen (HBsAg), HBV‐DNA, and hepatitis B core antibody (HBcAb). Following acute hepatitis B infection, the surface antigen and the core antibody commonly become detectable in the serum; both may remain in the serum even after viral clearance (Almeida 2001; Bolarinwa 2015). Based on this, both markers (HBsAg and HBcAb) are used as evidence of previous exposure to the virus. Detection of antibody to the surface antigen (hepatitis B surface antibody (HBsAb)) is generally assumed to depict immunity to HBV infection. It is a general assumption that the presence of the HBeAg in the serum depicts active HBV replication within hepatocytes, with attendant high risk of viral transmission, including mother‐to‐child transmission (MTCT) of HBV (Bolarinwa 2015; Schillie 2015). Correspondingly, the presence of the HBeAb in the serum (with HBsAg negativity) coincides with clinical remission in chronic HBV infection and equally offers some protection against MTCT of HBV (Lu 2014). Previously, the HBeAg was assumed to be a surrogate marker for the presence of HBV‐DNA, and people who were negative to HBeAg were thought to have achieved viral clearance; however, this assertion has been challenged following the discovery of people with HBeAg‐negative (HBeAb‐positive) chronic HBV infection, with very active disease (Hadziyannis 1995). In view of this, Hadziyannis and Vassilopoulos in 2001 revealed that people whose results are positive for HBeAb and HBsAg but negative for HBeAg may require further evaluation for the presence of HBV‐DNA and serum transaminases to distinguish them from those with inactive HBsAg carrier state (Hadziyannis 2001). For these reasons, prophylaxis (postexposure prophylaxis) of infants from all HBsAg‐positive mothers is recommended, regardless of the mother's HBeAg or HBeAb status (Hadziyannis 2001). The presence of HBeAg indicates the degree of infectivity of the person. The higher the concentration of HBeAg, the higher the degree of infectivity (Kumar 2007).
Trans‐placental transfer of HBV remains very important (Wiseman 2009; Eke 2011). This is because some fetuses that contact HBeAg early in embryonic development become immunologically tolerant to the antigen. This eventually leads to chronic HBV infection caused by the inability of the body to eliminate the virus (Gambarin‐Gelwel 2007). Zhang and coworkers measured concentrations of HBsAg in maternal decidual cells, trophoblastic cells, and villous mesenchymal cells, and showed that the main route of HBV transmission from mother to fetus is trans‐placental (Zhang 2004). Zhang and coworkers also detected HBV‐DNA in amniotic fluid samples and vaginal secretion samples, emphasising risk of transmission of HBV by these fluids during childbirth (Zhang 2004).
While antiviral medications may have a role in the prevention of vertical transmission of hepatitis B (Brown 2016), this topic is beyond the scope of the present review.
Description of the intervention
HBIG is a purified solution of human immunoglobulin from human plasma that has high titres of antibody to HBsAg (anti‐HBs) (Habib 2007). It is derived from plasma donated by people immune to HBV infection (Habib 2007). Numerous studies have been conducted to assess the beneficial and harmful effects of gamma globulin in preventing type B hepatitis (Habib 2007; Mathew 2008; Lee 2009). These investigations have evaluated the benefits and harms of HBIG in pre‐exposure and postexposure settings. Data suggest that HBIG containing some antibody to HBsAg may be effective for pre‐ and postexposure prophylaxis of HBV infection (Rothstein 1982).
It is advisable to inspect the HBIG solution for particulate matter and discolouration before administration. HBIG and hepatitis B vaccine may be given at the same time but at different sites. HBIG should not be mixed with other drugs in the same syringe (Szmuness 1981). Contraindications to its use include anaphylactic or severe systemic reaction to human globulin; as well as thrombocytopenia or a coagulation disorder that would contraindicate intramuscular injection (Ellis 1969). HBIG is administered intramuscularly, preferably in the anterolateral aspects of upper thigh or deltoid muscle. If the gluteal region is to be used, it is advisable to avoid the central region to reduce risk of injury to the sciatic nerve. The mean time to maximal concentration in the blood of HBIG after intramuscular infection is four to five days, with elimination half‐life of 17.5 to 25.0 days.
Adverse reactions noticed with HBIG include erythema, pain, tenderness at the injection site, headache, malaise, agitation, amnesia, essential tremor, fatigue, light‐headedness or fainting, pyrexia, angioedema, pruritus, rash, urticaria, nausea, vomiting, aphthous stomatitis, diarrhoea, dyspepsia, gingival hyperplasia, cold symptoms or influenza, anaphylactic reactions, myalgia, joint stiffness, back pain, hypotension, and hypertension (Ellis 1969). As with any intervention originating from human plasma, there is a risk of transmission of infective agents.
How the intervention might work
HBIG is widely administered to confer passive prophylactic immunity against the HBV because of the ability of anti‐HBs to neutralise hepatitis B virions (Habib 2007; Mathew 2008; Lee 2009). HBIG provides passive immunisation for people exposed to HBV as evidenced by a reduction in the attack rate of hepatitis B following its use. The administration of the usual recommended dose of HBIG generally results in a detectable level of circulating anti‐HBs, which persists for approximately two months or longer (Habib 2007).
Hepatitis B immunoglobulin seems to be an effective immunoglobulin, which is used for preventing MTCT of HBV (Li 2003). The possible mechanism in pregnant women is that HBsAb in HBIG can bind HBsAg and activate the complement system, strengthen humoral immunity, reduce HBV levels, prevent (or reduce) infection of healthy cells, and reduce replication of HBV (Shi 2010a; Yi 2016). In the process, it can clear the circulating hepatitis B virions and reduce the viral load in the maternal blood (Li 2003). It can also prevent and decrease hepatitis B multiplication in the maternal body fluids (Li 2003; Li 2004). Antibodies are transferred from the mother to the fetus through the placenta. After maternal administration of intramuscular hepatitis B immunoglobulin, protective hepatitis B antibodies are transmitted to the fetus, which makes it possible for the fetus to become protected via intrauterine passive immunisation. This subsequently prevents intrauterine infection of the fetus by the HBV (Li 2003). Passive immunisation obtained from pregnancy state could be responsible for its action (Shi 2010a). Despite this observation, the authors of Han 2007 and Xiao 2007 have challenged the proposed way the HBIG intervention works. The authors of Xiao 2007 confirmed the efficacy of HBIG application in pregnant women in the interruption of intrauterine infection but found no significant increase in newborn HBsAb seropositivity, while the authors of Han 2007 found no significant decrease in maternal HBV‐DNA load and that none of the newborns received HBsAb. Therefore, it is unclear whether HBIG injection at four‐week intervals will effectively decrease maternal HBV load or will permit HBIG to reach the fetal circulation through the placenta. Nevertheless, several studies showed that a significant decrease in maternal HBV‐DNA level or HBsAg titres occur following HBIG administration (Li 2003; Shi 2009). The authors of Yu 2006 revealed that within three to seven days after each HBIG injection, both maternal HBV‐DNA and HBsAg levels decreased, whereas four weeks after HBIG injection, maternal HBV‐DNA and HBsAg returned to the levels prior to injection (Yu 2006). Based on these findings, it was proposed that HBIG interruption of HBV intrauterine infection was mainly due to HBIG transportation through the placenta and less likely due to a reduced maternal HBV load (Yu 2006).
Why it is important to do this review
Infection with the HBV is considered a public health problem worldwide. According to World Health Organization (WHO) estimates, there are about 400 million carriers of the infection (WHO 2006). Every year, approximately one million people die because of the association between HBV and the development of chronic clinical forms, such as chronic active hepatitis, cirrhosis, and hepatic carcinoma (WHO 2006). Because hepatitis B acquired at birth leads to chronic infection in 60% to 90% of people, the identification of pregnant women infected with the virus and the institution of prophylactic measures aimed at preventing MTCT of women at risk is a prime target for the control of hepatitis B, even in low prevalence countries (Gambarin‐Gelwel 2007).
The asymptomatic carrier status has far‐reaching consequences, particularly for pregnant women, who vertically transmit the virus to their fetuses. Also, between 35% and 40% of all the HBV‐infected people diagnosed every year have resulted from transmission of HBV from mother to child (Shahnaz 2005). Moreover, the utilisation of childhood immunisation in most low‐income countries, especially African countries, is low. Children perinatally infected by their mothers may themselves be a source of horizontal transmission to their younger siblings and playmates, especially in overcrowded living conditions (Agbede 2007). Therefore, breaking the MTCT will interrupt most of the secondary routes of transmission as well (Agbede 2007).
The antenatal period may be a major access point for the antenatal population in limited resource settings to benefit from HBIG (Abou‐Zahr 2003). Successful interventions to prevent vertical transmission linked to antepartum rapid testing have been demonstrated in a variety of limited resources. Recommendation for pregnancy vaccination is determined by antenatal prevalence of HBsAg in clinical settings (Gambarin‐Gelwel 2007).
It has been recommended that administration of HBIG to the mother during pregnancy may prevent intrauterine infection (Zhu 2003), though controversy exist for its efficacy (Li 2004; Li 2010; Yi 2016). Administration of immunoprophylactic HBIG within 12 hours of birth and a three‐dose succession of HBV vaccine (joint immunoprophylaxis) reduces the frequency of MTCT of HBV to approximately 5% (Ma 2014). Disappointingly, high HBV viral load and HBeAg in pregnant women is a significant risk factor for failure of joint immunoprophylaxis (Yin 2013). This is because up to 1% to 9% of infants born to HBV‐carrying mothers still have HBV infection despite joint immunoprophylaxis (del Canho 1994; Yan 1999; Xu 2002; Guo 2013; Yi 2016). Since up to 1% to 9% of perinatal infection may occur in utero, it appears likely that no form of postnatal prophylaxis will be 100% effective, unless in utero prophylaxis (periodical antenatal HBIG) is instituted (Liu 2015). Additionally, the T‐cell function is not fully developed in the neonatal period, and accordingly newborns exhibit immune tolerance to HBsAg (Liu 2015). Thus, it is easier for neonates to become chronic carriers once infected with HBV (Liu 2015). This observable fact makes preventing maternally transmitted HBV infection a critical step in eliminating HBV infection. Therefore, a study on the benefits and harms of periodical HBIG administration to pregnant women during their third trimester of pregnancy for the prevention of MTCT of HBV infection, in addition to routine joint immunoprophylaxis for the newborn, is of paramount significance.
There have been published reviews (Shi 2009; Zhou 2012) and meta‐analyses (Jin 2014; Xu 2014) assessing the benefits and harms of HBIG during pregnancy for the prevention of MTCT of HBV. These non‐Cochrane reviews and meta‐analyses appear to have several limitations (Page 2016). For example, they overlooked the random‐effect principle or the unevenness of HBeAg status in the pregnant women studied, including study heterogeneity or dosages of HBIG (or both) (Shi 2009; Zhou 2012; Jin 2014). Cochrane systematic reviews are usually more thorough. Hence, despite the fact that published reviews and meta‐analyses already exist, we still decided to go ahead with this review.
Objectives
To determine the benefits and harms of hepatitis B immunoglobulin (HBIG) administration to pregnant women during their third trimester of pregnancy for the prevention of mother‐to‐child transmission of hepatitis B virus infection.
Methods
Criteria for considering studies for this review
Types of studies
The review included randomised clinical trials on HBIG aimed at preventing MTCT of HBV, irrespective of publication status, year of publication, or language. We did not include any quasi‐randomised studies or observational studies for the assessments of harms. We are aware that this is a limitation of our review.
Types of participants
Pregnant women who were positive for HBsAg or positive for HBeAg, or both.
Types of interventions
Experimental intervention
-
Hepatitis B immunoglobulin (HBIG).
Comparison
-
Placebo or no intervention.
Types of outcome measures
We sought the following outcomes at the end of treatment as well as at maximal follow‐up.
Primary outcomes
-
All‐cause mortality or other serious adverse events of the newborn. A serious adverse event, defined according to the International Conference on Harmonisation (ICH) Guidelines (ICH‐GCP 1997), was any untoward medical occurrence that resulted in death, was life threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, or was a congenital anomaly/birth defect.
-
All‐cause mortality or other serious adverse events of the mothers.
-
Serological signs of hepatitis B infection of the newborn. These were reported as newborns with HBsAg‐positive laboratory result; newborns with HBeAg‐positive laboratory result; newborns with HBV‐DNA‐positive laboratory result; and newborns with antibodies to hepatitis B core antigen.
Secondary outcomes
-
Non‐serious adverse events of the babies. Any untoward medical occurrence in a person or clinical investigation participant administered HBIG that did not meet the criteria in Primary outcomes was a non‐serious adverse effect.
-
Presence of local and systemic adverse events (serious and non‐serious) of the mothers.
-
Cost‐effectiveness of treatment.
Search methods for identification of studies
Electronic searches
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2016; 16 June 2016), the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5) in the Cochrane Library, MEDLINE Ovid (1946 to June 2016), Embase Ovid (1974 to June 2016), Science Citation Index Expanded (Web of Science; 1900 to June 2016), SCOPUS (1966 to June 2016), African Journals OnLine (AJOL) (1988 to June 2016), and INDEX MEDICUS (1879 to June 2016) (Royle 2003) using the search strategies and the time spans given in Appendix 1. We searched ClinicalTrials.gov (ClinicalTrials.gov) and portal of the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch) to identify ongoing trials on 22 December 2016. We also searched the China Biological Medicine Database (CBMdisc) to obtain the relevant randomised clinical trials. We contacted the Cochrane Vaccines Field and the Cochrane Pregnancy and Childbirth to identify further trials.
Searching other resources
We checked the reference list of relevant articles to identify further trials. We also contacted the authors of relevant papers and pharmaceutical companies that produce HBIG to inquire for any published or unpublished studies.
Data collection and analysis
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2016).
Selection of studies
Two authors (AE and UE) screened titles and abstracts of studies for inclusion. Two authors (UE and GE) independently applied the inclusion criteria to retrieve the full texts of the selected studies. For the papers in Chinese, two authors (YX and JL) extracted data, which two other authors (AE and GE) cross‐checked. Disagreements about inclusion was discussed among all the authors. If a consensus was not met, we contacted the contact editor (CG) of the review. We sought further information from the authors where papers contained insufficient information to make a decision about eligibility. We scrutinised each of the trial reports to ensure that multiple publications from the same trial were included only once. We listed all multiple publications referring to an included trial under the primary reference. We listed the excluded studies and gave reasons for their exclusion.
Data extraction and management
One author (AE) developed the data extraction forms. Thereafter, three authors (AE, UE, and GE) independently extracted data from the trials in English using the data extraction forms in duplicate. One author (YX) extracted data from the trials in Chinese. We resolved disagreements by consensus between the authors. In the case of missing or unclear data, we contacted the authors of the publications.
Assessment of risk of bias in included studies
We used Cochrane domains for assessing risk of bias of all eligible trials (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savović 2012a; Savović 2012b), as well as the Cochrane Hepato‐Biliary Group Module (Gluud 2016). All authors assessed the domains, which are listed below. We contacted the authors of the papers for any information that was not specified or was unclear.
Allocation sequence generation
-
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent adjudicator.
-
Unclear risk of bias: the trial was described as randomised, but the method of sequence generation was not specified.
-
High risk of bias: the sequence generation method was not, or may not have been, random. Quasi‐randomised studies, those using dates, names, or admittance numbers to allocate participants were inadequate and were excluded for the assessment of benefits but not for harms.
Allocation concealment
-
Low risk of bias: allocation was controlled by a central and independent randomisation unit, sequentially numbered, opaque, and sealed envelopes or similar, so that intervention allocations could not have been foreseen in advance of, or during, enrolment.
-
Unclear risk of bias: the trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
-
High risk of bias: if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding of participants and treatment providers
-
Low risk of bias: it was described that both participants and treatment providers were blinded to treatment allocation.
-
Unclear risk of bias: it was unclear if participants and treatment providers were blinded, or the extent of blinding was insufficiently described.
-
High risk of bias: no blinding or incomplete blinding of participants and treatment providers was performed.
Blinding of outcome assessment
-
Low risk of bias: it was mentioned that outcome assessors were blinded and this was described.
-
Unclear risk of bias: it was not mentioned if the outcome assessors were blinded, or the extent of blinding was insufficiently described.
-
High risk of bias: no blinding or incomplete blinding of outcome assessors was performed.
Incomplete outcome data
-
Low risk of bias: the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals.
-
Unclear risk of bias: the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
-
High risk of bias: the number or reasons for dropouts and withdrawals were not described.
Selective outcome reporting
-
Low risk of bias: morbidity and mortality, or clinically relevant and reasonably expected outcomes were reported on.
-
Unclear risk of bias: not all predefined, or clinically relevant and reasonably expected outcomes were reported on or were not reported fully, or it was unclear whether data on these outcomes were recorded or not.
-
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Vested interest bias
-
Low risk of bias: it was described that the trial was not sponsored by a pharmaceutical company, a person, or a group with a financial or other interest in a certain result of the trial.
-
Unclear risk of bias: it was unclear how the trial was sponsored.
-
High risk of bias: the trial was sponsored by a pharmaceutical company, a person, or a group with a certain financial or other interest in a given result of the trial.
Other bias
-
Low risk of bias: the trial appeared to be free of other bias domains that could put it at risk of bias.
-
Unclear risk of bias: the trial may or may not have been free of other domains that could put it at risk of bias.
-
High risk of bias: there were other factors in the trial that could put it at risk of bias.
Overall risk of bias
We judged trials to be overall low risk of bias if they were assessed as 'low risk of bias' in all the above domains. We judged trials to be at an overall high risk of bias if they were assessed as having an unclear risk of bias or a high risk of bias in one or more of the above domains.
We assessed the domains 'Blinding of outcome assessment', 'Incomplete outcome data', and 'Selective outcome reporting' for each outcome result. Thus, we were able to assess the bias risk for each outcome result in addition for each trial (overall risk of bias of each trial).
Measures of treatment effect
We used risk ratio (RR) as the measure of treatment effect for dichotomous data. We reported all outcomes using 95% confidence intervals (CI).
Unit of analysis issues
We allowed the inclusion of trials with multiple intervention arms. However, we included into the analysis only the arms relevant to this review. We combined all relevant experimental intervention arms of a trial into a single group, and all relevant control intervention arms into a single control group. For dichotomous outcomes, both the sample sizes and the numbers of people with events were summed across groups. For continuous outcomes, we planned to combine means and standard deviations (SD) using methods described in Section 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the number of randomised participants to calculate estimates of intervention effects and CIs.
Dealing with missing data
During selection of trials and data collection, we tried to obtain any missing information by contacting the author of correspondence of the publication by e‐mail or telephone. We performed analyses according to the intention‐to‐treat principle, using the following four possible scenarios (Gluud 2016):
-
assuming poor outcome (both groups): dropouts from both the treatment and control groups were considered failures, using the total number of participants as the denominator;
-
assuming good outcome (both groups): dropouts from both the treatment and control groups were considered successes, using the total number of participants as the denominator;
-
assuming good outcome in the intervention group, and assuming poor outcome in the control group;
-
assuming poor outcome in the intervention group, and assuming good outcome in the control group.
Assessment of heterogeneity
We attempted to assess heterogeneity in three ways (Higgins 2011): graphically, by using forest plots; by the Chi2 test where P values of less than 0.10 determined statistical significance; and by the I2 statistic. We read the I2 test value in the following way: from 0% to 40%, heterogeneity may not be important; from 30% to 60%, heterogeneity may be moderate; from 50% to 90%, heterogeneity may be substantial; and from 75% to 100%, heterogeneity may be considerable.
Assessment of reporting biases
There are several methods of assessing the occurrence of publication bias. The approach used in this review was based on scatter plots of the treatment effect estimated by individual studies versus a measure of study size or precision. In these graphical representations, larger and more precise trials were plotted at the top while smaller and less precise trials showed a wider distribution below. If there was no publication bias, the trials would be expected to be symmetrically distributed on both sides of the combined effect size line. Within a published report with potential publication bias, their analyses with statistically significant difference between intervention groups were more likely to be reported than non‐significant differences.
We assessed the reporting bias of the included trials as there were more than 10 trials included in this review and constructed funnel plots to look for evidence of publication bias. We ensured that all trials that fulfilled the inclusion criteria were included into the review, irrespective of the language of publication. We checked additional unpublished data for further information.
Data synthesis
Meta‐analysis
We performed the meta‐analyses according to the recommendations of Cochrane (Higgins 2011). The analyses were performed using Review Manager 5 (RevMan 2014). For meta‐analyses with more than one trial, we used both a fixed‐effect model (DeMets 1987) and a random‐effects model (DerSimonian 1986), along with an assessment of heterogeneity. We presented the results of dichotomous outcomes of individual trials as RR with 95% CI and the results of the continuous outcomes as mean difference (MD) with 95% CI.
Assessment of significance
We assessed our intervention effects with both random‐effects model meta‐analysis and fixed‐effect model meta‐analysis (Jakobsen 2014). If there were statistically significant discrepancies in the results (e.g. one giving a significant intervention effect and the other no significant intervention effect), we reported the more conservative point estimate of the two (Jakobsen 2014). The more conservative point estimate was the estimate closest to zero effect (the analysis with the highest P value) (Higgins 2011). If the two estimates were equal, we used the estimate with the widest CI as our main result of the two analyses. We assessed three primary outcomes; therefore, we considered a P value of 0.025 or less as statistically significant (Jakobsen 2014). Similarly, we planned to assess three secondary outcomes; therefore, we considered a P value of 0.025 or less as statistically significant (Jakobsen 2014). We used the eight‐step procedure to assess if the thresholds for significance were crossed (Jakobsen 2014).
Trial Sequential Analysis
The combination of meta‐analysis and trial sequential monitoring boundaries (a series of boundaries applied to sequence of tests on cumulative groups of participants randomised in a clinical trial) is referred to as Trial Sequential Analysis (Wetterslev 2008). Trial Sequential Analysis combines conventional meta‐analysis methodology with meta‐analytic sample size considerations (i.e. required information size) and methods already developed for repeated significance testing on accumulating data in randomised clinical trials (Wetterslev 2008). Traditional meta‐analysis runs the risk of random errors due to sparse data and repetitive testing of accumulating data when updating reviews. Therefore, we performed Trial Sequential Analysis (Wetterslev 2008; Wetterslev 2009; Jakobsen 2014) on the outcomes to calculate the required information size and assess the eventual breach of the cumulative Z‐curve of the relevant trial sequential monitoring boundaries for benefit, harm, or futility (Wetterslev 2008; Wetterslev 2009; Jakobsen 2014). To control random errors, we calculated the diversity‐adjusted required information size (DARIS) (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Wetterslev 2008; Thorlund 2011). DARIS was based on the proportion of participants in the control group with the outcome. Thereby, we controlled the risks of type I errors and type II errors. A more detailed description of Trial Sequential Analysis can be found at www.ctu.dk/tsa (Thorlund 2011; TSA 2011).
For dichotomous outcomes, we estimated DARIS based on the proportion of participants with an outcome in the control group, a relative risk reduction of 20%, an alpha of 2.5% for the primary and secondary outcomes, a beta of 10%, and the diversity suggested by the trials in the meta‐analysis (Jakobsen 2014). For continuous outcomes, we estimated the DARIS based on the SD observed in the control group of trials and a minimal relevant difference of 50% of this SD, an alpha of 2.5% for primary and secondary outcomes, a beta of 10%, and the diversity suggested by the trials in the meta‐analysis (Jakobsen 2014). The underlying assumption of Trial Sequential Analysis is that testing for statistical significance may be performed each time a new trial is added to the meta‐analysis. We added the trials according to the year of publication, and, if more than one trial was published in a year, we added trials alphabetically according to the last name of the first author. On the basis of the DARIS, we constructed the trial sequential monitoring boundaries for benefit, harm, and futility (Wetterslev 2008; Thorlund 2011). These boundaries determined the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the DARIS; if the trial sequential monitoring boundary for benefit or harm was crossed before the DARIS was reached, firm evidence may have been established and further trials can be considered superfluous. However, if the boundaries were not crossed, it was most probably necessary to continue doing trials to detect or reject a certain intervention effect. However, if the cumulative Z‐curve crossed the trial sequential monitoring boundaries for futility, no more trials may be needed.
Subgroup analysis and investigation of heterogeneity
We investigated heterogeneity using subgroup analyses. We analysed the various dosing regimen of HBIG administered during pregnancy. We also assessed the timing of HBIG administration (gestational age in pregnancy) since the risk of transmission depends on time of infection during pregnancy.
Sensitivity analysis
Sensitivity analyses were to be conducted, excluding trials with inadequate concealment of allocation and blinding of the outcome assessor.
'Summary of findings' table
We assessed the certainty of the evidence using the GRADE system (GRADEpro; tech.cochrane.org/revman/other‐resources/gradepro/download) to present review results in 'Summary of findings' table. When necessary, we planned to present 'Summary of findings' tables of the results from subgroup analyses only if they are meant to explain the heterogeneity in the overall results. A 'Summary of findings' table consists of three parts: information about the review, a summary of the statistical results, and the grade of the quality of evidence. The quality assessment of the available evidence is comprised of the number of studies; the types of studies (randomised); and five factors including risk of bias, unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses), indirectness of evidence (population, intervention, control, outcomes), imprecision of effect estimates (wide CIs), and publication bias that affected the quality of the evidence. We used these five factors to judge whether the certainty of the collected evidence should be downgraded if we were dealing with randomised clinical trials (or increased if we were dealing with observational studies).
We defined the levels of evidence as 'high', 'moderate', 'low', or 'very low' as follows.
-
High certainty: this research provided a very good indication of the likely effect; the likelihood that the effect will be substantially different was low.
-
Moderate certainty: this research provided a good indication of the likely effect; the likelihood that the effect will be substantially different was moderate.
-
Low certainty: this research provided some indication of the likely effect; however, the likelihood that it will be substantially different was high.
-
Very low certainty: this research did not provide a reliable indication of the likely effect; the likelihood that the effect will be substantially different was very high.
Results
Description of studies
See: Characteristics of included studies table; Characteristics of excluded studies table; Table 1.
| Study ID | Study location | Participants | Interventions | Outcomes | Funding |
| Zhejiang | 79 participants (44 intervention, 35 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Guangdong | 100 participants (50 intervention, 50 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Shenzhen | 94 participants (45 intervention, 49 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. | |
| Zhejiang | 141 participants (69 intervention, 72 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Zhejiang | 156 participants (86 intervention, 70 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, anti‐HBs | Not stated. | |
| Henan | 88 participants (45 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg anti‐HBs. | Not stated | |
| Guangdong | 216 participants (126 intervention, 90 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Zhejiang | 60 participants (29 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs, adverse events. | Not stated | |
| Shanghai | 223 participants (113 intervention, 110 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Jiangsu | 86 participants (40 intervention, 46 control) aged 22 to 32 years. | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Guangdong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBeAg, or both. | Not stated. | |
| Guangdong | 112 participants (57 intervention, 55 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both. | Not stated. | |
| Hubei | 448 participants (202 intervention, 246 control) aged 18 to 38 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. | |
| Guangdong | 122 participants (62 intervention, 60 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA. | Not stated. | |
| Shanghai | 117 participants (55 intervention, 62 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Henan | 86 participants (43 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Jiangxi | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. | |
| Guangzhou | 389 participants (262 intervention, 127 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both | Research supported by GlaxoSmithKline Research and Development Grant NUC30914; Science and Research Foundations of Sun Yat‐Sen University and Guangzhou Science Committee, No 1999‐J‐005‐01. | |
| Henan | 98 participants (55 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Shandong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA, and anti‐HBs. | Not stated. | |
| Shandong | 63 participants (32 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg and HBeAg | Not stated. | |
| Taizhou | 279 participants (159 intervention, 120 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBV‐DNA. | Not stated. | |
| Henan | 86 participants (46 intervention, 40 control) aged 22 to 28 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Supported by Technology Research Fund Committee of Henan province (No. 981170112). | |
| Shandong | 88 participants (44 intervention, 44 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA and anti‐HBs. 8‐month‐old babies positive for HBsAg, HBV‐DNA, and anti‐HBs. Maximum duration of surveillance: 8 months. Follow‐up time point: 8 months after birth. | Not stated. | |
| Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBeAg and HBV‐DNA. | Not stated. | |
| Jiangsu | 285 participants (163 intervention, 162 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg and HBV‐DNA | Not stated. | |
| Guangdong | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBeAg, anti‐HBs | Not stated. | |
| Shanghai | 83 participants (26 intervention I, 29 intervention II, 28 control) aged 20 to 33 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Guangxi | 61 participants (28 intervention, 33 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg | Not stated. | |
| Huizhou | 250 participants (117 intervention, 113 control) | Intervention: HBIG 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg; adverse effects of the immunoglobulins to the neonates and mothers | Supported by Huizhou Municipal Central hospital and Huizhou Science and Technology Bureau. | |
| Shanxi | 48 participants (34 intervention, 14 control) aged 20 to 33 years | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. | |
| Guangdong | 320 participants (163 intervention, 157 control) aged 19 to 36 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Guangdong | 184 participants (92 intervention, 92 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Shanghai | 204 participants (103 intervention, 101 control) aged 20 to 34 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg. | Not stated. | |
| Shanghai | 980 participants (487 intervention, 493 control) aged 19 to 35 years | Intervention: HBIG 200 IU or 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, and HBV‐DNA. | Supported by a grant from the Ministry of Public Health China (No. 97030223). |
anti‐HBc: anti‐hepatitis core; anti‐HBe: anti‐hepatitis B envelope; anti‐HBs: anti‐hepatitis B surface; HBIG: hepatitis B immunoglobulin; HBcAg: hepatitis B core antigen; HBeAg: hepatitis B envelope antigen; HBsAg: hepatitis B surface antigen; HBV‐DNA: hepatitis B virus DNA.
All the 36 included trials in this systematic review were randomised clinical trials published as full paper articles. We listed excluded studies in the Characteristics of excluded studies table with reasons for exclusion.
Results of the search
The search identified 1235 bibliographic references, 1196 through database searching and 39 through other sources.
We excluded 299 duplicates and screened the 936 remaining references. We excluded 870 clearly irrelevant references through reading of abstracts. Thus, we assessed 66 references for eligibility. After careful scrutiny, we excluded 30 of these references as they did not fulfil the inclusion criteria. Subsequently, 36 references describing 36 trials met the inclusion criteria for this systematic review.
The reference flow is shown in Figure 1.

Study flow diagram for searches on hepatitis B Immunoglobulin (HBIG).
Included studies
All the included trials were randomised clinical trials that compared HBIG with no intervention. None of the included trials compared HBIG with placebo.
Participants
The trials included 6044 participants. Most of the trials had specific inclusion criteria that included pregnant women who were HBsAg, HBeAg, or HBV‐DNA positive. Exclusion criteria included pregnant women who had abnormal liver function; women who tested positive for other hepatitis antigens such as A, C, E, and G; pregnant women with signs of threatened miscarriage/abortions, premature delivery, or pregnancy‐induced hypertension; women with medical/surgical complications of pregnancy and other pregnancy complications; and inability of the women to give informed consent for the trial. The age of participants in the included trials ranged from 17 to 46 years, with a mean of 24.6 years.
Apart from the universal inclusion criteria for these trials, which was pregnant women who tested positive for HBsAg or HBeAg, or both, 21 randomised clinical trials included pregnant women with normal liver function as inclusion criteria (Yue 1999; Chi 2002; Sui 2002; Chen 2003; Han 2003; Li 2003; Dai 2004; Li 2004; Lin 2004; Xu 2004; Yu 2005; Chen 2006a; Xu 2006; Yang 2006; Yuan 2006; Chen 2007; Liu 2007; Wang 2007; Wang 2008; Shi 2009; Xiao 2009). Seven trials considered women who tested negative to other hepatitis antigen such as A, C, D, E, and G as additional criteria for inclusion (Yue 1999; Sui 2002; Dai 2004; Li 2004; Chen 2006a; Li 2006; Wang 2007). Twelve trials included pregnant women with no signs of threatened miscarriage/abortions, premature delivery, and pregnancy‐induced hypertension as part of the inclusion criteria (Yue 1999; Chen 2003; Han 2003; Li 2003; Dai 2004; Luo 2004; Xu 2004; Yu 2005; Xu 2006; Chen 2007; Wang 2008; Xiao 2009). Seven trials considered women with no medical/surgical complications of pregnancy and other pregnancy complications as inclusion criteria (Chi 2002; Liang 2004; Zheng 2005; Yang 2006; Liu 2007; Wang 2007; Yu 2008). Six trials considered pregnant women with no any drug administration such as antiviral drugs, transfer factors, interferons, and immunomodulators as inclusion criteria, in addition to testing positive for HBsAg and HBeAg (Chi 2002; Ji 2003; Li 2004; Yuan 2006; Wang 2007; Wang 2008). In one trial, other inclusion criteria, apart from pregnant women who tested positive for HBeAg and HBsAg, were gestational age 28 weeks or less, exclusion of fetal anomalies by ultrasound scans, husbands who were not carriers of HBV, and the ability of the women to give informed consent for the trial (Li 2004).
Setting
All 36 trials were carried out in China. The regions where the trials were conducted were Guandong (Han 2003; Li 2003; Li 2004; Liang 2004; Yu 2005; Zheng 2005; Chen 2006a; Zhang 2007), Shenzhen (Chen 2007), Zhejiang (Chi 2002; Chen 2003; Ji 2003; Dai 2004; Wang 2008), Henan (Su 2000; Xing 2003; Guo 2006; Liu 2007), Shanghai (Zhu 1997; Zhu 2003; Lin 2004; Yu 2006; Ji 2007), Jiang Su (Jia 2001; Yang 2006), Hubei (Li 2006), Jiangxi (Luo 2004), Shandong (Sui 2002; Xu 2004; Wang 2007), Guangxi (Yu 2008), Guangzhou (Shi 2009), Shanxi (Yue 1999), Xinjiang (Xu 2006; Xiao 2009), and Huizhou (Yuan 2006).
Dose of hepatitis B immunoglobulin
Thirty‐one trials used a dose of HBIG 200 international units (IU) (Zhu 1997; Su 2000; Jia 2001; Chi 2002; Chen 2003; Han 2003; Ji 2003; Li 2003; Xing 2003; Dai 2004; Li 2004; Liang 2004; Lin 2004; Luo 2004; Xu 2004; Yu 2005; Zheng 2005; Chen 2006a; Guo 2006; Li 2006; Xu 2006; Yang 2006; Chen 2007; Ji 2007; Liu 2007; Wang 2007; Zhang 2007; Wang 2008; Yu 2008; Shi 2009; Xiao 2009). Two trials used HBIG 100 IU (Yue 1999; Sui 2002). Two trials used HBIG 400 IU HBIG (Yu 2006; Yuan 2006). One trial administered HBIG 200 IU, but used 400 IU for women who were both HBsAg‐ and HBeAg‐positive carriers (Zhu 2003).
Timing of hepatitis B immunoglobulin administration
The timing of administration of HBIG varied in the trials; 28 trials administered three doses of HBIG 200 IU at 28, 32, and 36 weeks of pregnancy (Zhu 1997; Su 2000; Jia 2001; Chi 2002; Chen 2003; Han 2003; Ji 2003; Li 2003; Xing 2003; Zhu 2003; Li 2004; Lin 2004; Luo 2004; Xu 2004; Yu 2005; Zheng 2005; Chen 2006a; Guo 2006; Xu 2006; Yu 2006; Chen 2007; Ji 2007; Liu 2007; Zhang 2007; Wang 2008; Yu 2008; Shi 2009; Xiao 2009). One trial administered HBIG 400 IU at 28, 32, and 36 weeks of pregnancy (Yuan 2006); one trial administered HBIG 200 IU at 32, 36, and 40 weeks of gestation (Li 2006);one trial administered HBIG 200 IU at 28, 30, 32, 34, 36, and 38 weeks of gestation (Yang 2006), at 30, 34, and 38 weeks of gestation (Dai 2004), and at 16, 20, 24, 28, 32, and 36 weeks of gestation (Wang 2007). Two trials administered HBIG 100 IU commencing from 20 weeks of gestation either at four‐weekly intervals (Sui 2002), or repeated at 22, 24, 26, 28, 30, 32, 34, 36, 37, 38, 39, and 40 weeks of gestation (Yue 1999). One trial administered HBIG at 12, 16, 20, 24, 28, 32, 36 and 40 weeks of gestation (Liang 2004) but did not indicate the dosing regimen.
Neonatal serological outcomes in the trials
Seven trials assessed and reported HBeAg in the neonates (Li 2003; Zhu 2003; Guo 2006; Xu 2006; Yang 2006; Yuan 2006; Wang 2007). Sixteen trials assessed and reported HBV‐DNA in the neonates (Sui 2002; Chen 2003; Zhu 2003; Dai 2004; Li 2004; Liang 2004; Luo 2004; Xu 2004; Zheng 2005; Li 2006; Xu 2006; Yang 2006; Yu 2006; Yu 2008; Shi 2009; Xiao 2009).
Fifteen trials reported just HBsAg in the neonates (Su 2000; Jia 2001; Chi 2002; Han 2003; Ji 2003; Xing 2003; Lin 2004; Liu 2007; Chen 2006a; Li 2006; Yang 2006; Chen 2007; Ji 2007; Zhang 2007; Wang 2008), and 11 trials reported HBsAg, HBeAg, or anti‐HBc (or a combination of these) (Zhu 1997; Yue 1999; Ji 2003; Xing 2003; Li 2003; Luo 2004; Yu 2005; Guo 2006; Yuan 2006; Wang 2007; Yu 2008), one trial reported HBeAg and HBV‐DNA (Xu 2006), two trials reported HBsAg, HBeAg and HBV‐DNA (Zhu 2003; Yang 2006), while five trials (Sui 2002; Dai 2004; Li 2004; Xu 2004; Shi 2009) reported HBsAg, HBV‐DNA, or anti‐HBs (or a combination of these).
Twenty‐nine trials reported HBsAg status in newborns at a median of 1.2 months of follow‐up after birth (range 0 to 12 months)(Zhu 1997; Yue 1999; Su 2000; Jia 2001; Chi 2002; Sui 2002; Han 2003; Ji 2003; Li 2003; Xing 2003; Zhu 2003; Li 2004; Lin 2004; Luo 2004; Xu 2004; Yu 2005; Chen 2006a; Guo 2006; Li 2006; Yang 2006; Yu 2006; Yuan 2006; Chen 2007; Ji 2007; Liu 2007; Zhang 2007; Wang 2008; Yu 2008; Shi 2009). Seven trials reported HBeAg status in newborns at a median 1.1 months of follow‐up after birth (range 0 to 12 months) (Li 2003; Zhu 2003; Guo 2006; Xu 2006; Yang 2006; Yuan 2006; Wang 2007).
Excluded studies
We excluded 30 studies for any of the following reasons: HBIG was administered to participants, but it was not a not randomised clinical trial (Goudeau 1983; Nair 1984; Chung 1985; Lo 1985; Theppisai 1987; Tsega 1988; Boutin 1990; Birnbaum 1992;Erdem 1994; Harold 1995; Boisier 1996; Euler 2003; Denis 2004; Zhu 2004; Zhang 2005; Chen 2006b; Pan 2006; Batham 2007; De Ruiter 2008; Da Conceicao 2009; Jonas 2009); women were pregnant, but they received both HBIG and hepatitis B vaccine together; women were pregnant but received HBIG and lamivudine (an antiretroviral medication); women received hepatitis B vaccine, HBIG, and lamivudine (Xu 2009); mothers only received only hepatitis B vaccine (Gupta 2003); a clinical review of hepatitis B in pregnancy (Edmunds 1996). We excluded five studies because the pregnant women did not receive HBIG during pregnancy, but their newborn babies received HBIG (Beasley 1981; Beasley 1983a; Beasley 1983b; Xu 1985; Esteban 1986). We excluded one trial because even though it was a randomised clinical trial using HBIG as the intervention and all women received HBIG, there was no placebo or control group (Xiao 2007). The treatment group (women with positive HBsAg and positive HBeAg) received HBIG while the second group (women with positive HBsAg and negative HBeAg) also received HBIG (see Characteristics of excluded studies table). We identified no ongoing studies.
Risk of bias in included studies
See Figure 2 and Figure 3 for detailed pictorial representation of the trials. From the analysis, all trials were classified at high risk of bias.
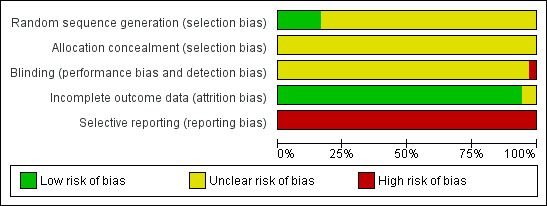
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
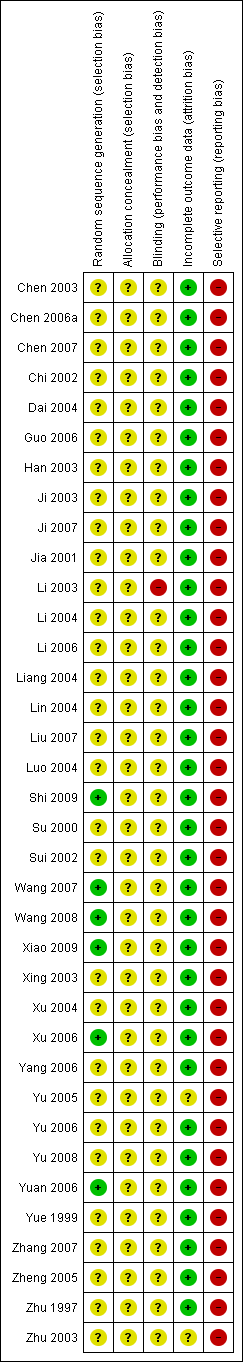
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Five trials reported adequate generation of allocation sequence (Xu 2006; Yuan 2006; Wang 2008; Shi 2009; Xiao 2009). In one of these trials, allocation sequence was computer‐generated (Yuan 2006). Generation of the allocation sequence was unclear in 31 trials (Zhu 1997; Yue 1999; Su 2000; Jia 2001; Chi 2002; Sui 2002; Chen 2003; Han 2003; Ji 2003; Li 2003; Xing 2003; Zhu 2003; Dai 2004; Li 2004; Liang 2004; Lin 2004; Luo 2004; Xu 2004; Yu 2005; Zheng 2005; Chen 2006a; Guo 2006; Li 2006; Yang 2006; Yu 2006; Chen 2007; Ji 2007; Liu 2007; Wang 2007; Zhang 2007; Yu 2008) (see Figure 3).
All 36 included trials were at unclear risk of bias regarding allocation concealment because the trials provided insufficient information to make a judgement.
Blinding
It was unclear in all 36 trials whether the investigators were blinded to assigning participants to treatment and control groups (see Figure 2). The impossibility of blinding investigators may have given rise to bias. Lack of blinding of participants could bias the results by affecting the actual outcomes of the participants in the trials. This may be due to lack of expectations in the control group, or due to differential behaviours across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co‐interventions). If the women were aware of the HBIG assignments, bias could also be introduced in the assessment of outcomes. Exchange of information between the intervention and control groups might have occurred as the intervention and control groups attended the same antenatal clinics. However, bias is likely to occur when people are provided health advice or asked to follow a protocol and maybe not in the situation when the intervention is an injection such as HBIG.
Incomplete outcome data
Thirty‐three out of 36 trials reported no dropout or withdrawal and so, all participants randomised were analysed (see Figure 2). Both Xiao 2009 and Xu 2006 trials had 8/52 (15.4%) mothers excluded according to the exclusion criteria, Zhu 2003 has unclear risk of bias in the incomplete outcome data since loss to follow‐up was not reported in the trial.
Selective reporting
None of the trials (including Zhu 2003) reported newborn and maternal mortality and morbidity and so all the trials were considered at high risk of selective reporting bias. Apart from the primary outcomes of the review, most included trials reported a range of other outcomes.
Vested interest (for‐profit) bias
Four out of 36 trials were at high risk of vested interest bias as they were sponsored by a pharmaceutical company (Shi 2009), or a group with a certain financial or other interest in a given result of the trials (Xing 2003; Zhu 2003; Yuan 2006). However, the remaining 32 trials were at unclear risk of vested interest bias because it was unclear how the trials were sponsored (Zhu 1997; Yue 1999; Su 2000; Jia 2001; Chi 2002; Sui 2002; Chen 2003; Han 2003; Ji 2003; Li 2003; Dai 2004; Li 2004; Liang 2004; Lin 2004; Luo 2004; Xu 2004; Yu 2005; Zheng 2005; Chen 2006a; Guo 2006; Li 2006; Yang 2006; Yu 2006; Yuan 2006; Chen 2007; Ji 2007; Liu 2007; Wang 2007; Zhang 2007; Wang 2008; Yu 2008; Xiao 2009).
Other potential sources of bias
All the trials were at unclear risk of other potential sources of bias such as baseline differences and early stopping.
Overall assessment of risk of bias
All trials were classified at high risk of bias.
Effects of interventions
Primary outcomes
All‐cause mortality or other serious adverse events of the newborn
None of the trials reported newborn mortality or other serious adverse events in the newborn.
All‐cause mortality or other serious adverse events of the mothers
None of the trials reported maternal mortality or other serious adverse events in the mother.
Serological signs of hepatitis B infection of the newborn
Newborn with hepatitis B surface antigen‐positive result
Twenty‐nine trials with 2769 participants in the intervention groups and 2541 participants in the control groups reported HBsAg as a primary outcome for HBV infection (Zhu 1997; Yue 1999; Su 2000; Jia 2001; Chi 2002; Sui 2002; Han 2003; Ji 2003; Li 2003; Xing 2003; Zhu 2003; Li 2004; Lin 2004; Luo 2004; Xu 2004; Yu 2005; Chen 2006a; Guo 2006; Li 2006; Yang 2006; Yu 2006; Yuan 2006; Chen 2007; Ji 2007; Liu 2007; Zhang 2007; Wang 2008; Yu 2008; Shi 2009). The results were reported after a median of 1.2 months of follow‐up after birth (range 0 to 12 months). Meta‐analysis of trials with treatment participants showed very low quality evidence that participants had a reduction in the transmission of HBsAg from mother to child favouring HBIG; 179/2769 (6%) participants with HBIG versus 537/2541 (21%) participants with no intervention tested positive for HBsAg (random‐effects model RR 0.30, 95% CI 0.24 to 0.38; I2 = 36%, P < 0.00001) (Analysis 1.1). We also used Trial Sequential Analysis to assess statistical significance. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) crossed the trial sequential monitoring boundary for benefit, thus yielding a robust statistically significant difference between HBIG and no intervention regarding the number of newborns with HBsAg‐positive results (Figure 4). However, all trials were at high risk of bias.
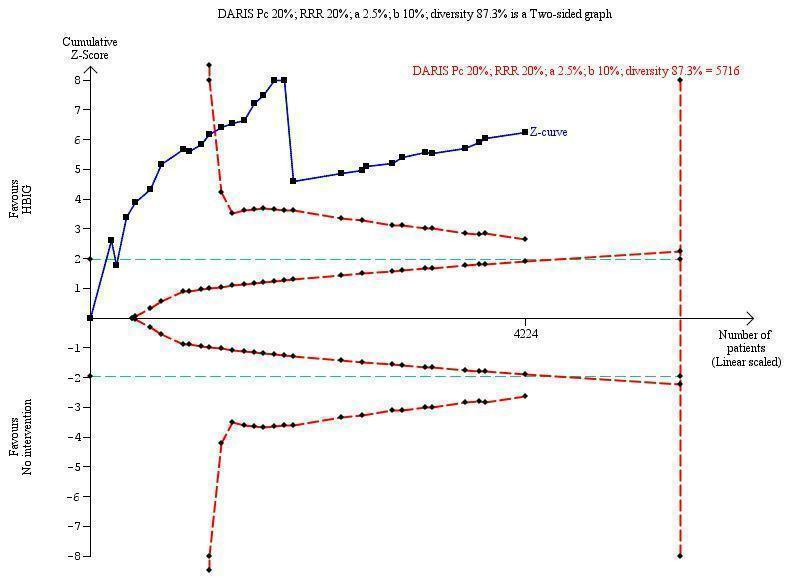
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with HBsAg‐positive results at end of follow‐up. The diversity‐adjusted required information size (DARIS) of 5716 participants was calculated based upon a proportion of 20% of babies tested positive for HBsAg in the control group, a relative risk reduction of a 20% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 87.3%. The actually accrued number of participants is 4224, which is 74% of the DARIS. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve crosses the red trial sequential monitoring boundary for benefit during the 11th trial. This implies that there is no risk of random error in the estimate of a beneficial effect of HBIG versus no intervention on the number of newborns with HBsAg‐positive results at end of follow‐up. The TSA‐adjusted confidence interval is 0.20 to 0.52.
Newborn with hepatitis B envelope antigen‐positive result
Seven trials with 889 participants in the intervention groups and 875 participants in the control groups reported HBeAg as a primary outcome for HBV infection (Li 2003; Zhu 2003; Guo 2006; Xu 2006; Yang 2006; Yuan 2006; Wang 2007). The results were reported after a median of 1.1 months of follow‐up after birth (range 0 to 12 months). Meta‐analysis of trials reporting on the number of newborns with HBeAg‐positive results showed no statistically significant difference between HBIG and no intervention; 184/889 (21%) participants with HBIG versus 232/875 (27%) participants with no intervention (random‐effects model RR 0.68, 95% CI 0.43 to 1.05; I2 = 90%, P = 0.08) (Analysis 1.2). Because our meta‐analysis did not reach the required information size (6492 participants), we used trial sequential monitoring boundaries, calculated with Trial Sequential Analysis, to adjust the thresholds for statistical significance accordingly. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) did not cross the trial sequential monitoring boundary for benefit, thus yielding an insignificant difference between the HBIG and no intervention regarding the number of newborns with HBeAg‐positive results at end of follow‐up (Figure 5).
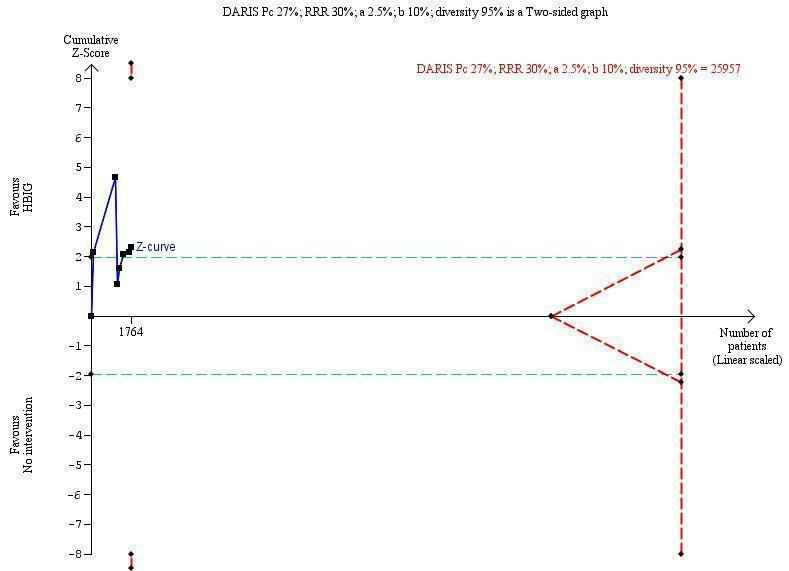
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with hepatitis B envelope antigen (HBeAg)‐positive results at end of follow‐up. The diversity‐adjusted required information size (DARIS) of 25,957 participants was calculated based upon a proportion of 27% of babies tested positive for HBeAg in the control group, a relative risk reduction of a 30% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 95%. The actually accrued number of participants is 1764, which is only 6.8% of the DARIS. (We planned to use a relative risk reduction of 20%, but this led to a DARIS of 60,715 participants and the TSA figure could not be drawn by the program; therefore, a relative risk reduction of 30% was adopted instead.) The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve does not cross the red inward sloping trial sequential monitoring boundaries for benefit or harm. Therefore, there is no evidence to support that HBIG influences number of newborns with HBeAg‐positive results at end of follow‐up. The cumulative Z‐curve does not reach the futility area, demonstrating that further trials are needed. The TSA‐adjusted confidence interval is wider than 0.04 to 6.37.
Newborns with antibodies to hepatitis B core antigen
None of the trials reported the effects of HBIG on antibodies to hepatitis B core antigen versus placebo or no intervention.
Newborn with hepatitis B virus DNA‐positive result
Sixteen trials with 996 participants in the intervention groups and 975 participants in the control groups reported HBV‐DNA as a primary outcome measure for HBV infection (Jia 2001; Sui 2002; Chen 2003; Zhu 2003; Dai 2004; Liang 2004; Luo 2004; Xu 2004; Zheng 2005; Li 2006; Yang 2006; Yu 2006; Ji 2007; Yu 2008; Shi 2009; Xiao 2009). The results were reported after a median of 1.2 months of follow‐up after birth (range 0 to 12 months). A total of 104/1112 (9%) participants with HBIG versus 382/1018 (38%) participants with no intervention were HBV‐DNA positive. The meta‐analysis showed low quality evidence in favour of HBIG versus no intervention in reducing transmission of HBV‐DNA from mother to child (random‐effects model RR 0.25, 95% CI 0.15 to 0.42; I2 = 84%, P < 0.00001) (Analysis 1.3). We also used Trial Sequential Analysis to assess statistical significance. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) crossed the trial sequential monitoring boundary for benefit, thus yielding a robust statistically significant difference between HBIG and no intervention regarding the number of newborns with HBV‐DNA‐positive results at end of follow‐up (Figure 6). However, all trials were at high risk of bias.
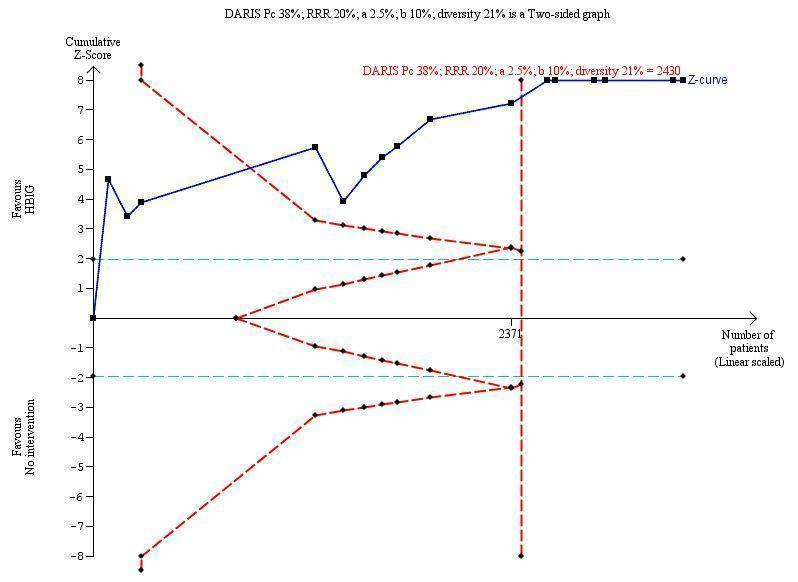
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with hepatitis B virus DNA (HBV‐DNA) positive results at end of treatment. The diversity‐adjusted required information size (DARIS) of n = 2430 participants was calculated based upon a proportion of 38% of babies tested positive for HBV‐DNA, a relative risk reduction of a 20% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 21%. The actually accrued number of participants is 2994, which is more than the DARIS of 2430 participants. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve crosses the red trial sequential monitoring boundary for benefit during the fourth trial. This implies that there is no risk of random error in the estimate of a beneficial effect of HBIG versus no intervention on the number of newborns with HBV‐DNA positive results at end of treatment. The TSA‐adjusted and 95% confidence intervals is from 0.22 to 0.37.
Subgroup analyses
Newborn hepatitis B surface antigen according to dosing regimen of hepatitis B immunoglobulin administration
Two trials administered HBIG 100 IU to prevent HBsAg transmission from mother to child (Yue 1999; Sui 2002). Out of 93 participants who received HBIG 100 IU, no (0%) newborn was HBsAg positive versus 14/66 (21%) newborns who received no intervention. The meta‐analysis showed statistically significant difference between the treatment group that received HBIG 100 IU and no intervention group on newborns with HBsAg‐positive results at end of follow‐up using both the fixed‐effect model and random‐effects model (fixed‐effect model RR 0.05, 95% CI 0.01 to 0.36; I2 = 0%, P = 0.003).
Twenty‐five trials administered HBIG 200 IU to prevent HBsAg transmission from mother to child (Zhu 1997; Su 2000; Jia 2001; Chi 2002; Chen 2003; Ji 2003; Li 2003; Xing 2003; Dai 2004; Li 2004; Lin 2004; Luo 2004; Xu 2004; Yu 2005; Chen 2006a; Li 2006; Yang 2006; Yu 2006; Chen 2007; Ji 2007; Liu 2007; Wang 2007; Zhang 2007; Wang 2008; Yu 2008). Out of 2043 participants who received HBIG 200 IU, 118 (6%) newborns were HBsAg positive versus 429/1812 (24%) newborns who received no intervention. The meta‐analysis showed a statistically significant difference between HBIG 200 IU and no intervention on newborns with HBsAg‐positive results at end of follow‐up (random‐effects model RR 0.26, 95% CI 0.21 to 0.33; I2 = 3%, P < 0.00001) (Analysis 2.1).
Two trials administered HBIG 400 IU to prevent HBsAg transmission from mother to child (Yu 2006; Yuan 2006). The meta‐analysis found no statistically significant between HBIG 400 IU and no intervention (fixed‐effect model RR 0.83, 95% CI 0.56 to 1.24; I2 = 67%, P = 0.36) (see Analysis 2.1).
Tests for subgroup differences showed significant differences in effect between trials assessing HBIG versus no intervention on newborn with HBsAg at end of follow‐up according to the dosing regimen of HBIG administration: 100 IU; 200 IU; and 400 IU (P = 0.02).
Newborn hepatitis B surface antigen according to timing of hepatitis B immunoglobulin administration
Twenty‐three trials administered HBIG to prevent HBsAg transmission from mother to child at 28, 32, and 36 weeks of gestation (Zhu 1997; Su 2000; Jia 2001; Chi 2002; Sui 2002; Chen 2003; Ji 2003; Li 2003; Xing 2003; Li 2004; Lin 2004; Luo 2004; Xu 2004; Yu 2005; Chen 2006a; Yang 2006; Yu 2006; Yuan 2006; Chen 2007; Ji 2007; Liu 2007; Zhang 2007; Yu 2008). Out of 1645 participants who received HBIG at gestational ages of 28, 32, and 36 weeks, 110 (7%) newborns were HBsAg positive versus 310/1433 (22%) newborns who received no intervention. The meta‐analysis showed a statistically significant difference between HBIG and no intervention at the end of follow‐up (random‐effects model RR 0.30, 95% CI 0.21 to 0.41; I2 = 45%, P < 0.00001) (see Analysis 3.1).
One trial administered HBIG to prevent HBsAg transmission from mother to child at 28, 30, 32 and 34 (Yang 2006); one trial at 30, 34, and 38 (Dai 2004); one trial at 32, 36, and 40 (Li 2006); one trial at 20, 22, 24, 26, 28, 30, 32, 34, 36 and 40 (Yue 1999); and one trial at 16, 20, 24, 28, 32 and 36 (Wang 2007) weeks of gestation. Apart from the 23 trials that reported HBsAg results in participants administered HBIG at gestational ages of 28, 32, and 36 weeks, no other timing (gestational age categories) was reported in more than one trial. Therefore, we could not perform a meta‐analysis on trials reporting subgroup differences of the newborn with HBsAg‐positive result at end of follow‐up according to timing of HBIG administration, other than that at gestational ages of 28, 32, and 36 weeks (see Analysis 3.1).
Tests for subgroup differences showed no significant difference in effect between trials assessing HBIG versus no intervention on newborns with HBsAg at end of follow‐up according to timing of HBIG administration (P = 0.05) (Analysis 3.1).
Newborn hepatitis B envelope antigen according to dosing regimen of hepatitis B immunoglobulin administration
None of the trials administered HBIG 100 IU to prevent HBeAg transmission from mother to child. However, four trials administered HBIG 200 IU to prevent HBeAg transmission from mother to child (Li 2003; Xu 2006; Yang 2006; Wang 2007). Out of 235 participants that received HBIG 200 IU, 102 (43%) newborns were HBeAg positive versus 115/203 (56%) newborns who received no intervention. The meta‐analysis found no statistically significant beneficial effect for HBIG in decreasing HBeAg in newborns (random‐effect model RR 0.54, 95% CI 0.26 to 1.12; I2 = 79%, P = 0.10). HBIG may not protect against HBeAg transmission; however, convincing lack of benefit could not be demonstrated due to considerable trial heterogeneity. One trial (Yuan 2006) administered HBIG 400 IU to prevent HBeAg transmission from mother to child (random‐effect model RR 1.27, 95% CI 0.51 to 3.18; I2 = not applicable, P = 0.61) (see Analysis 2.3).
Tests for subgroup differences showed no significant difference in effect between trials assessing HBIG versus no intervention on newborns with HBeAg at end of follow‐up according to dosing regimen of HBIG administration: 100 IU, 200 IU, and 200 IU to 400 IU (P = 0.15).
Newborn hepatitis B envelope antigen according to timing of hepatitis B immunoglobulin administration
Three trials administered HBIG to prevent HBeAg transmission from mother to child at 28, 32, and 36 weeks of gestation (Li 2003; Xu 2006; Yuan 2006). Twenty‐four out of 204 (11%) participants who received HBIG to prevent HBeAg transmission from mother to child at a gestational age of 28, 32, and 36 weeks had newborns who were HBeAg positive versus 44/215 (20%) participants who received no intervention. Meta‐analysis showed that administering HBIG at 28, 32, and 36 weeks did not significantly reduce MTCT of HBeAg (random‐effects model RR 0.59, 95% CI 0.26 to 1.32; I2 = 68%, P = 0.20). However, considering other gestational age (timing) of HBIG administration, one trial administered HBIG to prevent HBeAg transmission from mother to child at gestational ages of 28, 30, 32, 34, 36 and 38 weeks (Yang 2006), while another trial administered it at a gestational age of 16, 20, 24, 28, 32 and 36 weeks (Wang 2007). Since there was only one trial in each of these subgroups, we performed no meta‐analysis (see Analysis 3.3).
Tests for subgroup differences showed no significant difference in effect between trials assessing HBIG versus no intervention on newborns with HBeAg at end of follow‐up according to timing of HBIG administration (P = 0.44) (Analysis 3.3).
Newborn hepatitis B virus DNA according to dosing regimen of hepatitis B immunoglobulin administration
One trial administered HBIG 100 IU to prevent HBV‐DNA transmission from mother to child (Sui 2002). Six trials administered HBIG 200 IU to prevent HBV‐DNA transmission from mother to child (Chen 2003; Li 2004; Xu 2006; Yang 2006; Shi 2009; Xiao 2009). Out of 392 participants who received HBIG 200 IU 42 (11%) newborns were HBeAg positive versus 155/277 (56%) newborns who received no intervention. The meta‐analysis showed a statistically significant beneficial effect for HBIG 200 IU in decreasing HBsAg transmission of HBV‐DNA in newborns (random‐effects model RR 0.24, 95% CI 0.15 to 0.39; I2 = 56%, P < 0.00001) (see Analysis 2.2).
Tests for subgroup differences showed a significant difference in effect between trials assessing HBIG versus no intervention on newborn with HBV‐DNA at end of follow‐up according to dosing regimen of HBIG administration: 100 IU, 200 IU, and 200 IU to 400 IU (P = 0.67).
Newborn hepatitis B virus DNA according to timing of hepatitis B immunoglobulin administration
Twelve trials administered HBIG to prevent HBV‐DNA transmission from mother to child at 28, 32, and 36 weeks of gestation (Sui 2002; Chen 2003; Zhu 2003; Li 2004; Luo 2004; Xu 2004; Zheng 2005; Xu 2006; Yu 2006; Yu 2008; Shi 2009; Xiao 2009). Out of 641 participants who received HBIG, 73 (11%) newborns were HBV‐DNA positive versus 210/545 (39%) newborns who received no intervention. The meta‐analysis showed a statistically significant beneficial effect for HBIG when administered at gestational ages of 28, 32, and 36 weeks in decreasing HBsAg transmission of HBV‐DNA in the newborn (random‐effect model: RR 0.30, 95% CI 0.17 to 0.51; I2 = 81%, P < 0.0001). One trial administered HBIG to prevent HBsAg transmission of HBV‐DNA from mother to child at 28, 30, 32, 34, 36 and 38 weeks (Yang 2006), one trial at 30, 34, and 38 weeks (Dai 2004), one trial at 32, 36, and 40 weeks (Li 2006), and one trial at 12, 16, 20, and 24, 28, 32, 36, and 40 weeks (Liang 2004). As there was only one trial in each subgroup of timing of HBIG administration, we could not draw any conclusion or meta‐analysis (see Analysis 3.2).
Tests for subgroup differences showed a significant difference in effect between trials assessing HBIG versus no intervention on newborn with HBV‐DNA at end of follow‐up according to timing of HBIG administration (P = 0.02) (Analysis 3.2).
Secondary outcomes
Non‐serious adverse events of the babies
None of the trials reported non‐serious adverse events of the immunoglobulins on the babies.
Presence of local and systemic adverse events (serious and non‐serious) of the mothers
None of the trials reported the presence of local and systematic non‐serious adverse events on the mothers.
Cost‐effectiveness of treatment
None of the trials reported cost‐effectiveness of treatment.
Sensitivity analysis
We could not perform sensitivity analyses because all the included trials had unclear concealment of allocation and unclear blinding of outcome assessor.
Publication bias
We created funnel plots to obtain an overall picture of the trials that this review identified with respect to publication bias regarding the outcome of newborns with HBsAg‐positive serological results (Figure 7) or newborns with HBV‐DNA‐positive serological results (Figure 8). Tests for funnel plot asymmetry should be used only when there were at least 10 studies included in the meta‐analysis, because when there were fewer studies the power of the tests will be too low to distinguish chance from real asymmetry. Therefore, we could not construct funnel plots or perform tests for funnel plot asymmetry for newborns with HBeAg‐positive serological results, since only seven trials reported it. By visual inspection there were doubts about funnel asymmetry of Figure 7 and Figure 8. For each funnel plot, we chose a test for asymmetry in accordance with recent recommendations, and used a P < 0.10 to indicate statistical evidence of asymmetry. Hence, in newborns with HBsAg‐positive serological results, we examined the contour‐enhanced funnel plot of the 29 trials (see Figure 7). This showed symmetry, with small studies systematically having similar effect sizes with the larger studies (Peters' test for asymmetry, P = 0.478) (Peters 2006). Similarly, when we examined the contour enhanced funnel plot of the 16 trials that reported newborns with HBV‐DNA positive result, it also showed symmetry (Peters' test for asymmetry, P = 0.496) (Peters 2006). This also did not add strength to the notion that publication bias mechanisms might be operative in favour of HBIG‐lowering levels of HBV‐DNA in the serum.
Funnel plot of comparison: 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, outcome: 1.1 Newborn positive for HBsAg.
Funnel plot of comparison: 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, outcome: 1.3 Newborn positive for HBV‐DNA.
'Summary of findings' tables
We prepared a 'Summary of findings' table, summarising the results of all the reported outcomes (see Summary of findings table 1).
Discussion
Summary of main results
This systematic review included 36 randomised clinical trials with 6044 pregnant women. We identified no placebo‐controlled randomised clinical trials. Only seven trials reported inclusion of HBeAg‐positive mothers (Li 2003; Zhu 2003; Guo 2006; Xu 2006; Yang 2006; Yuan 2006, Wang 2007). All trials and trial results were at high risks of bias. None of the trials reposted mortality or other serious adverse events in the mothers or the neonates. Compared with no intervention, HBIG seemed to reduce MTCT of HBsAg (RR 0.30, 95% CI 0.24 to 0.38, 29 trials), HBV‐DNA (RR 0.25, 95% CI 0.15 to 0.42, 16 trials), but not HBeAg (RR 0.68, 95% CI 0.43 to 1.05, 7 trials) of the neonates. The median follow‐up after birth was only just above one month, which is too short. Regarding secondary outcomes, none of the included trials reported adverse events of the immunoglobulins on the neonates, presence of local and systemic adverse events (serious and non‐serious) on the mothers, and cost‐effectiveness of treatment. We did not assess harms reported in observational studies.
From this systematic review, it appeared that HBIG 100 IU or 200 IU is more effective in the prevention of MTCT of HBsAg than HBIG 400 IU, since there were statistically significant reductions in the levels of HBsAg in participants who received HBIG 100 IU (RR 0.05, 95% CI 0.01 to 0.36; 2 trials; I2 = 0%, P = 0.003) or 200 IU (RR 0.26, 95% CI 0.21 to 0.33; 25 trials; I2 = 3%, P < 0.00001) dose, but not at a dose of 400 IU (RR 0.67, 95% CI 0.30 to 1.53; 2 trials; I2 = 67%, P = 0.34). HBIG also appeared to be safe in pregnancy since there were no recorded incidences of rigours, rash, or dysfunction of the liver and kidney in the participants throughout administration and follow‐up periods.
Our meta‐analyses have showed a very low quality to low quality evidence that participants showed a reduction in the transmission of HBsAg, HBV‐DNA, and HBeAg compared with no intervention. Trial Sequential Analysis confirmed the meta‐analysis results for effects of HBIG on reducing the number of newborns with HBsAg‐ and HBV‐DNA‐positive results at the end of follow‐up. However, these outcomes are serological and should therefore be considered as surrogates. Therefore, more randomised placebo‐controlled trials may be needed before any conclusions about the effect of HBIG versus no intervention on the number of newborns with HBeAg‐positive results can be drawn. To the best of our knowledge, antenatal HBIG administration and combination treatment using HBIG plus active HBV immunisation (joint immune prophylaxis) in the newborn has not been compared with antenatal HBIG alone since it is considered unethical to withhold perinatal prophylaxis solely for research. Therefore, our systematic review has provided very low quality evidence for the benefits of HBIG administration in the antenatal period in addition to the combination treatment using HBIG plus active HBV immunisation (joint immune prophylaxis) in the newborn for preventing MTCT of HBV infection.
Overall completeness and applicability of evidence
This systematic review examined the evidence of 36 randomised clinical trials on HBIG compared with no intervention for the prevention of MTCT of HBV. All the included trials were available as full‐text publications. All trials and trial results were at high risks of bias. Therefore, the rather large benefits we demonstrated are likely to be a combination of real effects and overestimation of benefits due to bias. We are unable to determine how much is due to real effects and how much is bias. Moreover, we only assessed adverse effects based on the included randomised clinical trials. We did not assess quasi‐randomised studies or other observational studies for potential harms. The latter point is a shortcoming of this systematic review.
The evidence in this systematic review shows that HBIG seems to be effective in the prevention of MTCT of HBV. Having done this systematic review, certain conclusions have been reached. This systematic review suggests that HBIG could have a benefit when used for the prevention of MTCT of HBV, as well as preventing babies born to hepatitis B‐positive mothers from developing HBV markers of infection (HBsAg and HBV‐DNA). Therefore, based on the findings in this review, countries planning investments in a HBIG programme should exercise judgement on the expected magnitude of effectiveness vis‐a‐vis the cost of a vaccination programme (cost‐effectiveness analysis). Additionally, treatment options are advancing rapidly, and several new antiviral drugs apart from the HBIG have become available during the recent years. Evidence is accumulating that these antiviral therapies provide a cost‐effective means to reduce the morbidity and mortality associated with HBV infections, even in pregnancy (Buti 2013; Lu 2014; Ma 2014; Tsai 2014). However, this review was not designed to assess the effects of these agents, so we can draw no comparative conclusions.
Quality of the evidence
Risks of systematic errors and random errors
The quality of evidence reflects the extent to which the confidence in an estimate of effect is adequate to support recommendations (Guyatt 2008). The GRADE approach for assessing quality of evidence in systematic reviews involves making separate ratings for quality of evidence in each of the five aspects (risk of bias, inconsistency, imprecision, indirectness, and publication bias), and identifies how each of the five factors lowers the quality of the evidence (Guyatt 2008). Considering these five factors in this systematic review; first, all trials were at unclear risk of bias so our results may overestimate benefits and underestimate harms. We used the most conservative result, which takes account of the problems of fixed‐effect and random‐effects meta‐analyses (Jakobsen 2014). The main limitations in the reporting of the trials were the lack of clarity of the random sequence generation, lack of concealment of allocation, lack of blinding, and selective reporting bias. This review included 36 trials and only 6044 participants. Almost all our results had unclear allocation concealment and unclear blinding and so each of the aspects was downgraded by 1 level for serious risk of bias. There was also high risk of selective reporting in all the studies. However, the risk of bias is known to impact the estimated intervention effect. Trials with high risk of bias tend to overestimate beneficial effects and underestimate harmful effects. As we included trials more than 10 trials, we could assess the risk of publication bias as having high risk of selective reporting bias. This is a clear limitation of our review. Therefore, overall, the quality of evidence of this systematic review using the GRADE approach was of very low and low quality on maternal and newborn relevant outcomes, such as HBsAg and HBV‐DNA compared with no treatment. These effects passed the test of Trial Sequential Analysis. Accordingly, there is no imprecision regarding these outcomes. In contrast, Trial Sequential Analysis did not remove any bias. The evidence for lack of benefit of antenatal HBIG administration on maternal and newborn HBeAg compared with no intervention was of very low quality. The blue cumulative Z‐curve did not cross the red trial sequential monitoring boundary for benefit or harm or reach the area of futility (Figure 5). Accordingly, there was imprecision. This implies that more randomised clinical trials to estimate the beneficial effect of HBIG versus no intervention on the number of newborns with serological and clinical hepatitis B may be needed. Hence, from this systematic review, we found very low quality evidence or low quality evidence for the benefit of antenatal HBIG administration to the mother on the newborn's serological status compared to no treatment (Summary of findings table 1).
Heterogeneity
Heterogeneity among trials might be due, for example, to differences in timing and dose of HBIG, and to different reactions of the participants, all of which might affect the effects of HBIG in pregnancy. To reflect our concern about heterogeneity, we conducted all analyses using both a fixed‐effect analysis and a random‐effects analysis. Results from the two models differed only for the comparisons at subgroup analyses of newborn HBeAg at end of follow‐up according to 200 IU dose and timing of the HBIG at a gestational age of 28, 32, and 36 weeks. In such cases where there were statistically significant discrepancies in the results, we reported the more conservative point estimate (the analysis with the highest P value) of the two (Jakobsen 2014). Meta‐analysis of trials reporting on the number of newborns with HBeAg‐positive results showed considerable heterogeneity with an I2 statistic of 90%.
Low‐dose compared to high‐dose hepatitis B immunoglobulin subgroup analyses
Two trials used a higher dose (400 IU) of HBIG than the standard dose of 100 IU (two trials) or 200 IU (25 trials). The trials provided no information on adverse events. Therefore, more clinical trials of higher HBIG doses versus low HBIG doses may be needed to examine the potential of using high‐dose HBIG during pregnancy. Additionally, from this systematic review, it appears that low‐dose HBIG seems to work better than high dose. The reason for this finding is intricate. Random error is a possibility here, as we found no evidence of fraud. In one study of HBIG administration, the efficacy of high‐dose HBIG plus lamivudine combination therapy appeared similar to that of the low‐dose protocol (Angus 2000). However, the major disadvantage of high‐dose HBIG combination therapy is the cost.
Potential biases in the review process
There were some potential biases in the review process. While assessing trials for inclusion, we may have omitted some trials that used HBIG in the prevention of MTCT of HBV, as authors may not have included any of the search terms in their title or abstract. Also, the review included 36 trials, of which the majority had various methodological limitations capable of increasing the risk of bias, hence compromising the strength of evidence. A limitation of the randomised clinical trials used for this systematic review was that the maternal hepatitis B immunoglobulin levels were not consecutively measured. This might have been helpful in estimating the actual serum levels of the immunoglobulin needed for optimal prevention of MTCT of HBV. Another limitation of this review was the timing of the newborn testing at end of follow‐up (median 1.1/1.2 months (range 0 to 12 months), as planned, because the presence of both HBsAg and HBV‐DNA at birth are often transitory events and may not imply transmission of the infection (Papaevangelou 2012; Yin 2013). Vertical transmission of HBV is defined as positivity to HBsAg or HBV‐DNA in an infant at six to 12 months of life born to an infected mother (Borgia 2012; Yin 2013). Thus, detection of the infection when the child is six months old correlates with infection when the child is one year old and indicates chronicity of the infection (Yin 2013; Xu 2014). Additionally, the included randomised clinical trials could not distinguish the effects of antenatal HBIG administration in HBsAg‐positive and HBeAg‐negative mothers versus HBsAg‐positive and HBeAg‐positive mothers. HBeAg positivity is an independent risk factor for the mother‐to‐child‐transmission of HBV (Borgia 2012; Yi 2016). Factually, HBeAg can pass through the placenta via partial leakage from the placenta or through the 'cellular route' (Borgia 2012). The absence of HBeAg expression is associated with lower levels of viral replication and with a significantly lower risk of intrauterine transmission (Borgia 2012; Yi 2016). It is possible that antenatal HBIG administration could benefit maternal and newborn relevant outcomes, such as HBsAg and HBV‐DNA in HBsAg‐positive and HBeAg‐negative mothers but not in HBsAg‐positive and HBeAg‐positive mothers.
We did not plan HBV‐DNA laboratory result as an outcome in the protocol (post hoc analysis). Except for HBV‐DNA (which is a genuine marker of infectivity), the results on the third primary outcome (serological signs of hepatitis B infection of the newborn) are surrogate outcomes and we need to be critical while interpreting the findings. According to Guyatt 2011, these phenomena constitute indirectness of evidence. Guyatt 2011 states that direct evidence comes from research that directly compares the interventions in which we are interested when applied to the populations in which we are interested and measures outcomes important to patients. Evidence can be indirect if the outcomes differ from those of primary interest; for instance, surrogate outcomes that are not themselves important, but measured on the presumption that changes in the surrogate reflect changes in an outcome important to patients. When making comparisons between treatments under this circumstance, they are rated down in quality one or two levels depending on the extent of differences between the participant populations, co‐interventions, measurements of the outcome, and the methods of the trials of the interventions. This is because the commonly used markers to determine chronic infection with HBV are HBsAg, HBV‐DNA, and HBcAb. Following acute hepatitis B infection, HBsAg and HBcAb commonly become detectable in the serum; both may remain in the serum even after viral clearance (Almeida 2001; Bolarinwa 2015). Based on this, both markers (HBsAg and HBcAb) are used as evidence of previous exposure to the virus. Although it was previously thought that HBeAg was a surrogate marker for the presence of HBV‐DNA, and people who were negative to the HBeAg were thought to have attained viral clearance. Later discoveries refuted this statement (Hadziyannis 1995), and in 2001 Hadziyannis and Vassilopoulos revealed that people whose results were positive for HBeAb and HBsAg but negative for HBeAg may require further evaluation for the presence of HBV‐DNA and serum transaminases to distinguish them from people with inactive HBsAg carrier state (Hadziyannis 2001).
Another major limitation of the study was that even though the trials were all randomised clinical trials, they were all carried out in China. Hence, we need to be cautious when we generalise the findings of this review to other parts of the world. The review methods did not allow for detection of serious or rare adverse events since authors of the trials in this review did not report them even though they may have existed (selective reporting). None of the included trials compared antibodies to hepatitis B core antigen versus placebo or no intervention. Since maternal antibodies to HBcAb may persist for more than one year, testing for HBcAb may be difficult to interpret during this period and so this may be the reason why none of the included trials reported it.
Agreements and disagreements with other studies or reviews
Evidence concerning the efficacy of hepatitis B immunoglobulin in preventing MTCT of HBV is in tandem with the findings from one systematic review (Shi 2010b). This is because 33 of the 36 trials included in the present review (with the exception of Wang 2007; Wang 2008; and Xiao 2009 trials), were also included in a review of hepatitis B immunoglobulin injection in pregnancy to interrupt HBV MTCT in 2010 (Shi 2010b), which included 37 studies. The authors of this Shi 2010b review concluded that there is "strong evidence that multiple small doses of HBIG injection in late pregnancy, along with joint immune prophylaxis (strategy aimed at using both HBIG and HB vaccine together (Wong 2014)) beginning after birth, is effective and safe in the interruption of HBV intrauterine infection and mother‐to‐ child transmission in HBV carrier mothers with a high degree of infectiousness compared with joint immune prophylaxis (strategy aimed at using both HBIG and HB vaccine together (Wong 2014)) alone. For asymptomatic HBV carrier mothers, they also recommend HBIG administration as a complementary treatment to the routine immune prophylaxis in their newborns, beginning after birth."
In our present review, we did not include four of the 37 studies included by Shi 2010b review for various reasons (Zhu 2004; Zhang 2005; Chen 2006b; Pan 2006). For example, the four studies were not randomised clinical trials on HBV. One of the studies included in the Shi 2010b review stated in the abstract that "…later their infants were randomly divided into two groups" (Zhu 2004). However, in the methods section of the full article of the Zhu 2004 trial, they stated "pregnant women included were allocated into two groups according to their willingness either based on doctor's recommendation before birth or not."
One Cochrane systematic review found no randomised clinical trials that assessed the effects of hepatitis B vaccine during pregnancy for preventing infant infection, and using vaccine is different from using HBIG (Sangkomkamhang 2014).
One meta‐analysis of randomised clinical trials determined the clinical efficacy of various immune interventions on MTCT of HBV by retrieving different immune strategies on how to prevent MTCT reported in the literature from Chinese and English electronic databases from the viewpoint of intrauterine and extrauterine prevention (Jin 2014). The authors of the Jin 2014 meta‐analysis included 25 articles on intrauterine prevention and 16 on extrauterine prevention. Of the 25 articles in intrauterine prevention, seven were not included in the present review (Sun 2007; Zhao 2008; Liu 2009; Yan 2009; Yuan 2009; Cui 2011; Li 2013). While Liu 2009 and Yuan 2009 were not actually randomised clinical trials, we contacted the authors of the other five, but they did not reply (Sun 2007; Zhao 2008; Yan 2009; Cui 2011; Li 2013). Nevertheless, the results of the Jin 2014 meta‐analysis showed that intrauterine prevention could reduce infants' HBV infection rates (RR 0.36, 95% CI 0.28 to 0.45) and increase their anti‐hepatitis B surface positive rate (RR 2.42, 95% CI 1.46 to 4.01) at birth. The Jin 2014 meta‐analysis further revealed that compared with passive immunisation, passive‐active immunisation could reduce infants' HBV infection rates (RR 0.66, 95% CI 0.52 to 0.84) at birth, even at more than 12 months of age (RR 0.54, 95% CI 0.42 to 0.69) and their subgroup analysis demonstrated similar results. The authors of the Jin 2014 meta‐analysis concluded that the long‐term protective effect of pregnant women injected with HBIG during pregnancy should be further validated by large‐scale randomised trials and newborns of pregnant women who carried HBV should undergo a passive‐active immunisation strategy.
The authors of Xu 2014 performed a meta‐analysis to compare the effects of three measures for prevention of MTCT using randomised clinical trials and non‐randomised studies comparing five groups of pregnant women: HBIG administration, antiviral treatment, placebo, elective caesarean section, and vaginal delivery. Of the 37 references included in the Xu 2014 meta‐analysis, only eight references were related to HBIG (Zhu 1997; Yue 1999; Li 2003; Zhu 2003; Li 2004; Xu 2006; Yuan 2006; Wang 2008). We included these eight trials in our present review. The results of the Xu 2014 meta‐analysis revealed that, compared with the control group, the incidence of HBV intrauterine infection (RR 0.42, 95 % CI 0.27 to 0.64; P < 0.0001) and the number of infants with chronic hepatitis B (RR 0.44, 95 % CI 0.32 to 0.61; P < 0.00001) were lower in the HBIG group.
In a systematic review by Zhou 2012, published in Chinese, the authors evaluated the effects of HBIG intrauterine injection before delivery on interrupting MTCT of HBV using randomised clinical trials published between January 1992 and May 2012. We included all of the 12 RCTs included in the Zhou 2012 systematic review in our present review (Zhu 1997; Su 2000; Jia 2001; Chi 2002; Han 2003; Ji 2003; Li 2003; Zhu 2003; Dai 2004; Yuan 2006, Xu 2006; Ji 2007). The results indicated that the infant HBV infection rates in the HBIG group was 9.0% and in the control group was 25.5% (RR 0.36, 95% CI 0.30 to 0.43) at birth and funnel graphs showed that there was publication bias. Zhou 2012 concluded that injection of HBIG during pregnancy for HBV‐carrying mothers can effectively reduce the occurrence of HBV at birth.
One non‐systematic review co‐authored by Shi and published in 2014 (Ma 2014) stated "There is insufficient data to demonstrate major [harm] congenital malformation caused by HBIG in pregnant women. Because HBIG is recommended by WHO for newborns whose mothers are HBV carriers, and because most of the fetal organs are fully developed when HBIG was applied to the pregnant women (from the beginning of the third trimester), theoretically, three doses of 200 IU HBIG injection should be safe in late pregnancy with respect to congenital malformation”. In addition, the authors of Shi 2010b stated: “Few randomised clinical trials reported sufficiently on adverse events. It should also be noted that HBIG and the plasma‐derived HBVac have the potential for transmission of blood‐borne infections. Randomised clinical trials may overlook adverse events because of the relatively low numbers of participants or poor reporting of adverse events." They did not cite any reference among the 37 trials as having reported any harms. Therefore, it appears that their statements on harms may be speculation or extrapolation. This is also in line with the Ma 2014 non‐systematic review which concluded, "The efficacy of HBIG in HBV‐carrying mothers with differing severity of infectious status, and HBV mutant‐generating effects, are important safety issues that remain to be resolved."
Antivirals may have a role in preventing transmission during pregnancy. This is because the role of antivirals in addition to at‐birth prophylaxis of newborns of HBV‐infected mothers have been revealed in some published studies to be effective in reducing MTCT of HBV (Han 2011; Pan 2012; Celen 2013; Ayres 2014; Gentile 2014a; Gentile 2014b; Lu 2014; Tsai 2014).
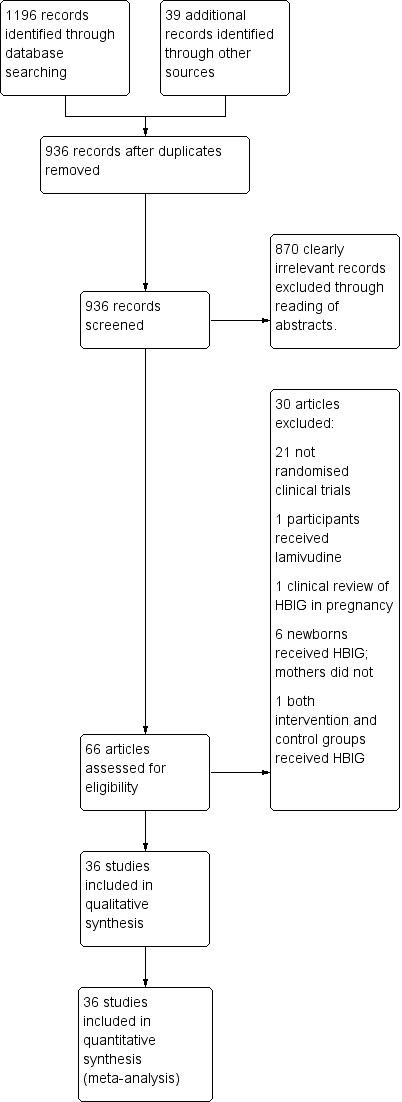
Study flow diagram for searches on hepatitis B Immunoglobulin (HBIG).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
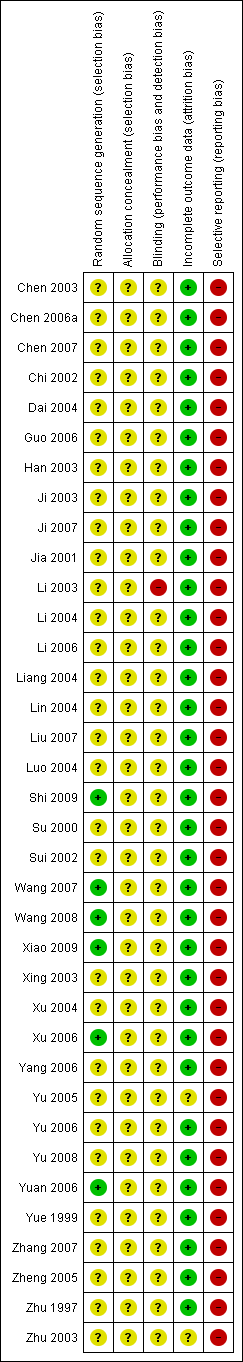
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
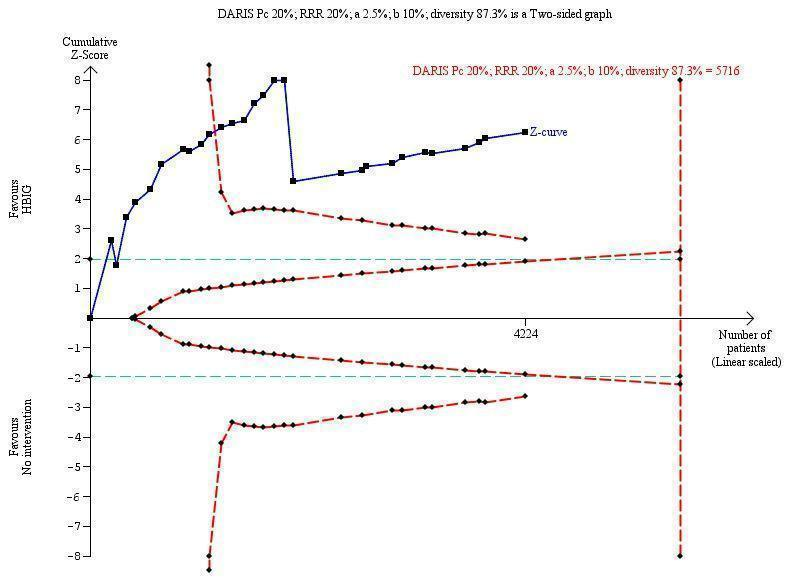
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with HBsAg‐positive results at end of follow‐up. The diversity‐adjusted required information size (DARIS) of 5716 participants was calculated based upon a proportion of 20% of babies tested positive for HBsAg in the control group, a relative risk reduction of a 20% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 87.3%. The actually accrued number of participants is 4224, which is 74% of the DARIS. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve crosses the red trial sequential monitoring boundary for benefit during the 11th trial. This implies that there is no risk of random error in the estimate of a beneficial effect of HBIG versus no intervention on the number of newborns with HBsAg‐positive results at end of follow‐up. The TSA‐adjusted confidence interval is 0.20 to 0.52.
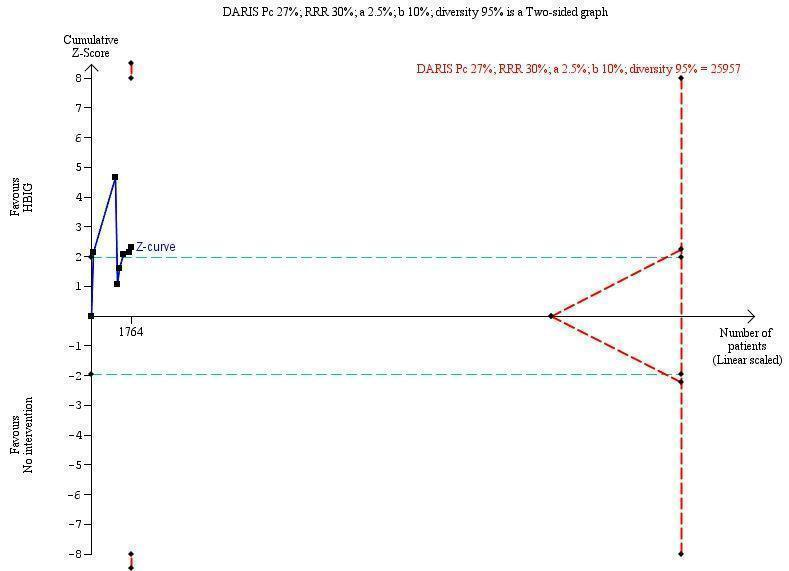
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with hepatitis B envelope antigen (HBeAg)‐positive results at end of follow‐up. The diversity‐adjusted required information size (DARIS) of 25,957 participants was calculated based upon a proportion of 27% of babies tested positive for HBeAg in the control group, a relative risk reduction of a 30% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 95%. The actually accrued number of participants is 1764, which is only 6.8% of the DARIS. (We planned to use a relative risk reduction of 20%, but this led to a DARIS of 60,715 participants and the TSA figure could not be drawn by the program; therefore, a relative risk reduction of 30% was adopted instead.) The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve does not cross the red inward sloping trial sequential monitoring boundaries for benefit or harm. Therefore, there is no evidence to support that HBIG influences number of newborns with HBeAg‐positive results at end of follow‐up. The cumulative Z‐curve does not reach the futility area, demonstrating that further trials are needed. The TSA‐adjusted confidence interval is wider than 0.04 to 6.37.
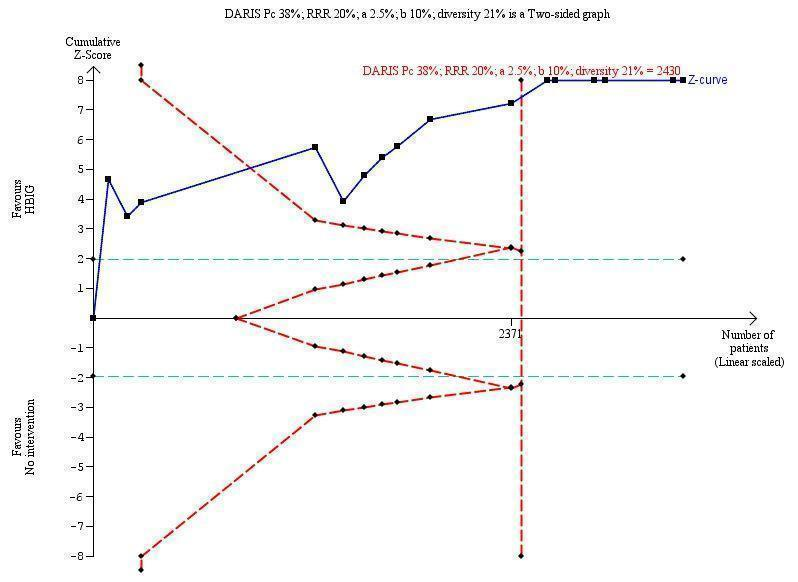
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with hepatitis B virus DNA (HBV‐DNA) positive results at end of treatment. The diversity‐adjusted required information size (DARIS) of n = 2430 participants was calculated based upon a proportion of 38% of babies tested positive for HBV‐DNA, a relative risk reduction of a 20% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 21%. The actually accrued number of participants is 2994, which is more than the DARIS of 2430 participants. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve crosses the red trial sequential monitoring boundary for benefit during the fourth trial. This implies that there is no risk of random error in the estimate of a beneficial effect of HBIG versus no intervention on the number of newborns with HBV‐DNA positive results at end of treatment. The TSA‐adjusted and 95% confidence intervals is from 0.22 to 0.37.
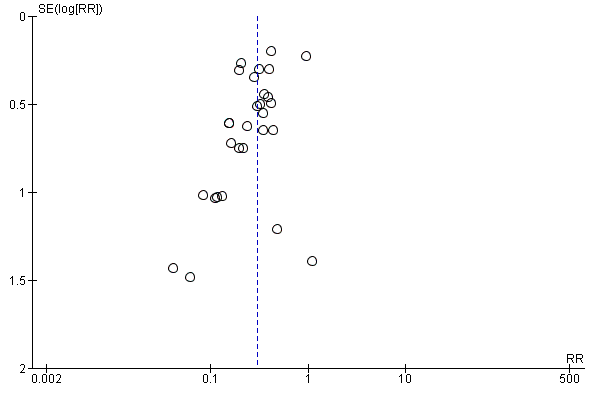
Funnel plot of comparison: 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, outcome: 1.1 Newborn positive for HBsAg.
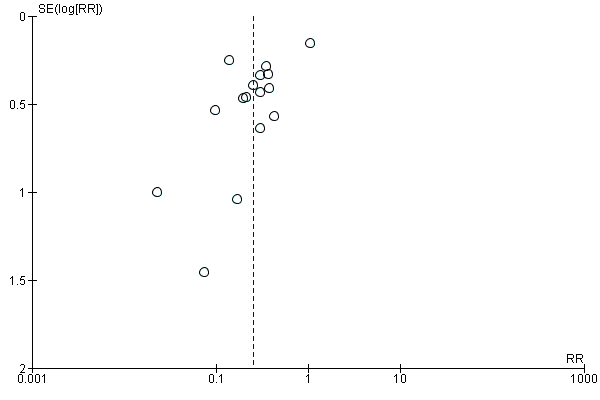
Funnel plot of comparison: 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, outcome: 1.3 Newborn positive for HBV‐DNA.

Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 1 Newborn positive for HBsAg.
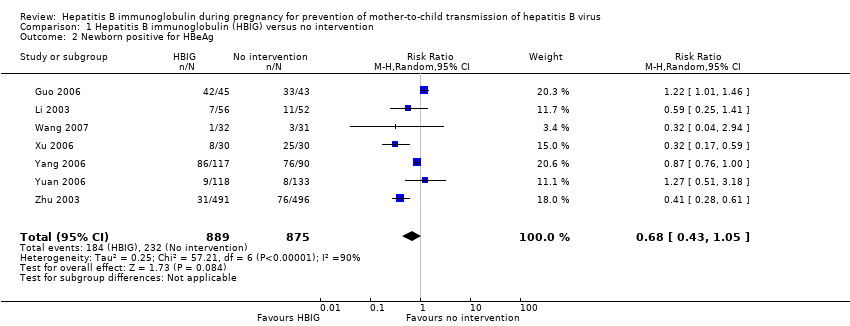
Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 2 Newborn positive for HBeAg.

Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 3 Newborn positive for HBV‐DNA.

Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 1 Newborn positive for HBsAg.
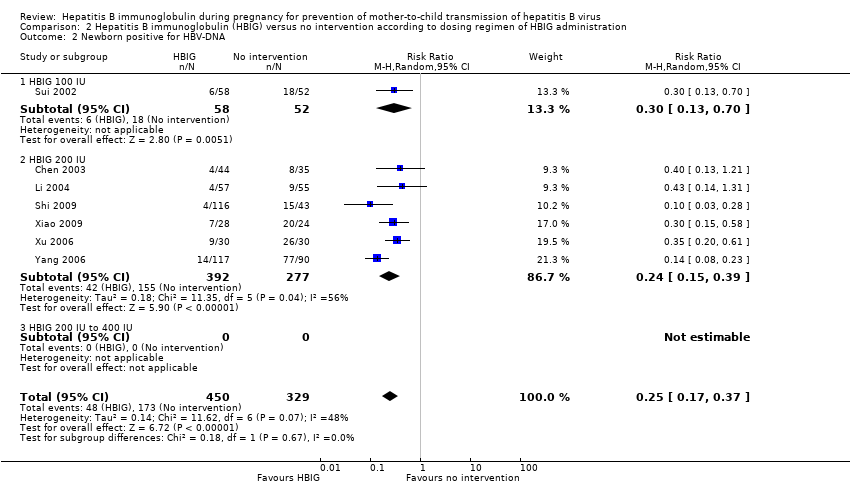
Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 2 Newborn positive for HBV‐DNA.
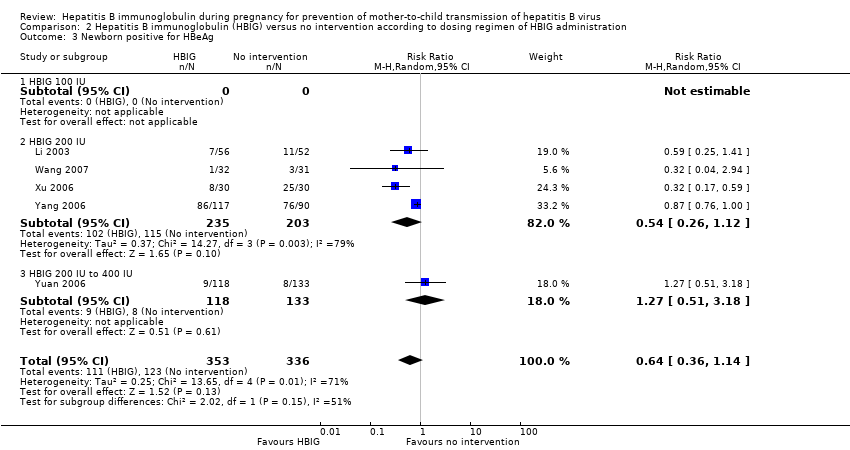
Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 3 Newborn positive for HBeAg.
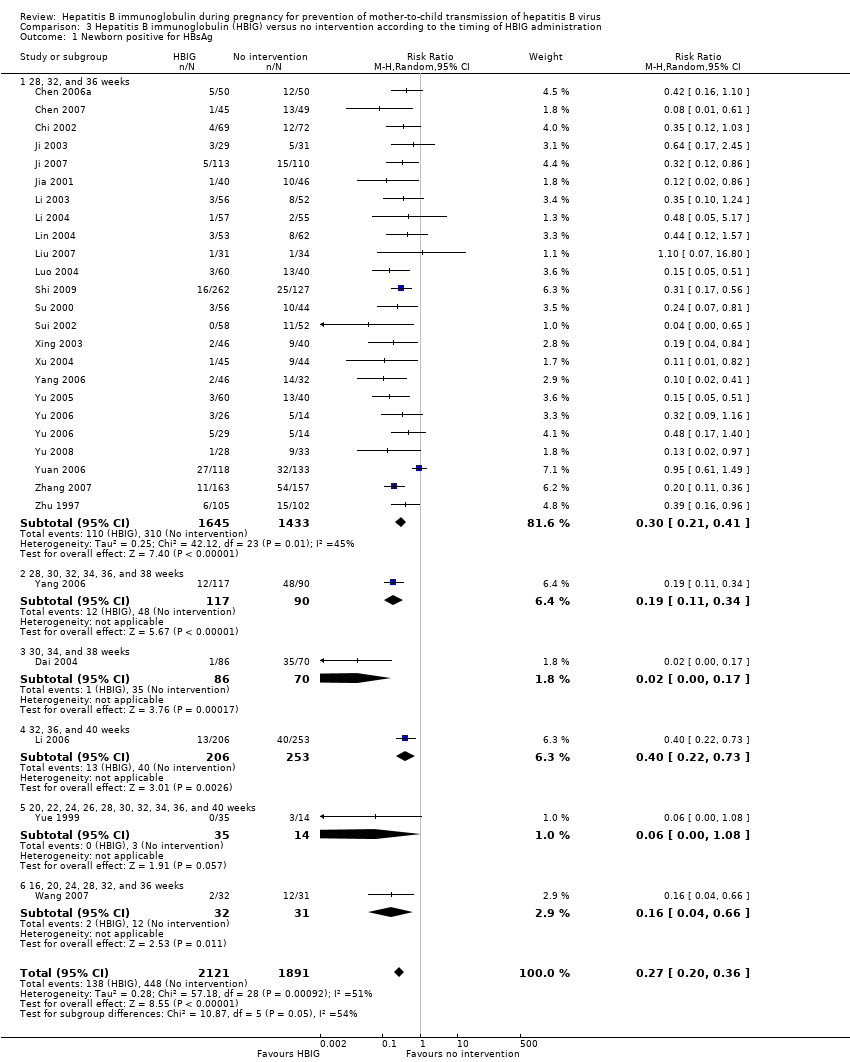
Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 1 Newborn positive for HBsAg.
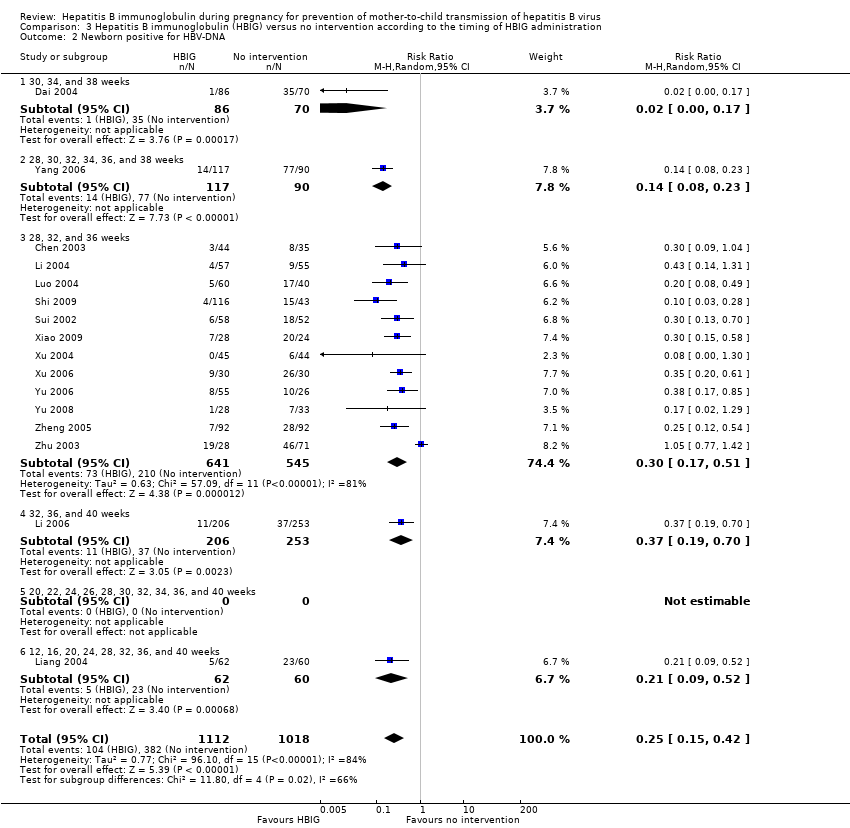
Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 2 Newborn positive for HBV‐DNA.
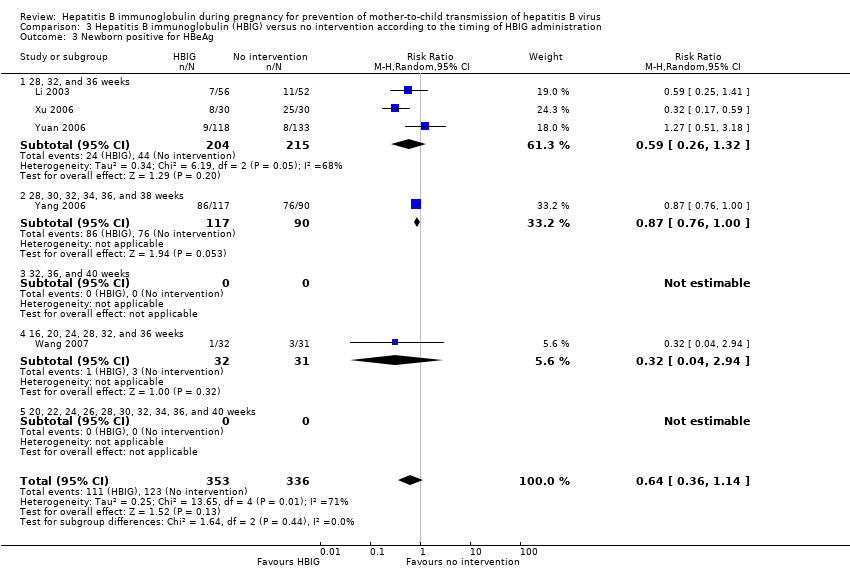
Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 3 Newborn positive for HBeAg.
| Hepatitis B immunoglobulin (HBIG) vs no intervention for prevention of mother‐to‐child transmission of hepatitis B virus | ||||||
| Participants: pregnant women positive for HBsAg or positive for HBeAg, or both. Comparison: no intervention. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | HBIG versus no intervention | |||||
| All‐cause mortality or other serous adverse events of the newborn | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| All‐cause mortality or other serous adverse events of the mothers | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Newborn with HBsAg‐positive result | Study population | RR 0.3 | 5310 | ⊕⊝⊝⊝ | ||
| 211 per 1000 | 63 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 64 per 1000 | |||||
| Newborn with HBeAg‐positive result | Study population | RR 0.68 | 1764 | ⊕⊝⊝⊝ | ||
| 265 per 1000 | 180 per 1000 | |||||
| Moderate | ||||||
| 212 per 1000 | 144 per 1000 | |||||
| Newborn with HBV‐DNA‐positive result | Study population | RR 0.25 | 2130 | ⊕⊕⊝⊝ | ||
| 375 per 1000 | 94 per 1000 | |||||
| Moderate | ||||||
| 366 per 1000 | 91 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Comment: Data for this outcome was not reported in any of the included trials. | ||||||
| Study ID | Study location | Participants | Interventions | Outcomes | Funding |
| Zhejiang | 79 participants (44 intervention, 35 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Guangdong | 100 participants (50 intervention, 50 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Shenzhen | 94 participants (45 intervention, 49 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. | |
| Zhejiang | 141 participants (69 intervention, 72 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Zhejiang | 156 participants (86 intervention, 70 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, anti‐HBs | Not stated. | |
| Henan | 88 participants (45 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg anti‐HBs. | Not stated | |
| Guangdong | 216 participants (126 intervention, 90 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Zhejiang | 60 participants (29 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs, adverse events. | Not stated | |
| Shanghai | 223 participants (113 intervention, 110 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Jiangsu | 86 participants (40 intervention, 46 control) aged 22 to 32 years. | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Guangdong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBeAg, or both. | Not stated. | |
| Guangdong | 112 participants (57 intervention, 55 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both. | Not stated. | |
| Hubei | 448 participants (202 intervention, 246 control) aged 18 to 38 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. | |
| Guangdong | 122 participants (62 intervention, 60 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA. | Not stated. | |
| Shanghai | 117 participants (55 intervention, 62 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Henan | 86 participants (43 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Jiangxi | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. | |
| Guangzhou | 389 participants (262 intervention, 127 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both | Research supported by GlaxoSmithKline Research and Development Grant NUC30914; Science and Research Foundations of Sun Yat‐Sen University and Guangzhou Science Committee, No 1999‐J‐005‐01. | |
| Henan | 98 participants (55 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Shandong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA, and anti‐HBs. | Not stated. | |
| Shandong | 63 participants (32 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg and HBeAg | Not stated. | |
| Taizhou | 279 participants (159 intervention, 120 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBV‐DNA. | Not stated. | |
| Henan | 86 participants (46 intervention, 40 control) aged 22 to 28 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Supported by Technology Research Fund Committee of Henan province (No. 981170112). | |
| Shandong | 88 participants (44 intervention, 44 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA and anti‐HBs. 8‐month‐old babies positive for HBsAg, HBV‐DNA, and anti‐HBs. Maximum duration of surveillance: 8 months. Follow‐up time point: 8 months after birth. | Not stated. | |
| Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBeAg and HBV‐DNA. | Not stated. | |
| Jiangsu | 285 participants (163 intervention, 162 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg and HBV‐DNA | Not stated. | |
| Guangdong | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBeAg, anti‐HBs | Not stated. | |
| Shanghai | 83 participants (26 intervention I, 29 intervention II, 28 control) aged 20 to 33 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Guangxi | 61 participants (28 intervention, 33 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg | Not stated. | |
| Huizhou | 250 participants (117 intervention, 113 control) | Intervention: HBIG 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg; adverse effects of the immunoglobulins to the neonates and mothers | Supported by Huizhou Municipal Central hospital and Huizhou Science and Technology Bureau. | |
| Shanxi | 48 participants (34 intervention, 14 control) aged 20 to 33 years | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. | |
| Guangdong | 320 participants (163 intervention, 157 control) aged 19 to 36 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Guangdong | 184 participants (92 intervention, 92 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Shanghai | 204 participants (103 intervention, 101 control) aged 20 to 34 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg. | Not stated. | |
| Shanghai | 980 participants (487 intervention, 493 control) aged 19 to 35 years | Intervention: HBIG 200 IU or 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, and HBV‐DNA. | Supported by a grant from the Ministry of Public Health China (No. 97030223). | |
| anti‐HBc: anti‐hepatitis core; anti‐HBe: anti‐hepatitis B envelope; anti‐HBs: anti‐hepatitis B surface; HBIG: hepatitis B immunoglobulin; HBcAg: hepatitis B core antigen; HBeAg: hepatitis B envelope antigen; HBsAg: hepatitis B surface antigen; HBV‐DNA: hepatitis B virus DNA. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 29 | 5310 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.24, 0.38] |
| 2 Newborn positive for HBeAg Show forest plot | 7 | 1764 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.05] |
| 3 Newborn positive for HBV‐DNA Show forest plot | 16 | 2130 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 28 | 4281 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.21, 0.37] |
| 1.1 HBIG 100 IU | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.36] |
| 1.2 HBIG 200 IU | 25 | 3855 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.21, 0.33] |
| 1.3 HBIG 400 IU | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.30, 1.53] |
| 2 Newborn positive for HBV‐DNA Show forest plot | 7 | 779 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.17, 0.37] |
| 2.1 HBIG 100 IU | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.70] |
| 2.2 HBIG 200 IU | 6 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.39] |
| 2.3 HBIG 200 IU to 400 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Newborn positive for HBeAg Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.14] |
| 3.1 HBIG 100 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 HBIG 200 IU | 4 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.12] |
| 3.3 HBIG 200 IU to 400 IU | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.51, 3.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 27 | 4012 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.20, 0.36] |
| 1.1 28, 32, and 36 weeks | 23 | 3078 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.21, 0.41] |
| 1.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.11, 0.34] |
| 1.3 30, 34, and 38 weeks | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.17] |
| 1.4 32, 36, and 40 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.22, 0.73] |
| 1.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.08] |
| 1.6 16, 20, 24, 28, 32, and 36 weeks | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.66] |
| 2 Newborn positive for HBV‐DNA Show forest plot | 16 | 2130 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.42] |
| 2.1 30, 34, and 38 weeks | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.17] |
| 2.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.08, 0.23] |
| 2.3 28, 32, and 36 weeks | 12 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.51] |
| 2.4 32, 36, and 40 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.19, 0.70] |
| 2.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 12, 16, 20, 24, 28, 32, 36, and 40 weeks | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.09, 0.52] |
| 3 Newborn positive for HBeAg Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.14] |
| 3.1 28, 32, and 36 weeks | 3 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.26, 1.32] |
| 3.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 3.3 32, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 16, 20, 24, 28, 32, and 36 weeks | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.94] |
| 3.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

