Иммуноглобулин против гепатита В во время беременности для предотвращения передачи вируса гепатита B от матери к ребенку.
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Zhejiang, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 44; placebo/no intervention 35; total 79. Inclusion criteria: not stated. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBV‐DNA. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Shantou, Guangdong, China. Mean age: intervention 26; no intervention 27; total not stated. Number of women: intervention 50; no intervention 50; total 100. Inclusion criteria: HBsAg‐positive pregnant women; no coinfection with hepatitis A, hepatitis C, hepatitis E, or hepatitis G; normal liver and kidney function. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBsAg within 24 hours after labour. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborns positive for HBsAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all women randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Longgang, Shenzhen, China. Mean age: intervention not stated; placebo/no intervention not stated; total not stated. Number of women: intervention 45; placebo/no intervention 49; total 94. Inclusion criteria: HBsAg‐ and HBeAg‐positive pregnant women; normal liver function; no signs of threatened abortion, threatened premature delivery, and pregnancy‐induced hypertension. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBsAg in 24 hours after labour. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborns positive for HBsAg and anti‐HBs. 12‐month‐old babies positive for HBsAg and anti‐HBs. Maximum duration of surveillance: 12 months. Follow‐up time point: 12 months after birth. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Wenzhou, Zhejiang, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 69; no intervention 72; total 141. Inclusion criteria: pregnant women with normal liver function; no medical or surgical complications and pregnancy complications; no drug administration such as transfer factor, interferon. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBsAg. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Yongjia, Zhejiang, China. Mean age: intervention not stated; placebo/no intervention not stated; total not stated. Number of women: intervention 86; placebo/no intervention 70; total 156. Inclusion criteria: HBsAg‐positive pregnant women; no signs of threatened abortion, threatened premature delivery, or pregnancy‐induced hypertension. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborns positive for HBsAg or HBV‐DNA. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 30, 34, and 38 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA positive and anti‐HBs. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported and trial was reported exclusively in Chinese. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Zhengzhou, Henan, China. Mean age: intervention not stated; placebo/no intervention not stated; total 23 to 33 years. Number of women: intervention 45; no intervention 43; total 88. Inclusion criteria: HBsAg‐positive pregnant women. Exclusion criteria: pregnant women coinfection with hepatitis A, C, E, or G; pregnant women received antiviral treatment. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn with HBsAg and HBeAg. 12‐month‐old babies positive for HBsAg and anti‐HBs. Maximum duration of surveillance: 12 months. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Huizhou, Guangdong, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 126; no intervention 90; total 216. Inclusion criteria: HBsAg‐positive pregnant women; normal liver function; no signs of threatened abortion or pregnancy‐induced hypertension. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborns and 1‐month‐old babies positive for HBsAg, negative for HBsAg within 1 year and remains positive, anti‐HBsAg positive. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborns positive for HBsAg and anti‐HBs. Maximum duration of surveillance: 12 months. Follow‐up time point: 1, 7, and 12 months after labour. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Leqing, Zhejiang, China. Mean age: intervention not stated; no intervention not stated; total 21 to 31 years. Number of women: intervention 29; no intervention 31; total 60. Inclusion criteria: pregnant women positive for both HBsAg and HBeAg. Exclusion criteria: pregnant women coinfection with hepatitis A, C, E, and G; pregnant women received antiviral treatment. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg and anti‐HBs. Adverse events (no adverse events found). | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Shanghai, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 113; no intervention 110; total 223. Inclusion criteria: HBsAg‐positive pregnant women. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg. 12‐month‐old babies positive for HBsAg. Maximum duration of surveillance: 12 months. Follow‐up time point: 12th month after birth. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Yangzhou, Jiangsu, China. Mean age: intervention not stated; no intervention not stated; total 22 to 32 years. Number of women: intervention 40; no intervention 46; total 86. Inclusion criteria: HBsAg‐positive pregnant women. Exclusion criteria: pregnant women coinfection with hepatitis A, C, E, or G; pregnant women received antiviral drugs. Newborn intrauterine infection definition: newborns positive for HBsAg and HBeAg. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Published language: English. | |
| Participants | Study location: Guangdong, China. Mean age: intervention I not stated; intervention II not stated; no intervention not stated; total not stated. Number of women: intervention I 56; intervention II43; no intervention 52; total 151. Inclusion criteria: HBsAg‐positive pregnant women; normal liver and kidney function; negative for hepatitis A, C, D, and E; no other severe complications; no other drugs) antivirus, cytotoxic, steroid hormones, or immune regulating drugs). Exclusion criteria: not stated. | |
| Interventions | Intervention group I: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Intervention group II (lamivudine group): Dosage of lamivudine: 100 mg/day orally. Duration: to the 30th day after labour. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg and HBeAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | High risk | It seems no blinding performed. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: English. | |
| Participants | Study location: Guangdong, China. Mean age (± SD): intervention 26.9 ± 1.8; no intervention 27.8 ± 2.8; total not stated. Number of women: intervention 57; no intervention 55; total 112. Inclusion criteria: HBsAg‐positive pregnant women; no signs of viral hepatitis. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg and HBV‐DNA. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Wuhan, Hubei, China. Mean age: intervention not stated; o intervention not stated; total 26.6 (range 18 to 38) years. Number of women: intervention 202; no intervention 246; total 448. Inclusion criteria: HBsAg‐positive pregnant women; no signs of viral hepatitis. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 32, 36, and 40 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA and HBsAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported and trial reported exclusively in Chinese. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Jiangmen, Guangdong, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 62; no intervention 60; total 122. Inclusion criteria: serum HBV‐DNA‐positive pregnant women; no pregnancy complications. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBV‐DNA. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 8. Gestational age at treatment: start from the 3rd month of gestation, once every month. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: English. | |
| Participants | Study location: Shanghai, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 55; no intervention 62; total 117. Inclusion criteria: HBsAg‐positive pregnant women; no signs of viral hepatitis. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Xinxiang, Henan, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 43; no intervention 43; total 86. Inclusion criteria: HBsAg‐ or HBeAg‐positive, HBV‐DNA‐negative pregnant women; HBV‐DNA‐negative pregnant women; normal liver function. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Ganzhou, Jiangxi, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 60; no intervention 40; total 100. Inclusion criteria: HBsAg‐positive pregnant women; normal liver function; no signs of threatened abortion, threatened premature delivery, and pregnancy‐induced hypertension. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBsAg or HBV‐DNA, or both. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA and HBsAg. 6‐month‐old babies positive for HBV‐DNA and HBsAg. Maximum duration of surveillance: 6 months. Follow‐up time point: 1st and 6th months after babies were born. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: English. | |
| Participants | Study location: Guangzhou, China. Mean age: intervention 28 years; no intervention 28 years; total 28 years. Number of women: intervention 262; no intervention 127; total 389. Inclusion criteria: HBsAg‐positive pregnant women; normal liver function; no signs of threatened abortion, threatened premature delivery, and pregnancy‐induced hypertension. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborns positive for HBsAg or HBV‐DNA, or both. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg and HBV‐DNA. Maximum duration of surveillance: 6 months. Follow‐up time point: 1st, 6th, 9th, and 12th months after babies were born. | |
| Notes | Research supported by GlaxoSmithKline Research and Development Grant NUC30914; Science and Research Foundations of Sun Yat‐Sen University and Guangzhou Science Committee, No 1999‐J‐005‐01. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers used. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Zhengzhou, Henan, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 55; no intervention 43; total 98. Inclusion criteria: HBsAg‐positive pregnant women. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 3, 2, and 1 month before delivery. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborns positive for HBsAg positive. 3‐month‐old and 9‐month‐old babies positive for HBsAg and anti‐HBsAg. Maximum duration of surveillance: 9 months. Follow‐up time point: 3 months and 9 months after birth. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Weihai, Shandong, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 56; no intervention 52; total 108. Inclusion criteria: HBsAg‐positive pregnant women; normal liver function; no history of hepatitis. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 100 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA, HBsAg, and anti‐HBs. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Qingdao, Shandong, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 32; no intervention 31; total 63. Inclusion criteria: midtrimester women positive for HBsAg and HBeAg; normal liver and kidney functions; hepatitis A, C, D, E negative; no surgical and pregnancy complications; no use of anti‐viral, anti‐cytotoxic, steroids, or immunomodulatory drugs. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 6. Gestational age at treatment: 16, 20, 24, 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg and HBeAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised number table used. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Taizhou, Zhejiang, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 159; no intervention 120; total 279. Inclusion criteria: HBsAg‐positive or both HBsAg‐ and HBeAg‐positive pregnant women; normal liver function; no signs of threatened abortion, threatened premature delivery, pregnancy‐induced hypertension, or pregnancy complications; with no use of antiviral therapy or hormonal drugs; aged 20 to 33 years; < 20 weeks of gestation. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Nodropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: English. | |
| Participants | Study location: Xinjiang, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 28; no intervention 24; total 52. Inclusion criteria: HBeAg‐positive pregnant women with good general condition; no threatened abortion or threatened premature labour, and hypertension; normal liver function; and to deliver at the same hospital. Exclusion criteria: need to stop pregnancy for some reasons; to deliver at other hospitals and lose follow‐up; to administer HBIG against protocol. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 8 (15.4%; i.e. < 20%) cases excluded according to the exclusion criteria. Judgement by review author: the exclusion criteria were not appropriate. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Luoyang, Henan, China. Mean age: intervention not stated; no intervention not stated; total 22 to 28 years. Number of women: intervention 46; no intervention 40; total 86. Inclusion criteria: HBsAg‐positive pregnant women. Exclusion criteria: pregnant women with co‐infection with hepatitis A, C, E, or G; pregnant women who received antiviral treatment. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg. Maximum duration of surveillance: 12 months. Follow‐up time point: 12 months after birth. | |
| Notes | Trial supported by Technology Research Fund Committee of Henan province (No. 981170112). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Qingdao, Shandong, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 44; no intervention 44; total 88. Inclusion criteria: HBsAg‐positive pregnant women; no history of hepatitis; normal liver function; no signs of threatened abortion, threatened premature delivery, or pregnancy‐induced hypertension. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for either HBsAg or HBV‐DNA. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg, HBV‐DNA, and anti‐HBs. 8‐month‐old babies positive for HBsAg, HBV‐DNA, and anti‐HBs. Maximum duration of surveillance: 8 months. Follow‐up time point: 8 months after birth. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: English. | |
| Participants | Study location: Xinjiang, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 28; no intervention 24; total 52. Inclusion criteria: positive‐HBeAg pregnant women and good general condition; no threatened abortion or threatened premature labour, and hypertension; normal liver function; to deliver at the same hospital. Exclusion criteria: to stop pregnancy for some reasons; to deliver at other hospitals and lose follow‐up; to administer HBIG against protocol. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBeAg and HBV‐DNA. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 8 (15.4%; i.e. < 20%) cases excluded according to the exclusion criteria. Judgement by review author: the exclusion criteria were not appropriate. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Nanjing, Jiangsu, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 163 (positive for HBsAg and HBeAg 117); no intervention 162 (positive for HBsAg and HBeAg 90); total 285. Inclusion criteria: HBsAg‐positive pregnant women; normal liver function. Exclusion criteria: pregnant women with diabetes, pregnancy‐induced hypertension. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3 or 6. Gestational age at treatment: 28, 32, and 36 weeks (HBsAg‐positive mothers); 28, 30, 32, 34, 36, and 38 weeks (HBsAg‐ and HBeAg‐positive mothers). All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg, HBeAg, and HBV‐DNA. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Zhaoqing, Guangdong, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 60 (HBsAg and HBeAg positive 13); no intervention 40 (HBsAg and HBeAg positive 10); total 100. Inclusion criteria: pregnant women; normal liver function; no signs of threatened abortion or pregnancy complications. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBsAg. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg and anti‐HBs. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropout or withdrawal reported and all participants randomised were analysed |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Shanghai, China. Mean age (± SD): intervention I 26.58 ± 3.76; intervention II 27.36 ± 4.24; no intervention 26.85 ± 4.01; total 20 to 33 years. Number of women: intervention I 26; intervention II 29; no intervention 28; total 83. Inclusion criteria: pregnant women of HBV carriers (HBsAg positive). Exclusion criteria: not stated. | |
| Interventions | Intervention group I: Dose of HBIG: 200 IU to 400 IU (HBsAg positive 200 IU; HBsAg and HBeAg positive 400 IU). Frequency: monthly. Number of doses: 3. Gestational age at treatment: 3, 2, and 1 month before delivery. Intervention group II: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 3, 2, and 1 month before delivery. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Guilin, Guangxi, China. Mean age: intervention not stated; no intervention not stated; total 22 to 39 years. Number of women: intervention 28 (HBsAg, HBeAg, HBcAb positive 12; HBsAg, HBeAb, HBcAb positive 13; HBsAg positive 3); no intervention 33 (HBsAg, HBeAg, HBcAb positive 10; HBsAg, HBeAb, HBcAb positive 14; HBsAg positive 9); total 61. Inclusion criteria: pregnant women with no pregnancy complications. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBV‐DNA. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA positive and HBsAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: English. | |
| Participants | Study location: Xi'an, Shanxi, China. Mean age (± SD): intervention 25.99 ± 2.39; no intervention 25.68 ± 2.67; total not stated. Number of women: intervention 117; no intervention 133; total 250. Inclusion criteria: pregnant women; no signs of threatened abortion, threatened premature delivery, and pregnancy‐induced hypertension; no history and symptoms of hepatitis; normal liver function. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 400 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 3, 2, and 1 month before delivery (starting at 28th week of gestation). All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg, anti‐HBs, HBeAg, anti‐HBe, and anti‐HBc. | |
| Notes | Study supported by Huizhou Municipal Central hospital and Huizhou Science and Technology Bureau. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: English. | |
| Participants | Study location: Xi'an, Shanxi, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 34; no intervention 14; total 48. Inclusion criteria: pregnant women; no signs of threatened abortion, threatened premature delivery, and pregnancy‐induced hypertension; no history and symptoms of hepatitis; normal liver function. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 100 IU. Frequency: weekly. Number of doses: 11. Gestational age at treatment: 20, 24, 28, 30, 32, 34, 36, 37, 38, 39, and 40th week. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg and anti‐HBs. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Shantou, Guangdong, China. Mean age: intervention not stated; no intervention not stated; total 19 to 36 years. Number of women: intervention 163; o intervention 157; total 320. Inclusion criteria: HBsAg‐positive pregnant women. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: Chinese. | |
| Participants | Study location: Taishan, Guangdong, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 92; no intervention 92; total 184. Inclusion criteria: serum HBV‐DNA‐positive pregnant women; no pregnancy complications. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBV‐DNA. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBV‐DNA. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language ‐ Chinese. | |
| Participants | Study location: Shanghai, China. Mean age: intervention not stated; no intervention not stated; total not stated. Number of women: intervention 92; no intervention 92; total 184. Inclusion criteria: serum HBV‐DNA‐positive pregnant women; no pregnancy complications. Exclusion criteria: not stated. Newborn intrauterine infection definition: newborn positive for HBV‐DNA. 204 participants (103 intervention, 101 control) who were aged 20 to 34 years who used HBIG for prevention of mother‐to‐child transmission of hepatitis B virus. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU. Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg. | |
| Notes | Sources of funding not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No dropout or withdrawal reported and all participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
| Methods | Randomised clinical trial. Publication language: English. | |
| Participants | Study location: Shanghai, China. Mean age: intervention not stated; no intervention not stated; total 19 to 35 years (mean (± SD) 24 ± 3 years). Number of women: intervention 487; no intervention 493; total 980. Inclusion criteria: pregnant women who are asymptomatic HBsAg carriers. Exclusion criteria: not stated. | |
| Interventions | Intervention group: Dose of HBIG: 200 IU or 400 IU (for HBsAg HBeAg double‐positive carrier). Frequency: monthly. Number of doses: 3. Gestational age at treatment: 28, 32, and 36 weeks. All neonates received passive‐active immunisation after birth. Control group: No intervention. All neonates received passive‐active immunisation after birth. | |
| Outcomes | Newborn positive for HBsAg, HBeAg, and HBV‐DNA. | |
| Notes | Study supported by grant from Ministry of Public Health China (No. 97030223). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but not stated how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | High risk | Newborn and maternal mortality and morbidity not reported. |
anti‐HBc: antibody to hepatitis core antigen; anti‐HBe: antibody to hepatitis B envelope antigen; anti‐HBs: antibody to hepatitis B surface antigen; HBcAb: hepatitis B core antibody; HBcAg: hepatitis B core antigen; HBeAb: hepatitis B envelope antibody; HBeAg: hepatitis B envelope antigen; HBIG: hepatitis B immunoglobulin; HBsAb: hepatitis B surface antibody; HBsAg: hepatitis B surface antigen; HBV‐DNA: hepatitis B virus DNA; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomised clinical trial even though the study was on pregnant women. | |
| HBIG was given only to infants of women who were HBsAg positive. Mothers did not receive any treatment. | |
| HBIG was not given to pregnant women. It was only given to their infants at birth (infants of women that were HBeAg positive). | |
| A randomised clinical trial of HBIG and hepatitis B vaccine. However, it was only administered to infants. No HBIG was given to the infected mothers positive for HBsAg or HBeAg. | |
| Not a randomised clinical trial. This was a study on infants of hepatitis B‐positive mothers who received HBIG as prophylaxis. They also received hepatitis B vaccine. | |
| Not a randomised clinical trial on hepatitis B virus. | |
| Not a randomised clinical trial. Pregnant women and non‐pregnant women were sampled. Women did not receive HBIG. | |
| Not a randomised clinical trial on hepatitis B virus. | |
| HBIG was given to the mothers who were hepatitis B virus positive. HBIG was also given to the infants. Not a randomised clinical trial. | |
| Not a randomised clinical trial on hepatitis B virus. | |
| Not a randomised clinical trial on hepatitis B virus. | |
| Not a randomised clinical trial. The pregnant women did not receive HBIG. | |
| Review on hepatitis B virus in pregnancy. No HBIG given. | |
| Study on infants born to HBsAg‐positive mothers, not on pregnant women. Not a randomised clinical trial. | |
| Only newborn infants received treatment. Both groups (intervention and control) received treatment. | |
| Not a randomised clinical trial. Only screening for HBsAg was performed. | |
| Efficacy of HBIG and hepatitis B vaccine were tested together. The trial enrolled only on infants. Not a randomised clinical trial. | |
| Only hepatitis B vaccine was given. HBIG was not given to the pregnant women. | |
| Not a randomised clinical trial. Enrolled infants and children of hepatitis B‐positive mothers. | |
| Not a randomised clinical trial and did not enrol pregnant women. A clinical review. No HBIG was given. | |
| Not a randomised clinical trial. HBIG was only given to infants of infected mothers with hepatitis B virus infection. | |
| Not a randomised clinical trial; mothers did not receive HBIG, only the infants received it. | |
| Not a randomised clinical trial on hepatitis B virus. | |
| Not a randomised clinical trial. Only the infants of hepatitis B‐positive mothers received treatment. | |
| Not a randomised clinical trial. HBIG was not given. | |
| Randomised clinical trial. Both study and control groups (all women) received HBIG treatment. The criteria for considering study in this review was not fulfilled by this Xiao 2007 trial. This is because, while the intervention arm received HBIG, the control arm also received HBIG, instead of placebo or no intervention. Thus, the treatment group (women with positive HBsAg and positive HBeAg) received treatment while the control group (women with positive HBsAg and negative HBeAg) also received HBIG treatment. | |
| Only the infants received the HBIG. Pregnant mothers did not receive HBIG. | |
| Participants received hepatitis B vaccine, HBIG, and lamivudine. | |
| Not a randomised clinical trial on hepatitis B virus. | |
| Not a randomised clinical trial on hepatitis B virus. |
HBeAg: hepatitis B envelope antigen; HBIG: hepatitis B immunoglobulin; HBsAg: hepatitis B surface antigen.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 29 | 5310 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.24, 0.38] |
| Analysis 1.1  Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 1 Newborn positive for HBsAg. | ||||
| 2 Newborn positive for HBeAg Show forest plot | 7 | 1764 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.05] |
| Analysis 1.2  Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 2 Newborn positive for HBeAg. | ||||
| 3 Newborn positive for HBV‐DNA Show forest plot | 16 | 2130 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.42] |
| Analysis 1.3  Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 3 Newborn positive for HBV‐DNA. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 28 | 4281 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.21, 0.37] |
| Analysis 2.1  Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 1 Newborn positive for HBsAg. | ||||
| 1.1 HBIG 100 IU | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.36] |
| 1.2 HBIG 200 IU | 25 | 3855 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.21, 0.33] |
| 1.3 HBIG 400 IU | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.30, 1.53] |
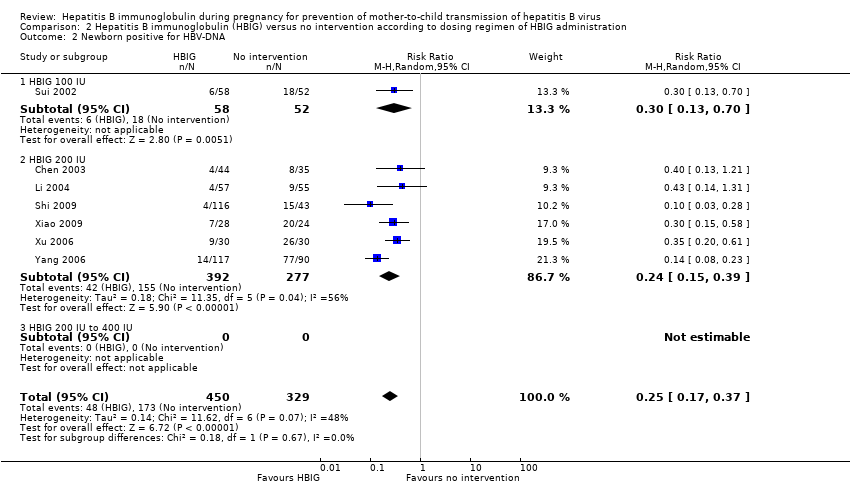
| 2 Newborn positive for HBV‐DNA Show forest plot | 7 | 779 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.17, 0.37] |
| Analysis 2.2  Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 2 Newborn positive for HBV‐DNA. | ||||
| 2.1 HBIG 100 IU | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.70] |
| 2.2 HBIG 200 IU | 6 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.39] |
| 2.3 HBIG 200 IU to 400 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
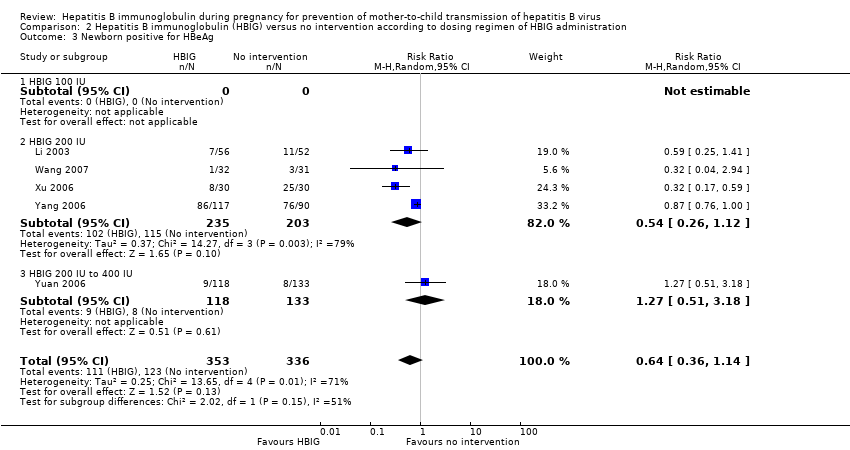
| 3 Newborn positive for HBeAg Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.14] |
| Analysis 2.3  Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 3 Newborn positive for HBeAg. | ||||
| 3.1 HBIG 100 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 HBIG 200 IU | 4 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.12] |
| 3.3 HBIG 200 IU to 400 IU | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.51, 3.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
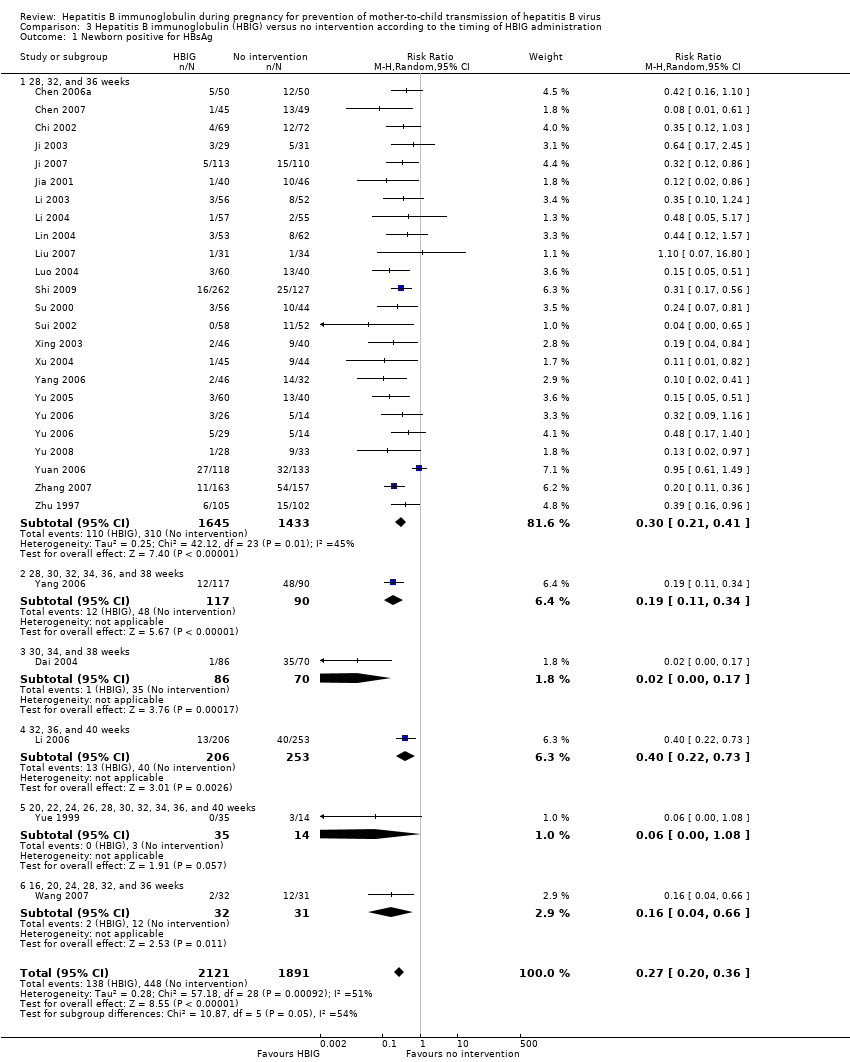
| 1 Newborn positive for HBsAg Show forest plot | 27 | 4012 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.20, 0.36] |
| Analysis 3.1  Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 1 Newborn positive for HBsAg. | ||||
| 1.1 28, 32, and 36 weeks | 23 | 3078 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.21, 0.41] |
| 1.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.11, 0.34] |
| 1.3 30, 34, and 38 weeks | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.17] |
| 1.4 32, 36, and 40 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.22, 0.73] |
| 1.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.08] |
| 1.6 16, 20, 24, 28, 32, and 36 weeks | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.66] |
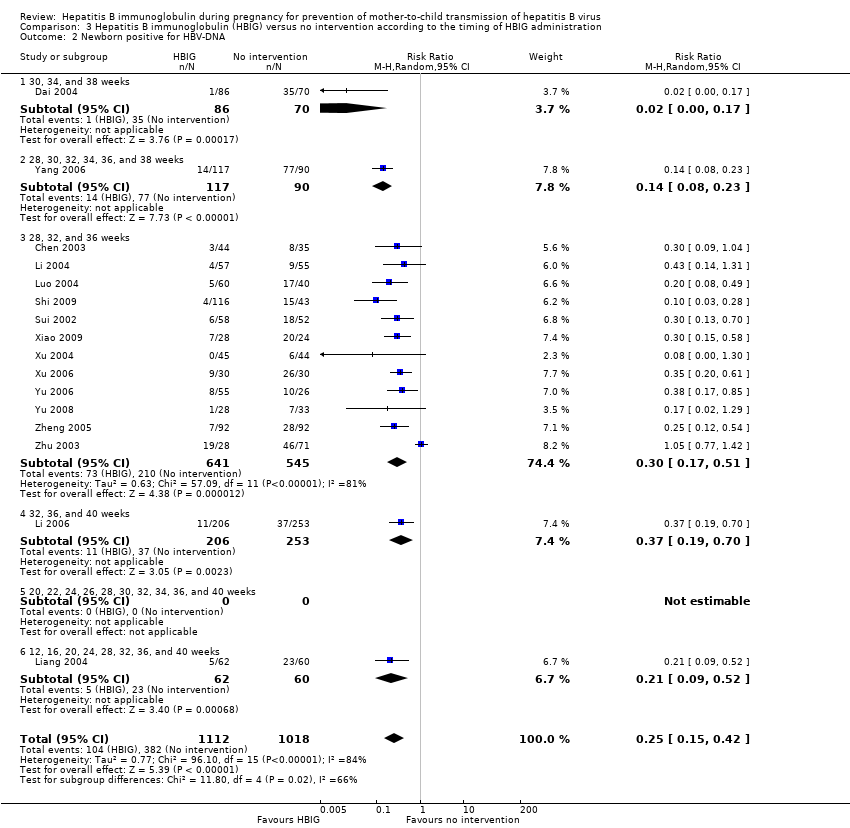
| 2 Newborn positive for HBV‐DNA Show forest plot | 16 | 2130 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.42] |
| Analysis 3.2  Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 2 Newborn positive for HBV‐DNA. | ||||
| 2.1 30, 34, and 38 weeks | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.17] |
| 2.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.08, 0.23] |
| 2.3 28, 32, and 36 weeks | 12 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.51] |
| 2.4 32, 36, and 40 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.19, 0.70] |
| 2.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 12, 16, 20, 24, 28, 32, 36, and 40 weeks | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.09, 0.52] |
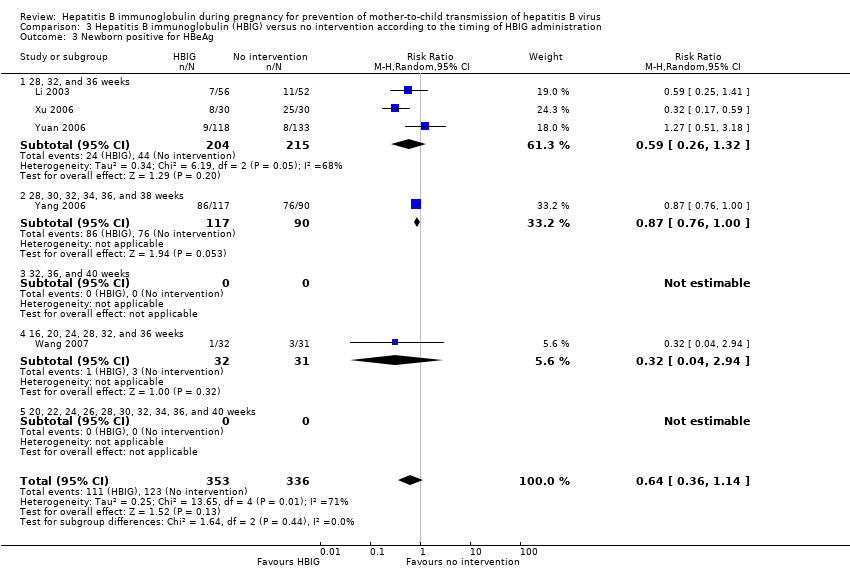
| 3 Newborn positive for HBeAg Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.14] |
| Analysis 3.3  Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 3 Newborn positive for HBeAg. | ||||
| 3.1 28, 32, and 36 weeks | 3 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.26, 1.32] |
| 3.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 3.3 32, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 16, 20, 24, 28, 32, and 36 weeks | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.94] |
| 3.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
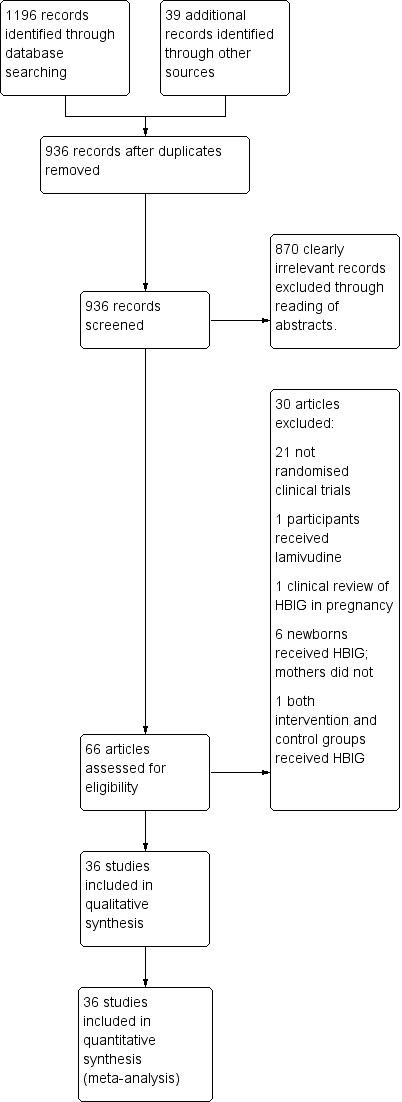
Study flow diagram for searches on hepatitis B Immunoglobulin (HBIG).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
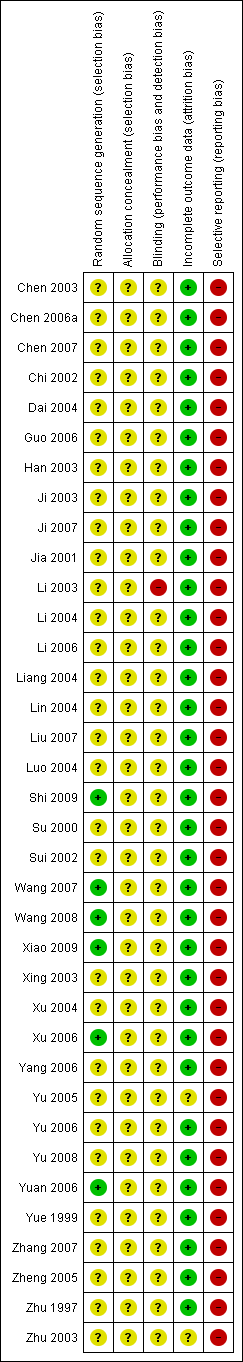
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
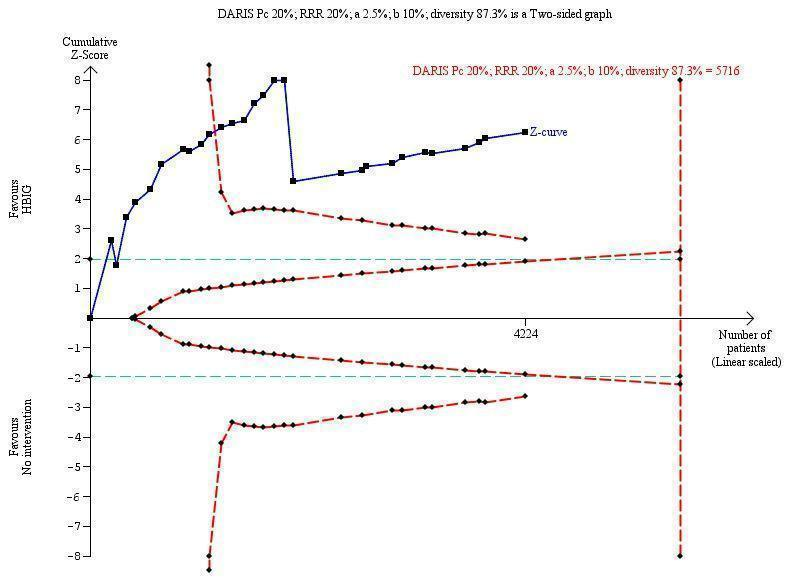
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with HBsAg‐positive results at end of follow‐up. The diversity‐adjusted required information size (DARIS) of 5716 participants was calculated based upon a proportion of 20% of babies tested positive for HBsAg in the control group, a relative risk reduction of a 20% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 87.3%. The actually accrued number of participants is 4224, which is 74% of the DARIS. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve crosses the red trial sequential monitoring boundary for benefit during the 11th trial. This implies that there is no risk of random error in the estimate of a beneficial effect of HBIG versus no intervention on the number of newborns with HBsAg‐positive results at end of follow‐up. The TSA‐adjusted confidence interval is 0.20 to 0.52.
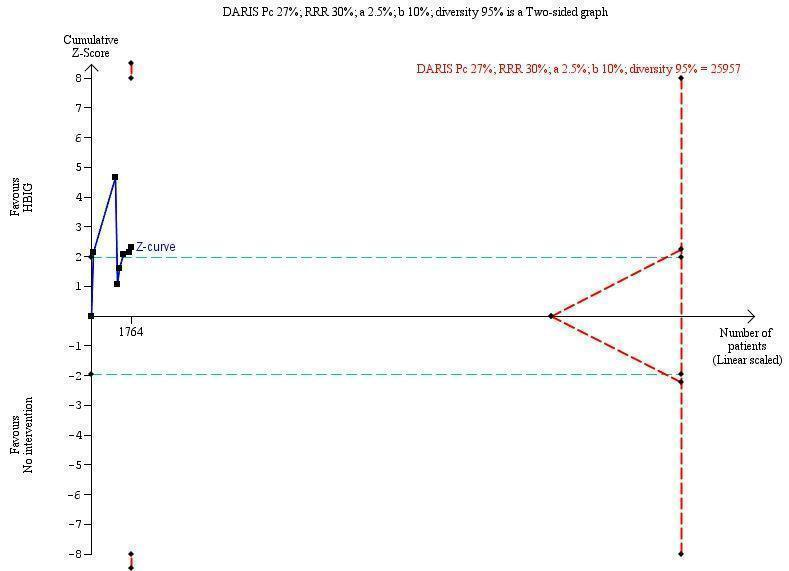
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with hepatitis B envelope antigen (HBeAg)‐positive results at end of follow‐up. The diversity‐adjusted required information size (DARIS) of 25,957 participants was calculated based upon a proportion of 27% of babies tested positive for HBeAg in the control group, a relative risk reduction of a 30% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 95%. The actually accrued number of participants is 1764, which is only 6.8% of the DARIS. (We planned to use a relative risk reduction of 20%, but this led to a DARIS of 60,715 participants and the TSA figure could not be drawn by the program; therefore, a relative risk reduction of 30% was adopted instead.) The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve does not cross the red inward sloping trial sequential monitoring boundaries for benefit or harm. Therefore, there is no evidence to support that HBIG influences number of newborns with HBeAg‐positive results at end of follow‐up. The cumulative Z‐curve does not reach the futility area, demonstrating that further trials are needed. The TSA‐adjusted confidence interval is wider than 0.04 to 6.37.
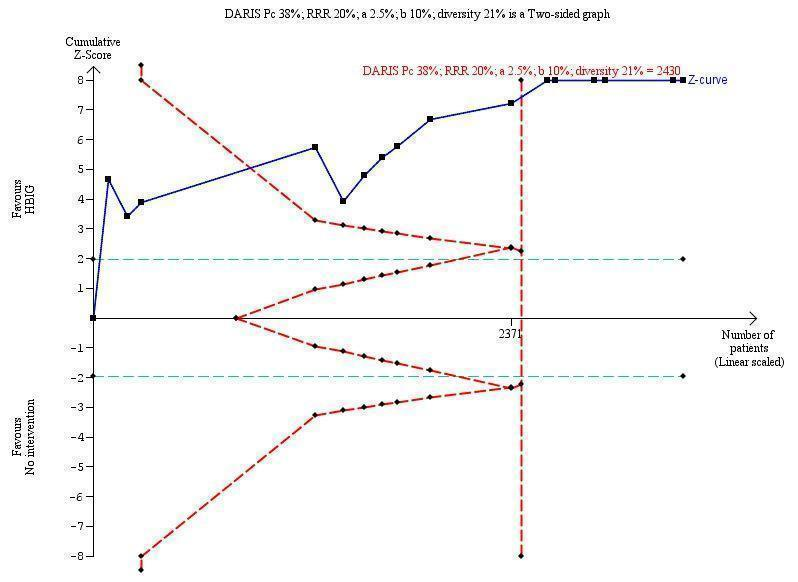
Trial Sequential Analysis (TSA) of the random‐effects meta‐analysis of the effect of hepatitis B immunoglobulin (HBIG) versus no intervention on the number of newborns with hepatitis B virus DNA (HBV‐DNA) positive results at end of treatment. The diversity‐adjusted required information size (DARIS) of n = 2430 participants was calculated based upon a proportion of 38% of babies tested positive for HBV‐DNA, a relative risk reduction of a 20% in HBIG group, an alpha (type I error) of 2.5%, a beta (type II error) of 10%, and a diversity (D) of 21%. The actually accrued number of participants is 2994, which is more than the DARIS of 2430 participants. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The blue cumulative Z‐curve crosses the red trial sequential monitoring boundary for benefit during the fourth trial. This implies that there is no risk of random error in the estimate of a beneficial effect of HBIG versus no intervention on the number of newborns with HBV‐DNA positive results at end of treatment. The TSA‐adjusted and 95% confidence intervals is from 0.22 to 0.37.
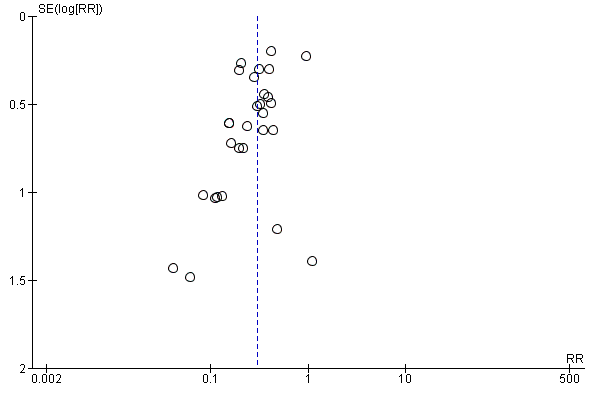
Funnel plot of comparison: 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, outcome: 1.1 Newborn positive for HBsAg.
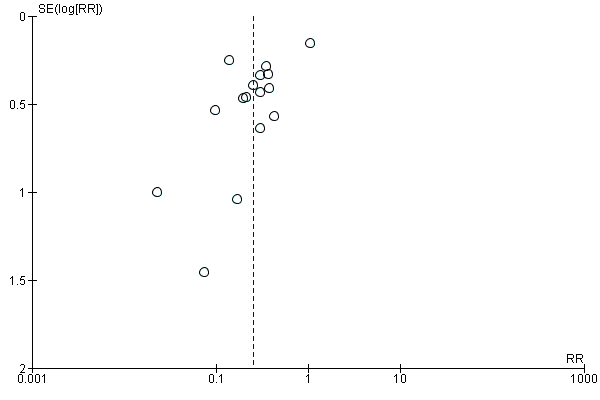
Funnel plot of comparison: 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, outcome: 1.3 Newborn positive for HBV‐DNA.

Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 1 Newborn positive for HBsAg.
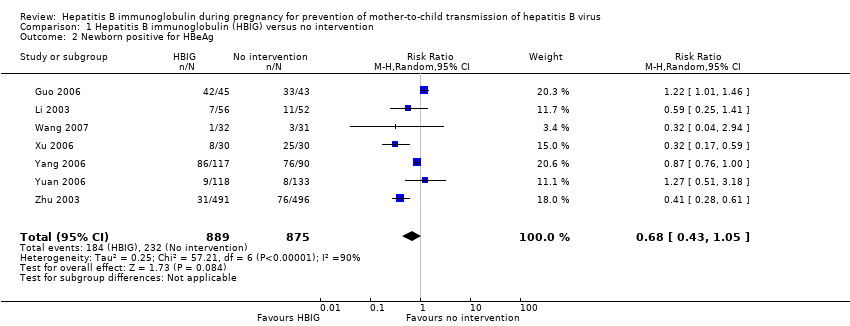
Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 2 Newborn positive for HBeAg.

Comparison 1 Hepatitis B immunoglobulin (HBIG) versus no intervention, Outcome 3 Newborn positive for HBV‐DNA.

Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 1 Newborn positive for HBsAg.
Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 2 Newborn positive for HBV‐DNA.
Comparison 2 Hepatitis B immunoglobulin (HBIG) versus no intervention according to dosing regimen of HBIG administration, Outcome 3 Newborn positive for HBeAg.
Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 1 Newborn positive for HBsAg.
Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 2 Newborn positive for HBV‐DNA.
Comparison 3 Hepatitis B immunoglobulin (HBIG) versus no intervention according to the timing of HBIG administration, Outcome 3 Newborn positive for HBeAg.
| Hepatitis B immunoglobulin (HBIG) vs no intervention for prevention of mother‐to‐child transmission of hepatitis B virus | ||||||
| Participants: pregnant women positive for HBsAg or positive for HBeAg, or both. Comparison: no intervention. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | HBIG versus no intervention | |||||
| All‐cause mortality or other serous adverse events of the newborn | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| All‐cause mortality or other serous adverse events of the mothers | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Newborn with HBsAg‐positive result | Study population | RR 0.3 | 5310 | ⊕⊝⊝⊝ | ||
| 211 per 1000 | 63 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 64 per 1000 | |||||
| Newborn with HBeAg‐positive result | Study population | RR 0.68 | 1764 | ⊕⊝⊝⊝ | ||
| 265 per 1000 | 180 per 1000 | |||||
| Moderate | ||||||
| 212 per 1000 | 144 per 1000 | |||||
| Newborn with HBV‐DNA‐positive result | Study population | RR 0.25 | 2130 | ⊕⊕⊝⊝ | ||
| 375 per 1000 | 94 per 1000 | |||||
| Moderate | ||||||
| 366 per 1000 | 91 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Comment: Data for this outcome was not reported in any of the included trials. | ||||||
| Study ID | Study location | Participants | Interventions | Outcomes | Funding |
| Zhejiang | 79 participants (44 intervention, 35 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Guangdong | 100 participants (50 intervention, 50 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Shenzhen | 94 participants (45 intervention, 49 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. | |
| Zhejiang | 141 participants (69 intervention, 72 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Zhejiang | 156 participants (86 intervention, 70 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, anti‐HBs | Not stated. | |
| Henan | 88 participants (45 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg anti‐HBs. | Not stated | |
| Guangdong | 216 participants (126 intervention, 90 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Zhejiang | 60 participants (29 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs, adverse events. | Not stated | |
| Shanghai | 223 participants (113 intervention, 110 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Jiangsu | 86 participants (40 intervention, 46 control) aged 22 to 32 years. | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Guangdong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBeAg, or both. | Not stated. | |
| Guangdong | 112 participants (57 intervention, 55 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both. | Not stated. | |
| Hubei | 448 participants (202 intervention, 246 control) aged 18 to 38 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. | |
| Guangdong | 122 participants (62 intervention, 60 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA. | Not stated. | |
| Shanghai | 117 participants (55 intervention, 62 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Henan | 86 participants (43 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg. | Not stated. | |
| Jiangxi | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg. | Not stated. | |
| Guangzhou | 389 participants (262 intervention, 127 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg or HBV‐DNA, or both | Research supported by GlaxoSmithKline Research and Development Grant NUC30914; Science and Research Foundations of Sun Yat‐Sen University and Guangzhou Science Committee, No 1999‐J‐005‐01. | |
| Henan | 98 participants (55 intervention, 43 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Shandong | 108 participants (56 intervention, 52 control) | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA, and anti‐HBs. | Not stated. | |
| Shandong | 63 participants (32 intervention, 31 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg and HBeAg | Not stated. | |
| Taizhou | 279 participants (159 intervention, 120 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBV‐DNA. | Not stated. | |
| Henan | 86 participants (46 intervention, 40 control) aged 22 to 28 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Supported by Technology Research Fund Committee of Henan province (No. 981170112). | |
| Shandong | 88 participants (44 intervention, 44 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBV‐DNA and anti‐HBs. 8‐month‐old babies positive for HBsAg, HBV‐DNA, and anti‐HBs. Maximum duration of surveillance: 8 months. Follow‐up time point: 8 months after birth. | Not stated. | |
| Xinjiang | 52 participants (28 intervention, 24 control) | Intervention: HBIG 200 IU. Control: no intervention. | newborn HBeAg and HBV‐DNA. | Not stated. | |
| Jiangsu | 285 participants (163 intervention, 162 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg and HBV‐DNA | Not stated. | |
| Guangdong | 100 participants (60 intervention, 40 control) | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBeAg, anti‐HBs | Not stated. | |
| Shanghai | 83 participants (26 intervention I, 29 intervention II, 28 control) aged 20 to 33 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Guangxi | 61 participants (28 intervention, 33 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA, HBsAg | Not stated. | |
| Huizhou | 250 participants (117 intervention, 113 control) | Intervention: HBIG 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg; adverse effects of the immunoglobulins to the neonates and mothers | Supported by Huizhou Municipal Central hospital and Huizhou Science and Technology Bureau. | |
| Shanxi | 48 participants (34 intervention, 14 control) aged 20 to 33 years | Intervention: HBIG 100 IU. Control: no intervention. | Newborn HBsAg, anti‐HBs | Not stated. | |
| Guangdong | 320 participants (163 intervention, 157 control) aged 19 to 36 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg | Not stated. | |
| Guangdong | 184 participants (92 intervention, 92 control) aged 22 to 39 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBV‐DNA | Not stated. | |
| Shanghai | 204 participants (103 intervention, 101 control) aged 20 to 34 years | Intervention: HBIG 200 IU. Control: no intervention. | Newborn HBsAg, HBeAg, antibodies to HBsAg, HBeAg, and HBcAg. | Not stated. | |
| Shanghai | 980 participants (487 intervention, 493 control) aged 19 to 35 years | Intervention: HBIG 200 IU or 400 IU. Control: no intervention. | Newborn HBsAg, HBeAg, and HBV‐DNA. | Supported by a grant from the Ministry of Public Health China (No. 97030223). | |
| anti‐HBc: anti‐hepatitis core; anti‐HBe: anti‐hepatitis B envelope; anti‐HBs: anti‐hepatitis B surface; HBIG: hepatitis B immunoglobulin; HBcAg: hepatitis B core antigen; HBeAg: hepatitis B envelope antigen; HBsAg: hepatitis B surface antigen; HBV‐DNA: hepatitis B virus DNA. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 29 | 5310 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.24, 0.38] |
| 2 Newborn positive for HBeAg Show forest plot | 7 | 1764 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.05] |
| 3 Newborn positive for HBV‐DNA Show forest plot | 16 | 2130 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 28 | 4281 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.21, 0.37] |
| 1.1 HBIG 100 IU | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.36] |
| 1.2 HBIG 200 IU | 25 | 3855 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.21, 0.33] |
| 1.3 HBIG 400 IU | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.30, 1.53] |
| 2 Newborn positive for HBV‐DNA Show forest plot | 7 | 779 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.17, 0.37] |
| 2.1 HBIG 100 IU | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.70] |
| 2.2 HBIG 200 IU | 6 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.39] |
| 2.3 HBIG 200 IU to 400 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Newborn positive for HBeAg Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.14] |
| 3.1 HBIG 100 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 HBIG 200 IU | 4 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.12] |
| 3.3 HBIG 200 IU to 400 IU | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.51, 3.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Newborn positive for HBsAg Show forest plot | 27 | 4012 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.20, 0.36] |
| 1.1 28, 32, and 36 weeks | 23 | 3078 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.21, 0.41] |
| 1.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.11, 0.34] |
| 1.3 30, 34, and 38 weeks | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.17] |
| 1.4 32, 36, and 40 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.22, 0.73] |
| 1.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.08] |
| 1.6 16, 20, 24, 28, 32, and 36 weeks | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.66] |
| 2 Newborn positive for HBV‐DNA Show forest plot | 16 | 2130 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.42] |
| 2.1 30, 34, and 38 weeks | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.17] |
| 2.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.08, 0.23] |
| 2.3 28, 32, and 36 weeks | 12 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.17, 0.51] |
| 2.4 32, 36, and 40 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.19, 0.70] |
| 2.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 12, 16, 20, 24, 28, 32, 36, and 40 weeks | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.09, 0.52] |
| 3 Newborn positive for HBeAg Show forest plot | 5 | 689 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.14] |
| 3.1 28, 32, and 36 weeks | 3 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.26, 1.32] |
| 3.2 28, 30, 32, 34, 36, and 38 weeks | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 3.3 32, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 16, 20, 24, 28, 32, and 36 weeks | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.94] |
| 3.5 20, 22, 24, 26, 28, 30, 32, 34, 36, and 40 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

