Fármacos orales como el citrato de clomifeno o los inhibidores de la aromatasa con gonadotrofinas para la estimulación ovárica controlada en pacientes sometidas a fecundación in vitro
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008528.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 02 noviembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Ginecología y fertilidad
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
For the 2017 update:
MSK: data searching, selection of studies, data extraction, drafting of update, assessment of studies for inclusion, interpretation and analysis of the data, and final editing of the review.
AM: input in selection of studies, and editing the final draft of the review.
SB: overall supervision, input in selection of studies, and editing the final draft of the review.
KYL: data searching, selection of studies, data extraction.
AG: data searching, selection of studies, data extraction, assessment of studies for inclusion, and contributed to final writing of the manuscript.
Sources of support
Internal sources
-
Reproductive Medicine Unit, Christian Medical College, Vellore, India.
MSK is working in Christian Medical College, Vellore
-
University of Aberdeen, UK.
AM and SB are currently working for the University of Aberdeen
-
Mansoura University, Egypt.
AG is currently working for Mansoura University
External sources
-
None, Other.
Declarations of interest
MSK: no conflicts of interest to declare.
AM: no conflicts of interest to declare.
SB: no conflicts of interest to declare.
KYL: no conflicts of interest to declare.
AG: no conflicts of interest to declare.
Acknowledgements
We thank:
-
Richard Kirubakaran, Cochrane South Asia, Prof. BV Moses Centre for Evidence‐Informed Health Care and Health Policy, Christian Medical College, Vellore, India;
-
Marian Showell, Information Specialist for the Cochrane Gynaecology and Fertility Group;
-
Editorial team of the Cochrane Gynaecology and Fertility Group for their support and assistance.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Nov 02 | Oral medications including clomiphene citrate or aromatase inhibitors with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilisation | Review | Mohan S Kamath, Abha Maheshwari, Siladitya Bhattacharya, Kar Yee Lor, Ahmed Gibreel | |
| 2012 Nov 14 | Clomiphene citrate in combination with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilization | Review | Ahmed Gibreel, Abha Maheshwari, Siladitya Bhattacharya | |
| 2010 Jun 16 | Clomiphene citrate for controlled ovarian stimulation in women undergoing in vitro fertilization | Protocol | Ahmed Fathy Gibreel, Abha Maheshwari, Siladitya Bhattacharya | |
Differences between protocol and review
We have changed the title of the review from 'Clomiphene citrate in combination with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilization' to 'Oral medications including clomiphene citrate or aromatase inhibitors with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilisation'.
There has been a change of authors and contact author.
We have widened the scope of the current update by including other oral medications such as aromatase inhibitors for controlled ovarian stimulation. This resulted in the following changes.
-
Type of intervention: Clomiphene citrate with or without gonadotropins (original) and aromatase inhibitors with or without gonadotropins (addition in update).
-
Type of participants: We added the word 'fresh' IVF. This was done to clearly indicate inclusion of only those women who had oocyte retrieval and embryo transfer in the same cycle and not those women who had all embryos frozen and transferred in subsequent cycles.
-
Primary outcomes: We included ovarian hyperstimulation syndrome as a primary outcome (adverse) along with live birth.
-
Risk of bias: We considered lack of blinding as low risk for performance and detection bias for the original review. However, with ovarian hyperstimulation syndrome being added as a primary outcome for the general IVF population, we no longer considered lack of blinding as low risk for this domain.
-
Measures of treatment effect: We used risk ratio instead of odds ratio for dichotomous outcomes as it is more intuitive and easier to understand. However, we used Peto odds ratio for dichotomous outcomes that were associated with low event rates.
-
Data synthesis: In the original protocol, the main comparison group was clomiphene citrate with gonadotropins (with or without gonadotropin‐releasing hormone (GnRH) antagonist) versus gonadotropin in GnRH agonist protocol in IVF. However, with the advent of newer drugs and protocol, we changed this comparison. Also, due to wider use of oral medications in poor responders, we evaluated the general population and poor responders in separate comparisons:
-
clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle GnRH antagonist) versus gonadotropins (with GnRH agonist or midcycle antagonist protocols) in IVF and intracytoplasmic sperm injection cycles in the general population;
-
clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle GnRH antagonist) versus gonadotropins (with GnRH agonist or midcycle antagonist protocols) in IVF and intracytoplasmic sperm injection cycles in a population of poor responders.
-
-
Effects of interventions: Given the two different comparisons, we also presented the Effects of interventions separately for the general population and poor responders.
-
'Summary of findings' table: Given the two different comparisons, we also presented separate 'Summary of findings' tables for the general population and poor responders.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Clomiphene [*administration & dosage];
- Drug Therapy, Combination [methods];
- Fertility Agents, Female [*administration & dosage];
- Fertilization in Vitro [*methods];
- Gonadotropin‐Releasing Hormone [antagonists & inhibitors];
- Gonadotropins [*administration & dosage];
- Letrozole;
- Live Birth [epidemiology];
- Nitriles [*administration & dosage];
- Oocyte Retrieval [statistics & numerical data];
- Ovarian Hyperstimulation Syndrome [chemically induced, epidemiology];
- Ovulation Induction [*methods];
- Pregnancy Rate;
- Randomized Controlled Trials as Topic;
- Sperm Injections, Intracytoplasmic;
- Triazoles [*administration & dosage];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
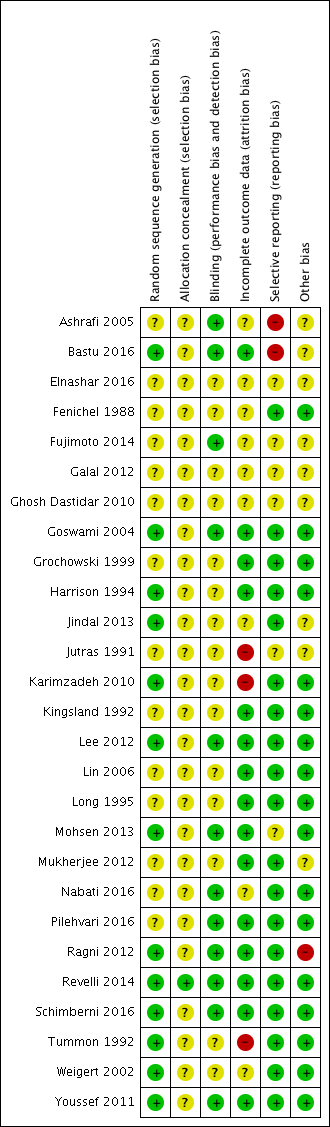
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, outcome: 1.1 Live birth.

Forest plot of comparison: 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, outcome: 1.2 Ovarian hyperstimulation syndrome.
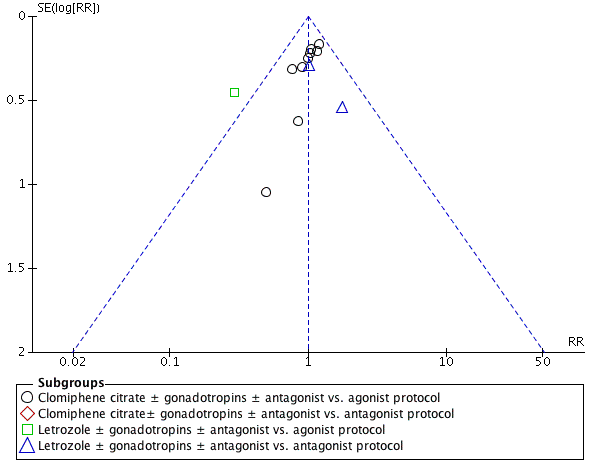
Funnel plot of comparison: 1 Clomiphene citrate or letrozole with gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins with GnRH protocols in IVF and ICSI cycles in general population, outcome: 1.4 Clinical pregnancy rate.
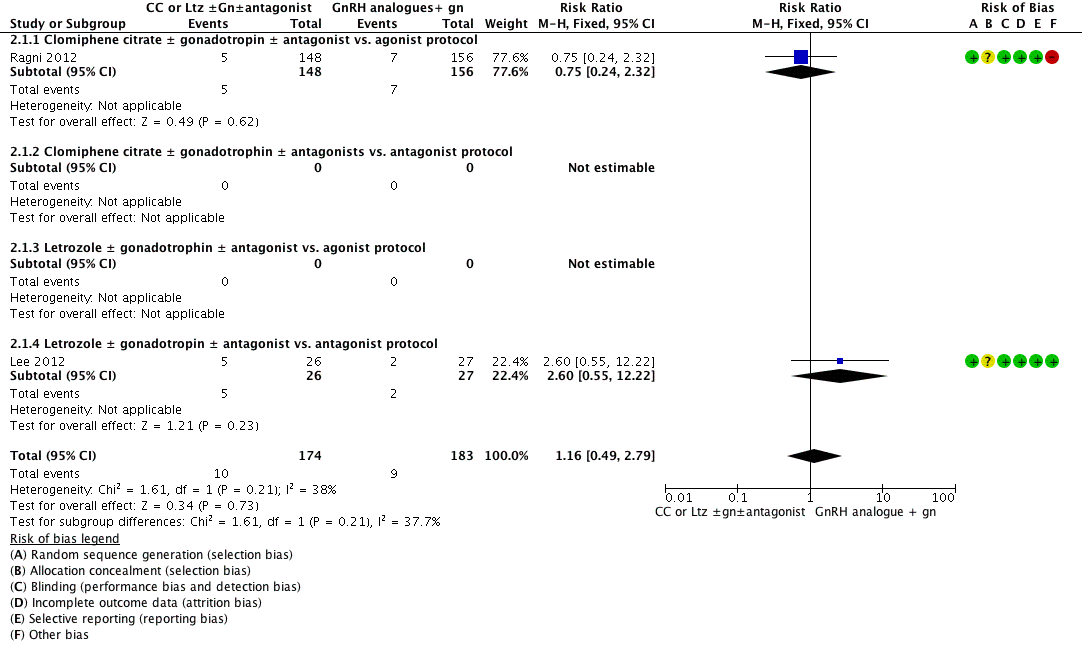
Forest plot of comparison: 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, outcome: 2.1 Live birth.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 1 Live birth.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 2 Ovarian hyperstimulation syndrome.
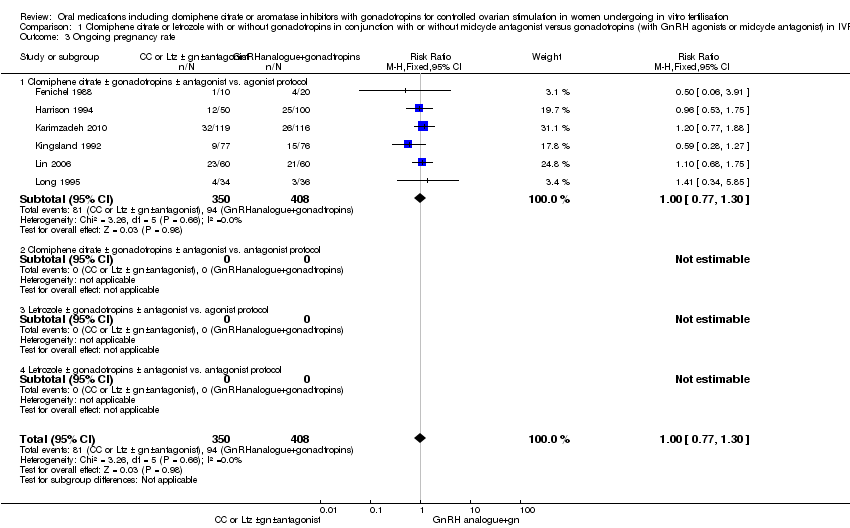
Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 3 Ongoing pregnancy rate.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 4 Clinical pregnancy rate.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 5 Cancellation rate.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 6 Mean number of ampoules used.
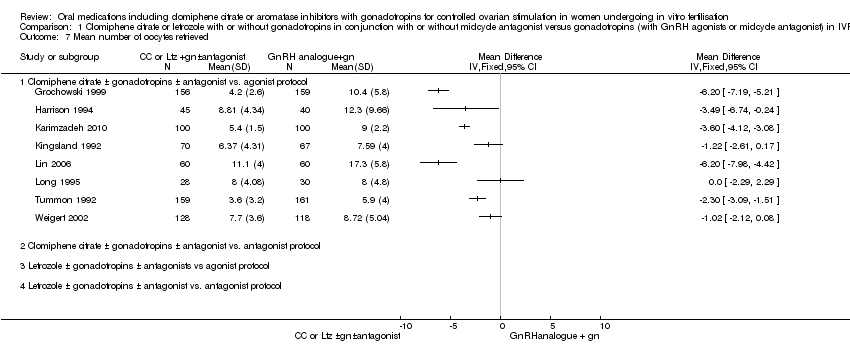
Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 7 Mean number of oocytes retrieved.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 8 Multiple pregnancy rate.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 9 Rate of miscarriage.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 10 Rate of ectopic pregnancy.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 11 Rate of foetal abnormalities.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 1 Live birth.
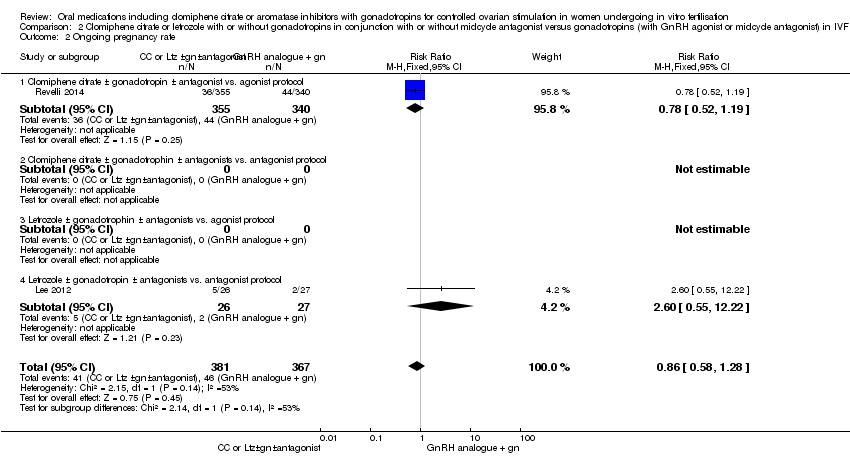
Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 2 Ongoing pregnancy rate.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 3 Clinical pregnancy rate.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 4 Cancellation rate.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 5 Mean number of ampoules used.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 6 Mean number of oocytes retrieved..

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 7 Multiple pregnancy rate.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 8 Rate of miscarriage.
| Clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle antagonist) compared to gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population for controlled ovarian stimulation | ||||||
| Patient or population: Women undergoing controlled ovarian stimulation in IVF and ICSI cycles (general population) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with gonadotropins (with GnRH agonists or midcycle antagonist) | Risk with clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle antagonist) | |||||
| Live birth per woman | 235 per 1000 | 216 per 1000 | RR 0.92 | 493 | ⊕⊕⊝⊝ | |
| Ovarian hyperstimulation syndrome per woman | 63 per 1000 | 14 per 1000 | Peto OR 0.21 | 1067 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate per woman | 248 per 1000 | 248 per 1000 | RR 1.00 | 1998 | ⊕⊕⊕⊝ | |
| Cancellation rate per woman | 80 per 1000 | 150 per 1000 | RR 1.87 | 1784 | ⊕⊕⊝⊝ | |
| Mean number of gonadotropin ampoules used per woman | The mean number of ampoules used in the control group ranged from 18 to 50. | In all studies CC plus gonadotropins was associated with use of fewer ampoules. The mean difference ranged from 5.6 to 24.6 ampoules | ‐ | 1098 | ⊕⊕⊕⊝ | |
| Mean number of oocytes retrieved per woman | The mean number of oocytes retrieved in the control group ranged from 5 to 17. | In seven studies CC plus gonadotropins was associated with retrieval of fewer oocytes, with the mean difference ranging from 1.02 to 6.20 oocytes. The difference was statistically significant in five of these studies. The eighth study found no evidence of a difference between the groups | ‐ | 1481 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level (serious risk of bias). All included studies had unclear risk of bias for allocation concealment. | ||||||
| Clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle antagonist) compared to gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders for controlled ovarian stimulation | ||||||
| Patient or population: Women undergoing controlled ovarian stimulation in IVF and ICSI cycles (poor responders) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with gonadotropins (with GnRH agonist or midcycle antagonist) | Risk with clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle antagonist) | |||||
| Live birth per woman | 49 per 1000 | 57 per 1000 | RR 1.16 | 357 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate per woman | 128 per 1000 | 109 per 1000 | RR 0.85 | 1462 | ⊕⊕⊝⊝ | |
| Cancellation rate per woman | 145 per 1000 | 212 per 1000 | RR 1.46 | 1601 | ⊕⊕⊝⊝ | |
| Mean number of gonadotropin ampoules used per woman | The mean number of ampoules used in the control group ranged from 39 to 71. | There were fewer ampoules used in the intervention groups (CC plus gonadotropins: MD ‐23.98, 95% CI ‐27.41 to ‐20.56; participants = 87; studies = 2); letrozole plus gonadotropins: MD ‐46.24, 95% CI ‐50.93 to ‐41.55; participants = 49; studies = 1). | ‐ | 136 | ⊕⊕⊕⊝ | |
| Mean number of oocytes retrieved per woman | The mean number oocytes retrieved in the control group ranged from 2 to 5. | In three of four studies CC plus gonadotropins versus gonadotropins in an agonist protocol was associated with retrieval of fewer oocytes, with the mean difference ranging from 0.75 to 2.10 oocytes. The difference was statistically significant in two of these studies. One study found no evidence of difference between CC plus gonadotropin versus gonadotropin in an antagonist protocol. Of three studies comparing letrozole plus gonadotrophins, one study reported significantly lower oocyte retrieval while the other two studies found no clear evidence of a difference. | ‐ | 1203 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level (serious risk of bias). Many of the included studies had unclear risk of bias for allocation concealment. | ||||||
| Study ID | Downregulation used | Type of FSH used | Starting dose of FSH | Dose of clomiphene citrateor letrozole | Cycle monitoring | Luteal support | Timing of hCG |
| Buserelin | hMG | 150 to 225 IU/day | 100 mg CC | Ultrasound | Progesterone | Leading follicle 17 mm | |
| Antagonist (cetrorelix subcutaneous) | hMG and recombinant FSH | hMG and recombinant FSH but in different doses: Group 1: 225 IU hMG + 225 IU rFSH Group 2: 150 IU hMG + 150 IU rFSH Group 3: 5mg Ltz + 150 IU rFSH | 5 mg Ltz | Ultrasound | Progesterone | Leading follicle > 17 mm | |
| Antagonist (ganirelix (Orgalutran) subcutaneous) for the Ltz group and triptorelin subcutaneous in the agonist control group | FSH | 75 IU for the Ltz group versus 150 to 225 IU for the control FSH/agonist group | 10 mg Ltz | Not mentioned | Not mentioned | Not mentioned | |
| Triptorelin intramuscular | hMG | hMG 125 to 300 IU/day | 200 mg CC | Ultrasound and oestradiol | hCG | Leading follicle 17 mm | |
| Ganirelix | hMG | Not mentioned | 100 mg CC | Not mentioned | Not mentioned | Not mentioned | |
| Not mentioned | hMG | 150 to 225 IU | 10 mg Ltz | Not mentioned | Not mentioned | Not mentioned | |
| Not mentioned | Recombinant FSH | 100 to 150 IU in the CC + gonadotropins group; 200 to 225 IU in the gonadotropins + GnRH agonist group) | Not mentioned | Not mentioned | Not mentioned | Not mentioned | |
| Triptorelin intramuscular depot | hMG | 150 to 225 IU/day | 100 mg CC | Ultrasound and oestradiol | Progesterone | Leading follicle 18 mm | |
| Triptorelin intramuscular and buserelin intranasal | hMG | 150 IU/day | 100 mg CC | Ultrasound and oestradiol | Progesterone | Leading follicle 17 mm | |
| Antagonist (cetrorelix subcutaneous) for the Ltz or CC group and GnRH agonist for the control group, type not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | |
| Leuprorelin | hMG | 150 IU/day | 50 mg CC | Ultrasound and oestradiol | Not mentioned | Leading follicle 15 mm | |
| Buserelin | Recombinant FSH | 150 to 225 IU/day | 100 mg CC | Ultrasound | Progesterone | Leading follicle 18 mm | |
| Buserelin nasal spray | hMG | According to age (225 IU for women < 35 years and 300 IU for women > 35 years) | 100 mg CC | Ultrasound and oestradiol | hCG | Leading follicle 17 mm | |
| Antagonist (cetrorelix subcutaneous) | hMG | 225 IU | 2.5 mg Ltz | Ultrasound and oestradiol | Progesterone | Leading follicle 18 mm | |
| Antagonist (cetrorelix subcutaneous) | hMG | 150 to 300 IU/day | 100 mg CC | Ultrasound, serum oestradiol, LH, and progesterone | Progesterone | Leading follicle 18 mm | |
| Leuprorelin (Lupron) | hMG | 150 IU/day | 50 mg CC | Ultrasound and oestradiol | None | Leading follicle 15 mm | |
| Antagonist (cetrorelix subcutaneous) for the Ltz group and agonist (leuprorelin) for the conventional agonist group | hMG | 150 IU for the Ltz group versus 300 IU for the control hMG/agonist group | 2.5 mg Ltz | Ultrasound and oestradiol | Progesterone | 18 mm | |
| Antagonist (ganirelix (Orgalutran) subcutaneous) | Recombinant FSH | 75 IU for the Ltz group versus 150 to 225 IU for the control FSH/antagonist group | 5 mg Ltz | Ultrasound and oestradiol | Progesterone | 18 mm | |
| The type of antagonist used was not mentioned in the study while the agonist used in the control group was buserelin | Recombinant FSH | 300 IU for the Ltz group versus 450 IU for the control FSH/agonist group | 5 mg Ltz | Ultrasound | Progesterone | 17 mm | |
| Antagonist (cetrorelix subcutaneous) | hMG | 150 IU for the CC group versus 300 IU for the control hMG/antagonist group | 100 mg CC | Ultrasound | Progesterone | 17 to 18 mm | |
| Buserelin | Recombinant FSH | 450 IU | 150 mg CC | Ultrasound and oestradiol | Progesterone | 18 to 20 mm | |
| Antagonist (cetrorelix or ganirelix (Orgalutran) subcutaneous); agonist was leuprorelin | hMG | 150 IU for the CC group versus 300 to 450 IU for the control hMG/antagonist group | 100 mg CC | Ultrasound and oestradiol | Progesterone | 18 to 20 mm | |
| Antagonist (cetrorelix subcutaneous) | Recombinant FSH | 450 IU for both groups | 100 mg CC | Ultrasound and oestradiol | Progesterone | 18 mm | |
| Leuprorelin subcutaneous | hMG | According to body weight (less than 52 kg would start with 75 IU/day, 52 to 75 kg would start with 112.5 IU/day, and 150 IU/day for women who weighed more than 75 kg) | 100 mg CC | Ultrasound and oestradiol | Progesterone | Leading follicle 16 mm | |
| Buserelin | Recombinant FSH | 150 IU/day | 100 mg | Ultrasound | Progesterone | Leading follicle 18 mm | |
| Buserelin | hMG | 225 to 300 IU/day | 100 mg | Ultrasound | Progesterone | Not mentioned | |
| CC: clomiphene citrate | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.27] |
| 1.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol. | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.27] |
| 1.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Ovarian hyperstimulation syndrome Show forest plot | 5 | 1067 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.11, 0.41] |
| 2.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 4 | 973 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.11, 0.47] |
| 2.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.03, 0.68] |
| 3 Ongoing pregnancy rate Show forest plot | 6 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 3.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 6 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 3.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Clinical pregnancy rate Show forest plot | 12 | 1998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.16] |
| 4.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| 4.2 Clomiphene citrate± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.72] |
| 4.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.71, 1.94] |
| 5 Cancellation rate Show forest plot | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.43, 2.45] |
| 5.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.43, 2.45] |
| 5.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean number of ampoules used Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Letrozole ± gonadotropins ± antagonists vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mean number of oocytes retrieved Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 8 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Letrozole ± gonadotropins ± antagonists vs agonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Multiple pregnancy rate Show forest plot | 5 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.39, 1.43] |
| 8.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 4 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.57] |
| 8.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.04, 3.82] |
| 9 Rate of miscarriage Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.47] |
| 9.1 Clomiphene citrate ± gonadotropins ± antagonists vs. agonist protocol | 6 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.61, 1.75] |
| 9.2 Clomiphene citrate ± gonadotropins ± antagonists vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Letrozole ± gonadotropins ± antagonists vs. antagonists protocol | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.35, 1.66] |
| 10 Rate of ectopic pregnancy Show forest plot | 2 | 223 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.56 [0.47, 120.94] |
| 10.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 2 | 223 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.56 [0.47, 120.94] |
| 10.2 Clomiphene citrate ± gonadotrophins ± antagonists vs. antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Letrozole ± gonadotropins ± antagonists vs. agonists protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Letrozole ± gonadotropins ± antagonists vs. antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Rate of foetal abnormalities Show forest plot | 1 | 74 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Clomiphene citrate ± gonadotropins ± antagonists vs. GnRHagonists or antagonist protocol | 1 | 74 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Letrozole ± gonadotropins ± antagonists vs. GnRH agonist or antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.49, 2.79] |
| 1.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.24, 2.32] |
| 1.2 Clomiphene citrate ± gonadotrophin ± antagonists vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Letrozole ± gonadotrophin ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Letrozole ± gonadotropin ± antagonist vs. antagonist protocol | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.55, 12.22] |
| 2 Ongoing pregnancy rate Show forest plot | 2 | 748 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.58, 1.28] |
| 2.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.19] |
| 2.2 Clomiphene citrate ± gonadotrophin ± antagonists vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Letrozole ± gonadotrophin ± antagonists vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Letrozole ± gonadotropin ± antagonists vs. antagonist protocol | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.55, 12.22] |
| 3 Clinical pregnancy rate Show forest plot | 8 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.12] |
| 3.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 3 | 1069 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.27] |
| 3.2 Clomiphene citrate ± gonadotrophin ± antagonists vs. antagonists protocol | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.05, 12.84] |
| 3.3 Letrozole ± gonadotropin ± antagonists vs. agonists protocol | 3 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.13] |
| 3.4 Letrozole ± gonadotropin ± antagonists vs. antagonists protocol | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.38, 2.86] |
| 4 Cancellation rate Show forest plot | 10 | 1601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.18, 1.81] |
| 4.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 4 | 1155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.20, 2.10] |
| 4.2 Clomiphene citrate ± gonadotropin ± antagonists vs. antagonists protocol | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.39, 1.53] |
| 4.3 Letrozole ± gonadotropin ± antagonist vs. agonists protocol | 3 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.10, 3.13] |
| 4.4 Letrozole ± gonadotropin ± antagonists vs. antagonists protocol | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.67, 2.01] |
| 5 Mean number of ampoules used Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Clomiphene citrate ± gonadotropin ± antagonists vs. agonist protocol | 2 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐23.98 [‐27.41, ‐20.56] |
| 5.2 Clomiphene citrate ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Letrozole ± gonadotropin ± antagonist vs. agonist protocol | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐46.24 [‐50.93, ‐41.55] |
| 5.4 Letrozole ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean number of oocytes retrieved. Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Clomiphene citrate ± gonadotropin ± antagonists vs. agonist protocol | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Clomiphene citrate ± gonadotropin ± antagonist vs. antagonist protocol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Letrozole ± gonadotropin ± antagonists vs. agonist protocol | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Letrozole ± gonadotropin ± antagonists vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Multiple pregnancy rate Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.75] |
| 7.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.75] |
| 7.2 Clomiphene citrate ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Letrozole ± gonadotrophin ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Letrozole ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Rate of miscarriage Show forest plot | 3 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.45, 2.12] |
| 8.1 Clomiphene citrate ± gonadotropin ± antagonists vs. agonist protocol | 2 | 765 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.55, 3.01] |
| 8.2 Clomiphene citrate ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Letrozole ± gonadotrophin ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Letrozole ± gonadotropin ± antagonists vs. antagonist protocol | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.73] |

