نقش مصرف مکمل ویتامین A در پیشگیری از بروز مرگومیر (mortality) و بیماری (morbidity) در کودکان سنین شش ماه تا پنج سال
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Cluster‐randomised trial conducted in Uttar Pradesh, India | |
| Participants | Eligibility: all children below 6 years of age were eligible for inclusion in the trial. Children with xerophthalmia were excluded. Sample: a total of 16 clusters (subcentres) were randomly selected and divided into 4 subdivisions (4 subcentres in each), with drugs A (vitamin A) and B (placebo) distributed in 2 each randomly. At the end of the study, investigators found that vitamin A was distributed in 3 subdivisions (12 subcentres) and placebo in 1 only (4 subcentres) by mistake. A total of 17,778 children were approached but only 15,247 children were included in the final analysis based on the fact that they received at least 1 dose of vitamin A. | |
| Interventions | Children in the experimental group received vitamin A along with small amounts of vitamin E. The dosages were 50,000 IU of vitamin A and 10 IU of vitamin E for children aged 1‐6 months and 100,000 IU of vitamin A and 20 IU of vitamin E for children aged 7‐72 months. The intervention was delivered every 4 months and continued for 12 months. | |
| Outcomes | All‐cause and cause‐specific mortality due to diarrhoea, pneumonia, measles, and meningitis | |
| Notes | The trial was conducted in 2 phases. The first phase consisted of 15 months (i.e. 3 months for registration and 12 months for intervention and measurement of relevant outcomes). In the second phase, mortality was measured in a sub‐sample of initially‐included children, exactly 12 months after termination of first phase. The cause of death was assigned by using a verbal autopsy tool. Baseline mortality rates for children below 6 years of age were 27.7 and 23.3 per 1000 for intervention and control group, respectively, with significant differences in the 2 groups (P < 0.01). According to WHO, India is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Out of the total 43 subcentres, 16 were randomly selected, four subdivisions (4 subcentres in each) were made and drugs A and B distributed in two each randomly" Comment: authors do not specify the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Other bias | Unclear risk | Comment: insufficient information to permit judgment |
| Methods | Factorial design, individually‐randomized trial conducted in Dhaka, the capital city of Bangladesh | |
| Participants | Eligibility: children aged 2‐5 years of either sex, vitamin A deficiency (serum retinol level < 20 mg/dL; and nutritional status corresponding to a weight‐for‐age score that was 61% of the median National Center for Health Standards standard were included. Children who had received vitamin A supplementation during the preceding 6 months or who had a history of night blindness or sickness due to underlying illnesses such as diarrhoea or respiratory tract infections were excluded. Sample: 256 children | |
| Interventions | 4 groups:
| |
| Outcomes | Vibriocidal antibody response to cholera vaccine | |
| Notes | No clinical outcomes were available so no data were included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Bottles of syrup were serially numbered according to the randomizations list" Comment: most likely done |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomizations code was broken after completion of the study" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The zinc syrup and its placebo syrup looked very similar, as did the vitamin A syrup and its placebo syrup." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The randomization code was broken after completion of the study" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The randomization code was broken after completion of the study" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: minimal attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: no trial registration number was available |
| Other bias | Low risk | Comment: this study seems to be free of other bias |
| Methods | Individually‐randomised trial conducted in New Delhi, India | |
| Participants | Eligibility: infants aged 9‐12 months attending the immunisation clinic of Safdurjung hospital in New Delhi were eligible for inclusion in the trial. Sick infants requiring hospitalisation excluded Sample: 256 infants, with equal numbers (i.e. 128) in vitamin A and placebo group. Mean age of participants was 9 months | |
| Interventions | The experimental group received a single dose of 100,000 IU of vitamin A in arachis oil. The control group received placebo in peanut oil. Both vitamin A and placebo were administered at the time of measles vaccination. At the end of the study, the vitamin A group received placebo, and the placebo group received vitamin A. | |
| Outcomes | Incidence of side effects in first 24 hours (vomiting, loose motions, fever, irritability, bulging fontanelle) | |
| Notes | Study participants were not significantly different in sex, age, and weight distribution, and nutritional status at the baseline.The baseline prevalence of vomiting, loose stools, fever, and irritability during the 24 hours prior to dosing was similar in both groups. 97.3% of the included infants had normal serum retinol level before the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The infants were randomised . . . according to the order of arrival at hospital. Randomisation was done by the nurse who gave measles vaccine to these children." Comment: probably not done |
| Allocation concealment (selection bias) | Unclear risk | Comment: children were randomised according to their entry into hospital |
| Blinding (performance bias and detection bias) | Low risk | Quote: "This double‐blind, randomised . . . supplied in small dark bottles marked '1' and '2'." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "This double‐blind, randomised . . . supplied in small dark bottles marked '1' and '2'." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "This double‐blind, randomised . . . supplied in small dark bottles marked '1' and '2' . . . Two clinicians examined each of the infants at both first and second visits. Neither clinician knew the bottle code." |
| Incomplete outcome data (attrition bias) | High risk | Comment: a total of 39 (15.2%) infants were lost to follow‐up with similar distribution in both the groups. Reasons for loss to follow‐up not given |
| Selective reporting (reporting bias) | High risk | Comment: methods describe that the clinicians did physical examinations and recorded weight, nutritional status, any signs of vitamin A deficiency, heart rate, respiratory rate, temperature, and systemic examination, especially neurological examination including the state of the fontanelle, reflexes, motor and sensory functions, etc. But bulging fontanelle not reported as an outcome, nor other variables mentioned in the results |
| Other bias | Low risk | Comment: no other apparent bias |
| Methods | Individually‐randomised study conducted in an urban slum of Delhi, India | |
| Participants | Eligibility: infants aged 6‐9 months were identified and enrolled into the study when they turned 9 months old. Infants who had a previous history of measles, contact with a case of measles or measles immunisation, or had received a dose of vitamin A in the previous 4 months were excluded . Participants with serious illness requiring hospitalisation or having clinical signs of vitamin A deficiency (i.e. xerophthalmia, Bitot's spots, etc.) were also excluded. Sample: 618 infants randomised either to vitamin A (N = 309) or placebo group (N = 309). 50% of the study population consisted of male infants. | |
| Interventions | Participants in the intervention group were given a single dose of 30 mg (100,000 IU) of vitamin A in the form of retinol palmitate, and the control group received soybean oil as placebo. Children were followed for 4 months. | |
| Outcomes | Antibody response to measles vaccine, incidence of measles during study period, and side effects (like vomiting, drowsiness,etc.) in first 48 hours were also reported. | |
| Notes | The primary objective of the study was to determine the response to measles vaccine when administered along with vitamin A at 9 months of age. The study found no significant difference in antibody titres between the 2 groups at 3 months after the administration of intervention.The baseline prevalence of clinical vitamin A deficiency in children aged 1‐5 years in the study area was 3.5% and that of biochemical vitamin A deficiency was 37%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infants were randomly assigned to receive vitamin A or a placebo by using a simple randomisation scheme with random permuted blocks of size eight, i.e. four infants each out of every eight infants enrolled were randomised to receive vitamin A or a placebo." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Low risk | Quote: "This scheme ensured that all infants received 30 mg vitamin A by 12 mo of age without interfering with the double‐blind design of the study." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: adequate masking of vitamin A and placebo should have meant that providers were adequately blinded |
| Blinding (performance bias and detection bias) | Low risk | Comment: adequate masking of vitamin A and placebo should have meant that outcome assessors were adequately blinded |
| Incomplete outcome data (attrition bias) | High risk | Comment: losses to follow‐up and exclusions described. Missing data excluded from the analysis. It is not possible to ascertain whether the exclusion of data from 17% of participants (equally distributed between treatment groups) would have impacted on the results. The investigators state that the reason for their exclusion is that a follow‐up serum sample could not be ascertained. |
| Selective reporting (reporting bias) | High risk | Comment: data on harms are incompletely disclosed in the study report |
| Other bias | Low risk | Comment: this study appears to be free of other bias |
| Methods | Individually‐randomised trial conducted in Serrinha, Brazil | |
| Participants | Eligibility: children aged 6‐48 months were eligible for inclusion in the trial. The exclusion criteria was presence of xerophthalmia or measles infection within the previous 30 days. Children who received a high dose of vitamin A supplementation in the previous 6 months or had weight‐for‐age less than 60% of the statistical median were also excluded. Sample: a total of 1240 children were included, 620 in vitamin A group and 620 in placebo. Mean age of participants was 28 months, and proportion of boys was 52% | |
| Interventions | The experimental group received vitamin A in a dose of 100,000 IU for children younger than 12 months and 200,000 IU for children older than 12 months. The control group received placebo only. The intervention was delivered every 4 months for 1 year. | |
| Outcomes | All‐cause mortality, incidence and prevalence of diarrhoea and respiratory tract disease, incidence of measles and xerophthalmia | |
| Notes | The study area had inadequate pubic health services. A previous survey in the area showed a biochemical deficiency (serum vitamin A concentration < 0.35 mmol/L) rate of 7.4% in children of this age group. According to WHO criteria, vitamin A deficiency should be considered a pubic health problem in this area. The surveillance for morbidity outcome was done 3 times/week for 1 year, so the recall period was 48‐72 hours. We took data for incidence of measles and xerophthalmia from account of attrition in Results section. According to WHO, Brazil does not have a high child mortality rate (i.e. < 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children were randomly assigned to receive vitamin A or placebo four times‐at the start of the trial and every 4 months thereafter." Comment: authors do not specify the method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: ". . .only an external investigator had the codes for the individually wrapped and numbered capsules." Comment: although specific details were not disclosed, the available information suggests that allocation was adequately concealed. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The gelatinous capsules of vitamin A and placebo (supplied by Hoffman La Roche) were identical in appearance and were unwrapped just before administration." Comment: the study was double‐blind, with identical presentation and dosing of vitamin A and placebo. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The gelatinous capsules of vitamin A and placebo (supplied by Hoffman La Roche) were identical in appearance and were unwrapped just before administration." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The study was kept double‐blind and only an external investigator had the codes for the individually wrapped and numbered capsules." Comment: if the assessors were not involved in the allocation process as suggested by the available information, outcome assessors were likely to have been blinded to treatment group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The total loss in follow‐up time was 10.3%, equally distributed between the study groups." Comment: the rate of attrition was balanced between the 2 treatment groups and was primarily attributable to migration. On that basis, attrition bias is not likely to have impacted on the results of the review. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study was not available and, as such, this aspect of the reporting of the study could not be assessed. |
| Other bias | Low risk | Comment: this study appears to be free of other potential bias. |
| Methods | Individually‐randomised trial conducted in Belem and Mindra, 2 districts in Bissau, the capital of Guinea‐Bissau | |
| Participants | Eligibility: infants aged 6‐9 months were eligible for inclusion in the trial. Those with signs of xerophthalmia, history of previous vitamin A supplementation, history of measles infection before 9 months of age, or who had a positive haemagglutinin‐inhibition assay (HIA) titre at 9 months of age were excluded. All infants reported to have had measles at 9‐18 months of age were also excluded. Sample: a total of 462 infants were randomised to either intervention or control group. The mean age of participants was 8.7 months, and proportion of boys was 51%. | |
| Interventions | There were 3 study groups:
Vitamin A was supplemented in a single dose of 100,000 IU dissolved in 1 mL of vegetable oil along with 40 IU of vitamin E. The placebo was 40 IU of vitamin E dissolved in 1 mL of vegetable oil. | |
| Outcomes | Antibody response to measles vaccine, all‐cause mortality, incidence of measles | |
| Notes | The primary objective of the study was to calculate the antibody response to measles vaccine when given with vitamin A. The results for antibody response to measles vaccine showed no significant difference between the groups. The study concluded that simultaneous administration of measles vaccine and vitamin A has no negative effect on measles immunity. Similarly, vitamin A supplementation was shown to have no significant effect on immune response of CD4 and CD8 T‐cell in children without clinical vitamin A deficiency. Vitamin A or placebo was given only at 9 months of age in all 3 study groups. The only difference among the groups was the frequency and type of vaccine administered. We therefore added all the numbers for all 3 intervention and placebo groups to report the outcomes of interest to our review. We primarily took data from trial flow diagram and calculated the effect sizes accordingly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation sequence was computer generated." |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was kept in sealed envelopes and only released when all clinical laboratory analyses were completed." |
| Blinding (performance bias and detection bias) | Low risk | Quote: ". . .because of the young age of the participants, any difference in taste was irrelevant . . ." Comment: identical presentation; probably adequate |
| Blinding (performance bias and detection bias) | Low risk | Quote: "None of the staff involved knew whether the bottles contained vitamin A or placebo . . ." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "None of the staff involved knew whether the bottles contained vitamin A or placebo . . ." Comment: masking of treatment group assignment and treatment to study personnel likely to have been maintained throughout. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: number lost to follow‐up and those excluded were explicitly described and equal in both the groups. Loss to follow‐up exceeded the number of deaths and children with measles. Reasons for missing data (migration) probably unrelated to treatment |
| Selective reporting (reporting bias) | Low risk | Comment: some evidence of selective outcome reporting around malaria; however, deaths and prevalence of measles reported |
| Other bias | Unclear risk | Comment: authors report imbalance in self‐reported disease in the children aged 6 months at baseline. It is unclear how big an impact this will have had as the variable is not specific |
| Methods | Individually randomised, placebo‐controlled trial conducted in Gobinda‐Khatick slum area of eastern Calcutta, India | |
| Participants | Eligibility: children aged 12‐71 months were eligible for inclusion in the study. Participants with signs of vitamin A deficiency (for example, xerophthalmia) were excluded. Sample: 180 children were randomised either to vitamin A or placebo group. Mean age of children and proportion of boys were not specified in the study. | |
| Interventions | The experimental group received 200,000 IU of vitamin A in the form of retinyl palmitate. The control group received placebo. Only a single dose of intervention was administered and children were followed for 6 months. | |
| Outcomes | Incidence of diarrhoea and acute respiratory tract infection | |
| Notes | The baseline age and nutritional characteristics were similar in both the groups. The surveillance for morbidity outcomes was done twice monthly. For respiratory disease morbidity, we took data for lower respiratory tract infection only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For each strata, a restricted randomisation list was prepared . . . a random permutated block of block length 6 was used." Comment: block randomisation by age and weight; probably done |
| Allocation concealment (selection bias) | Low risk | Quote: ". . . randomisation was done by a pharmacist of the drug manufacturing company." Comment: assuming that the pharmacist was independent of the study team, this was probably adequate |
| Blinding (performance bias and detection bias) | Low risk | Quote: ". . . identical (colour and taste) placebo. Both drug and placebo were prepared and dispensed in a single dose amber coloured glass ampoule by a local pharmaceutical company." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "For keeping the trial totally blinded to all participants (for example, patients, investigators, surveyor), randomisation was done by a pharmacist of the drug manufacturing company. Samples of drug (or placebo) were identified by the code number of the respective child." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "For keeping the trial totally blinded to all participants (for example, patients, investigators, surveyor), randomisation was done by a pharmacist of the drug manufacturing company. Samples of drug (or placebo) were identified by the code number of the respective child." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: ". . . data was analysed for 174 children due to attrition of 6 children for various reasons (for example, 5 children were hospitalised due to illnesses unrelated to the study objectives and the death of 1 child due post‐measles bronchopneumonia)." Comment: attrition was low and reported not to relate to treatment. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol was not available to permit a clear judgement. Study aims were to measure diarrhoea and respiratory infection; both outcomes were reported in full in the study report. 1 child died and the treatment group assignment was not disclosed. |
| Other bias | Low risk | Comment: this study appears to be free of other bias. |
| Methods | Factorial design, individually randomised trial conducted in Chengdu City, China | |
| Participants | Eligibility: children aged 3‐6 years, apparently good health, haemoglobin (Hb) concentration > 60 g/L, serum C‐reactive protein (CRP) < 10 mg/L, parental or guardian's approval for participation and parental or guardian's agreement to avoid additional use of vitamin A and iron supplements during the investigation were eligible for inclusion. Children with evidence of recent acute or chronic illnesses and/or Hb <60 g/L were excluded. Sample: 387 children were included in the study | |
| Interventions | 4 groups:
| |
| Outcomes | Incidence of diarrhoea and LRTI | |
| Notes | The study setting was a periurban area in Huayuan Town, Pixian County of Chengdu City, Sichuan Province, western China, from March to September 2011. Supplementation was given in schools. The paper did not have a study flow diagram. The data from the factorial design were included in 2 data sets. The first data set (Chen 2013a) is the comparison between Vitamin A and placebo while the second data set (Chen 2013b) is the comparison between vitamin A + iron vs iron only. The data for meta‐analysis was taken from table 2 and we calculated the rate ratio based on the number of events in the intervention and control groups with the denominator as person‐days at risk. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The RAND function of Excel (Microsoft, Redmond,WA, USA) was used to generate computer randomly permutated codes" |
| Allocation concealment (selection bias) | Low risk | Quote: "The health care workers, outcome assessors, data analyst and children were not made aware of the intervention assignment until the completion of data analysis." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Children were not made aware of the intervention". |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The health care workers, outcome assessors, data analyst and children were not made aware of the intervention. . ." |
| Blinding (performance bias and detection bias) | Low risk | Quote: ". . . outcome assessors, data analyst and children were not made aware of the intervention . . ." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: loss to follow‐up was 13% and balanced in each group with similar reasons for attrition. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the trial registration number was not given. Authors do mention that they could not report some of the a priori mentioned serum biochemical markers, as they could not collect enough blood samples. |
| Other bias | Low risk | Comment: the study seems to be free of other bias. |
| Methods | — | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | Same as Chen 2013a above | |
| Methods | Randomised trial conducted in a rural area of China | |
| Participants | Eligibility: children aged 6 months to 3 years were eligible for inclusion in the trial Sample: 198 children were randomised either to vitamin A or placebo group. There were 105 children in the vitamin A group and 81 in the placebo group. Mean age of children and proportion of boys were not specified in the study. | |
| Interventions | Vitamin A was supplemented in a dose of 200,000 IU for children aged > 12 months and 100,000 IU for children aged < 12 months. The control group received placebo in the form of vegetable oil. Interventions were given every 4 months for 12 months. | |
| Outcomes | Incidence of diarrhoea and respiratory disease, all‐cause hospitalisations, diarrhoea‐specific hospitalisations, pneumonia‐specific hospitalisations, mean vitamin A serum levels | |
| Notes | Baseline serum levels of retinol were similar in both groups. Measurement of biochemical vitamin A levels in the study area fulfilled the WHO criterion for an action to be triggered at a pubic health level. Morbidity surveillance was done twice a month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "198 children who were randomly assigned on a 3:2 allocation to treatment (105) and control (81) groups." Comment: no more information was provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Administration was double blind: neither parents nor doctors knew whether the child was in a treatment or control group." Comment: placebo capsules contained vegetable oil and were likely to have been indistinguishable from intervention. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Placebo capsules contained vegetable oil and were likely to have been indistinguishable from intervention." Comment: in view of the adequate blinding procedures, performance bias was unlikely to have influenced the results. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Data collected by doctors who were already blind to treatment group assignment." |
| Incomplete outcome data (attrition bias) | High risk | Comment: reasons for loss to follow‐up were not provided. The number randomised and those reported after loss to follow‐up do not match. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol of study was not available to permit a clear judgement |
| Other bias | Low risk | Comment: this study appears to be free of other bias |
| Methods | Individually‐randomised trial conducted in Vellor, India | |
| Participants | Eligibility: infants aged 9‐12 months were eligible for inclusion in the study. Participants with a previous history of measles vaccination or an exanthematous illness, with moderate or severe malnutrition, clinical signs of vitamin A deficiency, known immune deficiency or on immunosuppressive therapy, and those who had received blood or blood products in the previous 6 months were excluded. Sample: 395 infants were randomised to either vitamin A or placebo group. There were 198 infants in the vitamin A group and 197 in the placebo group. Mean age of participants was 9.8 months, and proportion of boys was 52% | |
| Interventions | Infants in experimental group received a single dose of vitamin A in a dose of 100,000 IU. The control group received placebo only. Interventions were given out at the time of measles vaccination. | |
| Outcomes | Antibody response to measles vaccine | |
| Notes | The primary objective of the study was to measure the antibody response to measles vaccine when given with and without vitamin A. This study found no significant inhibitory or enhancing influence on antibody response to measles vaccine when administered concomitantly with vitamin A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The infants who were immunized with monovalent measles vaccine were randomly assigned, in blocks of eight, to concomitantly receive 100,000 IU of Vitamin A in arachis oil or a placebo containing carboxymethylcellulose prepared in the hospital pharmacy." Comment: authors do not specify the method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: ". . . arachis oil or a placebo containing carboxymethylcellulose prepared in the hospital pharmacy." Comment: probably done since hospital pharmacy was responsible for preparing the order of vitamin A and placebo, and not likely to have been internal to the study team. |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: ". . . Vitamin A in arachis oil or a placebo containing carboxymethylcellulose . . ." Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) | High risk | Comment: the proportion of children providing adequate samples is low at 6 months, and there is insufficient detail about the reasons for missing data |
| Selective reporting (reporting bias) | High risk | Comment: there is no mention of mortality or any morbidity of measles or diarrhoea |
| Other bias | Unclear risk | Comment: insufficient information to permit judgment |
| Methods | Individually‐randomised trial conducted in urban slums of Chandigarh, India | |
| Participants | Eligibility: children aged < 10 years were eligible for inclusion in the study. Children with xerophthalmia and previous history of vitamin A supplementation were excluded. Sample: 1520 children were randomised either to vitamin A or placebo group. There were 756 children in the vitamin A group and 759 in the placebo group. Mean age of participants was 51 months, and proportion of boys in study sample was 50% | |
| Interventions | The experimental group received vitamin A in a dose of 50,000 IU for children aged < 6 months; 100,000 IU for children aged 6‐12 months and 200,000 for children aged > 1 year. The control group received placebo. The intervention was given every 4 months for 15 months. | |
| Outcomes | All‐cause mortality; cause‐specific mortality due to diarrhoea, pneumonia, and meningitis; incidence of diarrhoea, pneumonia, and measles. Measuerement of subclinical vitamin A deficiency status was by conjunctival impression cytology. | |
| Notes | Baseline sociodemographic and anthropometric characteristics were similar in both the groups. The study population had a high prevalence of vitamin A deficiency. Children were contacted every 15 days by home visits to obtain information on morbidity and mortality. The study included children < 10 of years of age; however, the mean age of the children was 51 months. Study methods were not explicitly described. According to WHO, India is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "From three slums of Chandigarh, 1520 non‐xerophthalmic children of less than 10 years of age were individually randomised in equal number to receive vitamin A or placebo." |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "An equivalent volume of arachis oil was given as placebo." Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) | High risk | Comment: although attrition rates were balanced, the rates of mortality were lower than the rate of withdrawal. This could impact on the reliability of the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Other bias | Unclear risk | Comment: study not sufficiently reported in order to assess this item fully |
| Methods | Cluster‐randomised, non‐placebo controlled trial conducted in Jumla district, Nepal | |
| Participants | Eligibility: children aged 1‐59 months were eligible for inclusion in the trial. Sample: 16 clusters were randomly assigned either to vitamin A or control group. These included 7197 children, of which 3786 children were in the vitamin A group and 3411 were in the control group. Proportion of boys was 51%. | |
| Interventions | In experimental group, vitamin A was given in doses of 200,000 IU for children aged 12‐59 months; 100,000 IU for children aged 6‐12 months; and 50,000 IU for children aged < 6 months old. Vitamin A was supplemented once only and children were followed for 5 months | |
| Outcomes | All‐cause mortality and cause‐specific mortality due to diarrhoea, pneumonia, and measles | |
| Notes | The study site was a remote, mountainous region of northwestern Nepal with a total population of about 80,000, with 12,000 children under 5 years of age. This area was considered as one of the poorest and most medically underserved areas of the country. The infant mortality rate was 189 deaths per 1000 live births and child (1‐4 years) mortality rate was 52 per 1000 per year. Malnutrition was prevalent in the study area, and 26% of children aged 1‐4 years were suffering from substantial malnutrition. A survey of 3651 children under 5 years of age showed active xerophthalmia in 1.3% to 2% of population and 1% to 5% among infants, which is high for this age group. Disaggregated data on mortality was available according to different age groups. We have used data for children aged 6‐59 months according to the objectives of our review. According to WHO, Nepal is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly selected by card eight of the 16 sub‐districts for vitamin A supplementation." Comment: probably done |
| Allocation concealment (selection bias) | High risk | Comment: author contacted and replied. Quote from author: "No effort was made to conceal the allocation sequence." |
| Blinding (performance bias and detection bias) | High risk | Quote: "There was no placebo or blinding." |
| Blinding (performance bias and detection bias) | High risk | Quote: "There was no placebo or blinding." |
| Blinding (performance bias and detection bias) | High risk | Quote: "There was no placebo or blinding." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there was no loss to follow‐up; coverage of intervention described in detail |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Other bias | Low risk | Comment: this study appears to be free of other bias. |
| Methods | Factorial design, cluster‐randomised trial conducted in Northern India | |
| Participants | Eligibility: children aged 1‐6 years were eligible for inclusion in the review. Sample: total clusters were 72, of which 36 clusters received vitamin A supplementation while 36 acted as control. Authors claimed to include 1 million children in the trial. | |
| Interventions | Children in the experimental group received 200,000 IU of vitamin A every 6 months for 5 years. Vitamin A was supplemented on mass treatment days by village child care workers. Capusles were open and poured into child's mouth. The control group did not receive any intervention (no placebo tablets). The factorial design was as follows:
| |
| Outcomes | All‐cause mortality; cause‐specific mortality due to diarrhoea, pneumonia, measles, and malnutrition; mean vitamin A serum levels; prevalence of Bitot's spots, and measles and pneumonia morbidity | |
| Notes | This study was conducted in Uttar Pradesh, India. The study utilised the infrastructure of the Integrated Child Development Services (ICDS), which maintains child care centres called Anganwadi child care (AWC) centres across the state. The other intervention as part of the factorial design was albendazole for deworming. The study was approved by King George's Medical University. Surveillance for disease outcomes was done every 6 months, and children were not selected randomly for that but chosen from AWC lists. Deaths were recorded by 18 full‐time, motorcycle village‐to‐village monitors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Neighbouring blocks (clusters), in groups of four (where possible in the same district), were randomly allocated in Oxford, UK", and "[a]part from the district each block was in, no relevant details of it were known to those generating the random allocation." Comment: most likely done |
| Allocation concealment (selection bias) | Low risk | Quote: "Apart from the district each block was in, no relevant details of it were known to those generating the random allocation". |
| Blinding (performance bias and detection bias) | High risk | Comment: the intervention was given on mass treatment days, and no placebo tablets were used. So participants most likely were not blinded to treatment allocation. |
| Blinding (performance bias and detection bias) | High risk | Comment: again, intervention was delivered on mass treatment days by AWC and treatment was known to AWCs. |
| Blinding (performance bias and detection bias) | High risk | Comment: outcomes assessors seems to be aware of the treatment allocation and control, as parents were asked if their children received intervention on mass treatment days. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: loss to follow‐up was 2% |
| Selective reporting (reporting bias) | Low risk | Comment: the trial was registered as NCT00222547, and pre‐specified outcomes were mentioned in protocol and analysed accordingly |
| Other bias | High risk | Comment: there are concerns that surveillance for implementation of intervention and assessment of outcomes are not rigorous. |
| Methods | Individually‐randomised trial conducted in 34 rural villages located on the southern coast of Central Java in Indonesia | |
| Participants | Eligibility: children aged 6‐47 months were eligible for inclusion. Children with cerebral palsy, epilepsy, flaccid paralysis, mental retardation, congenital or rheumatic heart disease were permanently excluded. Those with weight‐for‐height more than 3 SDs below the WHO growth reference mean or acute xerophthalmia were excluded for one cycle and treated with high‐dose vitamin A and then included. Sample: 1405 children were randomised to either the vitamin A group or the placebo group; proportion of boys was 50.9% | |
| Interventions | The intervention group received 206,000 IU of vitamin A in the form of retinyl ester plus 37 IU vitamin E for children aged > 12 months or 103,000 IU retinyl ester plus 17 IU vitamin E for children aged < 12 months of age. The control group received placebo that contained 17 IU or 37 IU vitamin E according to the age of the participant. The intervention was given every 4 months for 24 months. An average of 89% of the children received a treatment (vitamin A or placebo). | |
| Outcomes | All‐cause mortality, incidence of diarrhoea and respiratory disease, mean vitamin A serum level, proportion of vitamin A deficient, growth | |
| Notes | Baseline demographic, clinical and nutritional characteristics of the participants were the same, and the groups remained balanced at the start of each of the other 5 cycles. Children were visited every other day for 6 cycles. The longest recall period allowed was 4 days. Observed child‐days of ALRI of the vitamin A group and the control group were 280,186 and 273,630 respectively. According to WHO, Indonesia is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of the treatments was done with a 1:1 allocation ratio in blocks of eight, based on a table of random permutations of integers" Comment: likely to be adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "All investigators, field and laboratory staff, and participants were masked to the treatment code." Quote: "The capsules were packaged in opaque blister packs with a unique treatment code." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The oily contents of the vitamin A and placebo capsules were of similar taste and colour." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "All investigators, field and laboratory staff, and participants were masked to the treatment code." Comment: adequate allocation concealment and the identical presentation of placebo and vitamin A should have prevented providers becoming unblinded to treatment group assignment. Low risk of performance bias |
| Blinding (performance bias and detection bias) | Low risk | Quote: "All investigators, field and laboratory staff, and participants were masked to the treatment code." Comment: adequate allocation concealment and the identical presentation of placebo and vitamin A should have prevented outcome assessors becoming unblinded to treatment group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: complete details of those excluded and lost to follow‐up with reason were described. There was a low and balanced number of withdrawals between the treatment groups. The analytical method took account of the time on treatment (i.e. follow‐up time for each cycle), and this may have been adequate. |
| Selective reporting (reporting bias) | Low risk | Comment: lack of trial protocol hinders full assessment of this item. However, data on outcomes of relevance to the review were reported. |
| Other bias | Low risk | Comment: this study appears to be free of other bias. |
| Methods | Individually‐randomised, non‐placebo controlled trial conducted in South Kivu province of Congo | |
| Participants | Eligibility: children aged 0‐72 months were eligible for inclusion in the trial. Children were recruited as soon they were discharged from Kotive children's hospital. No exclusion criteria described Sample: 358 children were randomly assigned to vitamin A, mebendazole, or control group. Vitamin A group had 118 children and control group had 117. | |
| Interventions | There were 3 study groups. The first group was supplemented with vitamin A, the second group received mebendazole for deworming and the third group was simply observed as control. Children in the vitamin A group received retinol palmitate in a dose of 100,000 IU for children aged < 1 year and 200,000 IU for those > 1 year. Supplementation was repeated after 6 months and continued for 12 months | |
| Outcomes | All‐cause mortality, growth, and incidence of diarrhoea and respiratory disease morbidity | |
| Notes | Morbidity surveillance was done every 2 weeks for the first 3 months, then every 3 months until 12 months. Data on morbidity outcomes were presented in the form of odds ratios based on generalised estimating equation models. As we were using the data in the form of risk ratios, and no nominators were given in this study, we could not pool the data for diarrhoea and respiratory morbidity from this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "As soon as the children were discharged from the hospital, they were randomly assigned to one of the three groups." Comment: probably not done |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient details available to make a judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient details available to make a judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient details available to make a judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient details available to make a judgment |
| Incomplete outcome data (attrition bias) | Low risk | Comment: authors indicate that 6% were lost to follow‐up, not discussed in detail. Number died but not indicated how or by group data. Overall, 6% of the children were lost to follow‐up, with approximately equal proportions in each group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient details available to make a judgment |
| Other bias | Low risk | Comment: this study appears to be free of other bias. |
| Methods | Individually‐randomized, double‐blind trial conducted in Guinea‐Bissau | |
| Participants | Eligibility: children aged 6‐23 months were included. Exclusion criteria were vitamin A supplementation within the preceding month, and participation in another trial Sample: 7587 children were randomised to either intervention or control group | |
| Interventions | For those in the experimental group, vitamin A was given in an amount of 100,000 IU for children aged 6‐11 months and 200,000 IU for children aged 12‐23 months. For those in the control group, placebo was given in the same liquid amount as that in the intervention group. Supplementation was given at the time of vaccination. The vitamin A bottles contained vegetable oil with 200,000 IU vitamin A as retinyl palmitate and 40 IU vitamin E per mL oil; placebo bottles contained only 40 IU vitamin E per mL oil. | |
| Outcomes | All‐cause mortality, sex‐specific mortality, diarrhoea incidence, respiratory infection, adverse events | |
| Notes | Children who died because of accident were censored from mortality data analysis. We used the raw data to calculate the mortality and morbidity estimates (i.e. number of events in intervention group compared to control, with denominators as time of follow‐up) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The mother then drew a lot from an envelope prepared by the study supervisor." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Coded vitamin A and placebo supplements were prepared by Skanderborg Pharmacy, Denmark." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The dark brown bottles contained 10 ml." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: both the interventions were placed in a similar bottle so it is less likely that those provided knew the allocation. |
| Blinding (performance bias and detection bias) | Low risk | Comment: study investigators were not aware of allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 27 loss to follow‐up in vitamin A group and 21 in placebo group. Reason for attrition were given, and they were similar in both groups. |
| Selective reporting (reporting bias) | Low risk | Comment: the trial was registered with number NCT00514891. All a priori outcomes are reported. |
| Other bias | Low risk | Comment: this study appears to be free of other bias |
| Methods | Individually‐randomised trial conducted in the municipalities of Pililla and Binangonan in the province of Rizal, Philippines | |
| Participants | Eligibility: children aged 1‐6 years were eligible for inclusion in the study. Any child with clinical signs of vitamin A deficiency was excluded from the trial. Sample: 2471 children were randomised to 3 intervention groups. Mean age of children was 3.4 years, and proportion of boys in study population was 49.5% | |
| Interventions | There were 3 study groups: 2 were supplemented with vitamin A and 1 with placebo. The first experimental group received a high dose of vitamin A (i.e. 200,000 IU), and the second experimental group received a medium dose of vitamin A (i.e. 100,000 IU). The control group received placebo only. Children were supplemented only once and were followed for 1 week. | |
| Outcomes | Incidence of side effects within 1 week (nausea and/or vomiting, headache, diarrhoea and fever) | |
| Notes | The study area had a high prevalence of malnutrition, and therefore vitamin A deficiency was likely to be prevalent. The study reported outcomes for the first 48 hours and within a week. We have pooled the data for the first week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "By use of a double‐blind study design, children were randomly assigned to three treatment groups." Comment: no qualifying information on what 'randomly assigned' means is provided. Difficult to assess sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient details available to make a judgment |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the researchers and field workers nor the subjects knew the contents of the preparations; the code was kept confidential by Hoffman La Roche until after the analysis of the results was completed." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the researchers and field workers nor the subjects knew the contents of the preparations; the code was kept confidential by Hoffman La Roche until after the analysis of the results was completed." Comment: blinding adequate and performance bias unlikely to have influenced results |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the researchers and field workers nor the subjects knew the contents of the preparations; the code was kept confidential by Hoffman La Roche until after the analysis of the results was completed." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: complete details of those excluded and lost to follow‐up were provided. Only 76 children lost; differences slight between groups |
| Selective reporting (reporting bias) | Low risk | Comment: though not explicitly stated, all reported measured outcomes have data reported in results with sufficient clarity and explanation. |
| Other bias | Low risk | Comment: no other apparent bias was noted. |
| Methods | Cluster‐randomised trial conducted in 5 rural councils in northern Sudan | |
| Participants | Eligibility: inclusion criteria was 9‐72 months of age. Children with xerophthalmia were excluded. Sample: randomisation was done by households. The study included a total of 28,753 children, of whom 14,455 were in vitamin A group and 14,298 were in placebo group. The proportion of boys in the study was 50.7%. | |
| Interventions | Children in the vitamin A group received 200,000 IU of retinol palmitate along with 40 IU of vitamin E. The comparison group received 40 IU of vitamin E only. The intervention was given every 6 months for 18 months. | |
| Outcomes | All‐cause mortality; cause‐specific mortality due to diarrhoea, measles, respiratory disease; incidence of diarrhoea, respiratory disease, and measles; incidence of xerophthalmia, Bitot's spots, and night blindness | |
| Notes | Authors used non‐specific terms for describing cause of death (in table 4) like "shortness of breath", "convulsions", and "fever", etc. We have pooled data for "shortness of breath" under the heading of mortality due to lower respiratory tract infection. This is because it is highly unlikely that a child will die of an upper respiratory tract infection, and lower respiratory tract infection is a more general term than pneumonia to cover this, as it includes pneumonia as well. According to WHO, Sudan is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomisation was done by household . . . Assignment to treatment group was achieved by the two interviewers visiting alternate households throughout the village. All eligible children in alternate households were assigned to receive, every 6 months, either a capsule of 60 mg (200 000 IU) of vitamin A and 40 mg (40 IU) of vitamin E or a capsule of 40 mg of vitamin E without vitamin A." Comment: does not appear to be randomised |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient details available to make a judgment |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The capsules were colour‐coded to avoid the possibility of mix ups, but none of the study team members was aware which was the experimental capsule and which was the placebo until the end of data collection. All eligible children in a household received capsules of the same colour." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The capsules were colour‐coded to avoid the possibility of mix ups, but none of the study team members was aware which was the experimental capsule and which was the placebo until the end of data collection. All eligible children in a household received capsules of the same colour." Comment: performance bias unlikely given that trialists and staff were blinded during the intervention |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Only the manufacturer knew the contents of the capsules until after data collection and preliminary analysis of the results." Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 3320 children did not receive 1 or 2 of the 3 vitamin A or placebo capsules. Most of this non‐compliant group consisted of children absent from the household at the time of follow‐up, whereas others had moved away or refused to take part further. As a group, the non‐compliant children tended to be from poorer households than those who continued in the study. However, there were no significant differences between vitamin A and placebo groups in the number of non‐compliant subjects or in their ages, sex, or nutritional status. With respect to the variables relevant to the intervention, the losses to follow‐up were not significantly different from those that remained in the study. |
| Selective reporting (reporting bias) | Unclear risk | Comment: does not reference a protocol or trial registration number and does not state that all measured outcomes are reported |
| Other bias | Unclear risk | Comment: insufficient details available to make a judgment |
| Methods | Individually‐randomised trial conducted in a suburban community of city Bandung, Indonesia | |
| Participants | Eligibility: children aged 12‐54 months were included in the study. No exclusion criteria were specified. Sample: 269 children were randomised either to vitamin A or placebo group. The vitamin A supplemented group had 126 children while the placebo group had 141 children. Mean age of study participants was 33 months, and proportion of boys was 51% | |
| Interventions | The experimental group received 200,000 IU of vitamin A once every 6 months for 12 months. The comparison group received placebo only. | |
| Outcomes | Incidence of respiratory disease, mean serum retinol levels | |
| Notes | Authors presented data on respiratory outcomes according to severity of disease. We have included data for "severe respiratory disease" only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children were selected by randomised stratified sampling from the almost 2000 under‐fives residing in Cikutra." Comment: insufficient details available to make a judgment |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail provided to make judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "All children participated in an age‐ and sex‐matched randomised, double blind vitamin A supplementation programme by receiving vitamin A 200,000 IU or placebo capsules orally, at the start and at the 6th month of the study." |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient detail provided to make judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient detail provided to make judgement |
| Incomplete outcome data (attrition bias) | High risk | Comment: insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient detail provided to make judgement |
| Other bias | Unclear risk | Comment: the methods of the study are not described very clearly |
| Methods | Individually‐randomised trial conducted in Fortaleza, the capital of the Ceara state in northeastern Brazil | |
| Participants | Eligibility: children aged 2 months to 9 years were eligible for inclusion in the study. Those participants who had fever > 38°C or were exclusively breastfed were excluded. Sample: 79 children were randomised either to vitamin A or placebo group. There were 39 participants in vitamin A group and 40 in placebo. Mean age of participants was 43.3 months, and proportion of boys was 57%. | |
| Interventions | Retinol palmitate was supplemented in a dose of 100,000 IU for children aged < 12 months and 200,000 IU for children aged > 12 months in the experimental group. The comparison group received Tocopherol (vitamin E) as placebo. Supplements were given at enrolment, 4 months, and 8 months. | |
| Outcomes | Mean serum retinol levels, growth, and adverse reactions to vitamin A | |
| Notes | The infant mortality rate in the study area was 35/1000 live births. The primary objective of the study was to measure the effect of vitamin A on barrier function of gastrointestinal tract. The study concluded that the prevalence of new parasitic infection, especially with Giardia species, was significantly decreased with vitamin A intervention, suggesting an immune regulatory modulation of this nutrient on parasitic intestinal infections. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: 79 children were randomly selected (using computer‐generated random numbers) |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail provided to make judgement |
| Blinding (performance bias and detection bias) | Low risk | Comment: the parent or guardian of the children, field study team, and investigators were blinded to treatment agent. |
| Blinding (performance bias and detection bias) | Low risk | Comment: the parent or guardian of the children, field study team, and investigators were blinded to treatment agent |
| Blinding (performance bias and detection bias) | Low risk | Comment: the parent or guardian of the children, field study team, and investigators were blinded to treatment agent. Indication that blinded field study teams assessed outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Comment: after 12‐month follow‐up, 22 children were withdrawn from the study for the following reasons: change of address (n = 16), parents or guardians did not co‐operate with the study (n = 5), and 1 had above the median z score for length or height at the time of the study initiation. The percentage of participants completing the study at 12 months was 72.2%. |
| Selective reporting (reporting bias) | High risk | Comment: the objective of study also included reporting of diarrhoea. Authors had reported the overall incidence of diarrhoea in the whole population but the figures had been presented in a way that they can not be used in the meta‐analysis. |
| Other bias | Low risk | Comment: no other apparent bias observed |
| Methods | Randomised, placebo‐controlled trial conducted in Wuhan, an industrial centre in central region of China | |
| Participants | Eligibility: inclusion criteria was age 2‐7 years. Children were recruited from kindergarten in the area. Those who had fever, diarrhoea or a recent preventive injection were excluded from the study. Underweight children with BMI age‐ and sex‐ specific 5th percentile of the first US National Health and Nutrition Examination Survey data were excluded. Children whose protein or energy intake met Chinese RDA were also excluded. Sample: 105 children were randomised to 3 intervention groups (described below). Mean age of study participants was 55 months, and proportion of boys was 61% | |
| Interventions | There were 3 study groups. 2 of these consisted of children who were vitamin A deficient and 1 with children who were vitamin A sufficient. Vitamin A was given only to children in 1 of the vitamin A deficient groups in a dose of 100,000 IU every month for 3 months. The other 2 groups received placebo. | |
| Outcomes | All‐cause mortality, mean serum vitamin A levels | |
| Notes | In this review, we have included data for vitamin A deficient children who were either supplemented with vitamin A or placebo. According to WHO, China does not have a high child mortality rate (i.e. < 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The remaining 70 vitamin A‐deficient children were randomly and equally divided into vitamin A deficient‐supplemented group and vitamin A‐deficient placebo group." Comment: the term 'randomised' is also used to describe a 3rd group that is clearly matched. This may not be an RCT. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail provided to make judgement |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Children of vitamin A‐deficient‐supplemented group were given 100 000 IU (retinol equivalent) vitamin A capsules every 2 weeks for 3 months (Grubesic, 2004). Children of vitamin A‐sufficient placebo group and vitamin A‐deficient placebo group received placebo capsules in the same way." |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: although study was double randomised trial, no details of how blinding was achieved was described in the district |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient detail provided to make judgement |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no attrition reported |
| Selective reporting (reporting bias) | High risk | Comment: main outcome data not reported in a manner that can be analysed |
| Other bias | Unclear risk | Comment: as blinding is not described, potential performance bias and other sources of bias cannot be assessed |
| Methods | Individually‐randomised trial conducted in rural China | |
| Participants | Eligibility: children aged 6 months to 7 years were included in the study. Those without informed consent or with acute and chronic diseases were excluded. Sample: 132 children were randomly allocated to 3 intervention groups. Mean age of children was 36.5 months and proportion of boys was 50%. | |
| Interventions | The 3 intervention groups included vitamin A, beta‐carotene, and placebo. The experimental group received 100,000 IU of vitamin A every month for 3 months. The placebo group received biscuits. | |
| Outcomes | Mean vitamin A serum levels | |
| Notes | We have included the results for vitamin A group versus placebo only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 50 severe vitamin A deficient children and 82 marginal vitamin A deficient children were randomly divided into three groups respectively by using a table with randomly assorted digits." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment are described in the text. |
| Blinding (performance bias and detection bias) | High risk | Quote: "Vitamin A intervening group were administered 100,000 IU vitamin A capsules . . .the beta‐carotene intervening group . . . was administered 4 mg purified beta‐carotene . . . dissolved in vegetable oil and dropped into a general little biscuit . . . the placebo group were just administered a general little biscuit." Comment: vitamin A and placebo were administered in 2 different forms. Vitamin A was administered in capsule form while placebo was given in the form of biscuits. |
| Blinding (performance bias and detection bias) | High risk | Comment: vitamin A and placebo were administered in 2 different forms. Vitamin A was administered in capsule form while placebo was given in the form of biscuits. |
| Blinding (performance bias and detection bias) | High risk | Comment: vitamin A and placebo were administered in 2 different forms. Vitamin A was administered in capsule form while placebo was given in the form of biscuits. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropouts reported, and numbers at baseline and follow‐up appear to be the same. |
| Selective reporting (reporting bias) | High risk | Comment: use of clinic services, hospitalisation, cause‐specific morbidity not reported |
| Other bias | Low risk | Comment: this study appears to be free of other bias. |
| Methods | Factorial design, individually randomised trial conducted in La Magdalena Atlicpac, Mexico | |
| Participants | Eligibility: children aged 6‐15 months were eligible for inclusion in the review. Children who were suffering from diseases causing immunosuppression and any congenital or acquired alteration of the digestive tract that could alter the absorption of micronutrients were excluded. Children who were taking vitamin supplements were also excluded from the study. Sample: 786 children were randomised to 4 intervention groups. Mean age of participants was 9.8 months; proportion of boys in study population was 51.7% | |
| Interventions | The 4 intervention groups were as follows:
Interventions were delivered every 2 months for 12 months | |
| Outcomes | Diarrhoea and respiratory disease morbidity | |
| Notes | We have included data of this factorial design trial in 2 sets. The first data set gives comparisons for vitamin A vs placebo, and the second set includes data for vitamin A + zinc vs zinc only. Data on respiratory morbidity was given with three definitions. We have pooled the data for "cough + difficulty breathing" under the heading of lower respiratory tract infection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was generated by using a random‐number table by project personnel from CENSIA, a division of the Mexican Ministry of Health." |
| Allocation concealment (selection bias) | Low risk | Quote: "These solutions were packaged in consecutively numbered, colour‐coded, opaque plastic droplet bottles to ensure that field personnel and the principal investigator were blinded." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The vitamin A, zinc, and vitamin A + zinc supplements were prepared by personnel at the National Institute of Nutrition in 5‐mL solutions that were similar in taste and appearance." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "This double‐blind randomised trial . . . These solutions were packaged in consecutively numbered, color‐coded, opaque plastic droplet bottles to ensure that field personnel and the principal investigator were blinded." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "This double‐blind randomised trial . . . These solutions were packaged in consecutively numbered, color‐coded, opaque plastic droplet bottles to ensure that field personnel and the principal investigator were blinded." Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: lost to follow‐up data given along with reasons for lost to follow‐up. 93 children were lost to follow‐up or excluded. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available so cannot assess or make any judgement |
| Other bias | Low risk | Comment: this study appears to be free of other bias. |
| Methods | — | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | As above (Long 2006a) | |
| Methods | Individually randomised trial conducted in Mexico | |
| Participants | Eligibility: children aged 5‐15 months were eligible for inclusion in the trial. Those who were immunosuppressed, had any congenital abnormality or chronic diarrhoea were excluded. Those who had a history of vitamin A supplementation were also excluded. Sample: 195 children were randomised, of which 97 were in vitamin A group and 98 in placebo group; proportion of boys in study population was 49.7% | |
| Interventions | The experimental group received vitamin A in a dose of 20,000 IU for those aged < 12 months and 45,000 IU for those > 12 months. Intervention was repeated every 2 months for 12 months | |
| Outcomes | Incidence of diarrhoea and respiratory disease | |
| Notes | The baseline sociodemographic characteristics of study children and households were similar between children who received vitamin A and those who were given the placebo. Children received monthly visits and referrals to the doctor, which appeared to exceed normal treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was generated by project personnel based at the National Institute of Public Health." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: personnel at the National Institute of Nutrition carried out the preparation of the supplements to assure that field personnel and the principal investigator were unaware of treatment regimen. Children in the vitamin A and placebo groups received a 5 mL solution, from identical opaque plastic droplet bottles numbered consecutively, administered by the field team. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Testing had been carried out at the National Institute of Nutrition to assure that the placebo and vitamin A water miscible solution were similar in taste, viscosity and colour." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Personnel at the National Institute of Nutrition carried out the preparation of the supplements to assure that field personnel and the principal investigator were unaware of treatment regimen." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Personnel at the National Institute of Nutrition carried out the preparation of the supplements to assure that field personnel and the principal investigator were unaware of treatment regimen." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: unclear what was done with data for 7 missing children, but dropout was small and similar between groups (4 intervention, 3 control) |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not referenced, though the grant applications may be available |
| Other bias | Low risk | Comment: this study appears to be free of other bias. |
| Methods | Cluster‐randomised trial in rural Nepal | |
| Participants | Eligibility: children aged 6 months to 10 years were eligible to participate in the study. Sample: from 100 potentially eligible cluster sites, 75 were randomised (approximately 25,301 children). Baseline data on the number in each treatment group, proportion of boys and mean age were not provided. | |
| Interventions | The intervention groups were:
Study duration: 24 months | |
| Outcomes | All‐cause mortality and Bitot's spots | |
| Notes | No details on loss to follow‐up were given. Inclusion/exclusion criteria were inadequately described. No nominators/denominators were available for Bitot's spots. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using random number tables and the reference number for each block . . ." |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail provided to make judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient detail provided to make judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient detail provided to make judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient detail provided to make judgment |
| Incomplete outcome data (attrition bias) | High risk | Comment: no information given as regards how incomplete outcome data were addressed |
| Selective reporting (reporting bias) | High risk | Comment: very specific outcomes reported. 5 types of examinations were administered to the study children: ophthalmic, physical, anthropometric, blood, and faecal; while data in results is given only for prevalence of Bitot's spots and all‐cause mortality |
| Other bias | Unclear risk | Comment: insufficient detail provided to make judgment |
| Methods | Individually randomised study in urban area of Australia | |
| Participants | Eligibility: children aged 1‐4 years of age in 3 general practices from Adelaide. Children with more than 15 days of cough or 3 separate episodes of respiratory illness during the preceding 3 months were eligible. Sample: 147 children were randomised to the treatment groups. Mean age was 39.3 months. 50% of participants were boys | |
| Interventions | Vitamin A administered orally as retinyl palmitate, 1160 mcg 3 times per week for 20 weeks, versus placebo | |
| Outcomes | Acute respiratory infections, pneumonia, mean serum vitamin A | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of treatment was achieved by combining active and placebo bottles in a sequence, which was determined by consulting a table of random numbers, and numbering the bottles accordingly." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The placebo was a similarly constituted syrup omitting retinyl palmitate and labelled and bottled identically." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "All staff connected with the study remained blind to the identity of the child's medication." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "All staff connected with the study remained blind to the identity of the child's medication." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: a high rate of attrition, but reasons for withdrawal given and that there were no significant changes in the distribution of major potential confounding factors between the 2 groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available |
| Other bias | Low risk | Comment: no other apparent bias was observed. |
| Methods | Individually randomised study in urban area of Australia | |
| Participants | Eligibility: children aged 0‐2 years with previous history of bronchiolitis and nasal culture positive for RSV were included. Children taking vitamin A, and those with cystic fibrosis, cardiopulmonary difficulties, major brain dysfunctions were excluded. Sample: 206 children were randomised to the treatment groups. Mean age was 58 months. 60% of participants were boys | |
| Interventions | Vitamin A administered as retinyl palmitate, 4.2 mg per week for 12 months versus placebo | |
| Outcomes | Diarrhoea, diarrhoea‐related hospitalisation, acute respiratory infections, pneumonia, pneumonia‐related hospitalisation, mean serum vitamin A | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was achieved by randomly allocating four of eight batch numbers to Vitamin A supplement and the remaining four to placebo." Quote: "the batch number code was retained by the manufacturer" |
| Allocation concealment (selection bias) | Low risk | Quote: "The batch number code was retained by the manufacturer. The bottles were then distributed sequentially according to batch number as children presented . . ." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The placebo had an identical appearance and formulation except for the active ingredient." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Both investigators and parents were blind as to the treatment status of the child." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Both investigators and parents were blind as to the treatment status of the child . . . The batch number code was retained by the manufacturer." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: complete details of those excluded and lost to follow‐up were provided. |
| Selective reporting (reporting bias) | High risk | Comment: outcomes mentioned in Methods not reported in Results |
| Other bias | Low risk | Comment: this study appears to be free of other bias |
| Methods | Individually randomised study conducted in an urban area of Bangladesh | |
| Participants | Eligibility: children aged 12‐35 months were eligible for inclusion in the study. Children who had received vitamin A within the previous 4 months; had severe malnutrition, with signs or symptoms of vitamin A or zinc deficiency; or with any systemic illness such as diarrhoea, respiratory infection, fever, or any other illness that warranted medical intervention at the time of enrolment were excluded. Sample: 800 children were enrolled (200 in each of the 4 treatment groups). Mean age of participants was between 23.5 and 24.2 months across the treatment groups. 56% of the participants were boys | |
| Interventions | There were 4 treatment groups:
Study duration: 6 months | |
| Outcomes | Diarrhoea, acute respiratory infections, serum vitamin A levels, and vitamin deficiency | |
| Notes | Data on treatment analysis was not presented. We have written to authors for data on each treatment arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The children were randomly assigned by a person not involved in the study who used permuted blocks of random numbers." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Sets of 2 bottles and 1 capsule for each child were serially numbered . . . A local pharmaceutical company prepared the study syrups (zinc and placebo) which were supplied in identical 50‐mL bottles . . . The vitamin A and placebo capsules looked identical." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The zinc and placebo syrups were supplied in bottles that looked identical, and the appearance and consistency of the syrups were similar. Vitamin A and placebo capsules were identical in appearance." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The randomisation code was kept sealed until the completion of the study." Comment: identical presentation; probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The treatment allocations were disclosed after the final analysis." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: data on loss to follow‐up given and also stated that the baseline characteristics of children who were excluded or lost to follow‐up were comparable to those of the children who continued in the study. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available |
| Other bias | Low risk | Comment: no other apparent bias |
| Methods | Cluster‐randomised trial conducted in Trichy district of Tamil Nadu in southern India | |
| Participants | Eligibility: children aged 6‐60 months were included in the study. Sample: clustering unit was 'panchyat' (local government areas). 206 clusters were formed, and the majority of them consisted of 50‐100 children. The included clusters had a total of 15,419 children, of whom 7764 were in vitamin A group and 7655 in placebo group. | |
| Interventions | Children in experimental group received weekly doses of 8333 IU vitamin A and 20 mg vitamin E. The control group received 20 IU of vitamin E only in peanut oil. Any children diagnosed with xerophthalmia at baseline, midterm, or final examination were given a high dose (200,000 IU) supplement of vitamin A and continued in the study. Supplementations were given for 52 weeks. Children who missed 7 consecutive dosages were excluded from the analysis. | |
| Outcomes | All‐cause mortality; cause‐specific mortality due to diarrhoea, measles, and respiratory disease; incidence of diarrhoea and respiratory disease morbidity | |
| Notes | The baseline characteristics of the 2 groups were similar in terms of age and sex, 1‐month history of diarrhoea and respiratory disease, anthropometric indexes of nutritional status, xerophthalmia status, 5‐year retrospective history of mortality of children under 5, household economic, household hygienic status, and serum retinol levels. On average > 90% of the children were contacted each week, and the lowest coverage in any single week was 88%. 11% had clinical evidence of xerophthalmia, while about 38% had serum retinol concentrations < 0.35 mmol/L at baseline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The clusters were arranged according to population size; after a random start, they were assigned alternately to the treated or control groups." Comment: exact method of sequence generation was not provided |
| Allocation concealment (selection bias) | Low risk | Quote: ". . . no one associated with the study was aware of the colour code, which was held by the Hoffmann‐LaRoche until the study ended." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The appearance and taste of the solutions were identical . . . no one associated with the study was aware of the colour code, which was held by the Hoffmann‐LaRoche until the study ended." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The appearance and taste of the solutions were identical . . . no one associated with the study was aware of the colour code, which was held by the Hoffmann‐LaRoche until the study ended . . . masked controlled . . ." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The appearance and taste of the solutions were identical . . . no one associated with the study was aware of the colour code, which was held by the Hoffmann‐LaRoche until the study ended . . . masked controlled . . ." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "There was no difference in rates of contact between the treated and control groups. The reasons for lack of contact included moving from the study area . . ." Comment: reasons for loss to follow‐up given with a note that there was no difference in contact rates between the 2 groups |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes given in results as mentioned in the Methods section |
| Other bias | Low risk | Comment: no other apparent bias |
| Methods | Individually randomised trial conducted in rural India | |
| Participants | Eligibility: children aged 6‐36 months were eligible for inclusion in the trial. Those with ophthalmic signs of xerophthalmia, serious diseases, or severe malnutrition (< 60% of weight‐for‐age or < 85% of height‐for‐age of the National Center for Health Statistics median) were excluded and received appropriate treatment, including vitamin A. Sample: 583 children were included; 309 in vitamin A group and 274 in placebo group. Mean age of children was 18.6 months and proportion boys was 49.9%. | |
| Interventions | Children in experimental group received vitamin A in a dose of 100,000 IU for children aged < 1 year and 200,000 IU for children aged > 1 year. The comparison group received only placebo. The interventions were given every 4 months for 12 months. | |
| Outcomes | Incidence of diarrhoea and respiratory disease | |
| Notes | Definition used for respiratory disease was too generalised to be included under lower respiratory tract infection. It mainly covered upper respiratory tract infections. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study design was a randomised, double‐blind, placebo controlled intervention trial in which every 4 mo the treatment group received a high‐dose vitamin A supplement and the control group received a placebo." Comment: insufficient detail provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The study design was a randomised, double‐blind, placebo controlled intervention trial in which every 4 mo the treatment group received a high‐dose vitamin A supplement and the control group received a placebo." Comment: statement that blinding occurred, no further details provided |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The study design was a randomised, double‐blind, placebo controlled intervention trial in which every 4 mo the treatment group received a high‐dose vitamin A supplement and the control group received a placebo." Comment: statement that blinding occurred, no further details provided |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The study design was a randomised, double‐blind, placebo controlled intervention trial in which every 4 mo the treatment group received a high‐dose vitamin A supplement and the control group received a placebo." Comment: statement that blinding occurred, no further details provided |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Out of the 660 children who were eligible, a final group of 592 children who had both pre‐ and post‐anthropometric measurements were used in this analysis. The losses at follow‐up due to migration (n = 50), death (n = 10) and incomplete measurements (n = 8) were similar for both groups." Comment: losses were not large and balanced between groups; unlikely to introduce substantial bias here. Clinically relevant impact unlikely |
| Selective reporting (reporting bias) | High risk | Quote: "The examination for ophthalmic signs of vitamin A deficiency, using WHO criteria (27), was conducted by trained ophthalmologists from the Department of Ophthalmology, CMCH, at baseline and at the end of the 1‐y follow‐up period. Blood samples were also taken (from finger pricks) at the beginning and the end of the study by using 250‐pt capillary tubes. Serum retinol concentrations were estimated by using reversed‐phase HPLC at the Wellcome Research Laboratory, CMCH, Vellore, using retinyl acetate and all trans‐retinol (Sigma Chemical Co, St Louis) as standards." Comment: though measured, serum retinol results are never reported. |
| Other bias | Low risk | Comment: no other apparent bias |
| Methods | Individually randomised trial conducted in India | |
| Participants | Eligibility: children aged 12‐60 months and having recurrent respiratory tract infections were eligible for inclusion in the trial. Those with mild or moderate asthma; children who were on vitamin supplements or who had received a massive dose of vitamin A in the previous 6 months; those with pre‐existing congenital heart disease, chronic lung disease, pulmonary tuberculosis or immunodeficiency disorders; those on immunosuppressive drugs; and those with clinically apparent vitamin A deficiency were excluded. Sample: 61 children were randomised; 30 were in the vitamin A group and 31 in the placebo group. The mean age of children was 35.7 months, and proportion of boys was 60.7%. | |
| Interventions | Children in experimental group received a single dose of vitamin A in a dose of 200,000 IU. The comparison group was given placebo in arachis oil. Follow‐up period was 6 months | |
| Outcomes | Incidence of respiratory disease, mean vitamin A serum levels | |
| Notes | Definition of respiratory illness used was not specific enough | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible children were randomly allocated to receive either 200,000 IU of vitamin A in arachis oil or a placebo containing arachis oil without vitamin A." Comment: details of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "Eligible children were randomly allocated to receive either 200,000 IU of vitamin A in arachis oil or a placebo containing arachis oil without vitamin A." |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Of the 61 included children, seven (3 in the placebo group and four in vitamin A group) did not return for follow‐up." (second page) Comment: authors do not address the reasons for losses to follow‐up, and given the small size of this trial, bias may or may not be introduced depending on why the losses occurred by group. Given this lack of discussion, it is difficult to judge whether or not there is a low or high risk of bias, but it is likely to be high. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Details of doctor or outpatient visits and hospital cough, wheezy breathing, shortness of breath and fever. Details of doctor or outpatient visits and hospital admissions during the study period were also recorded. During each monthly follow‐up visit, the entries in the monthly calendar were reviewed with the parent." Comment: hospitalisation was not reported though it was collected |
| Other bias | Unclear risk | Comment: very little information provided in the paper; difficult to assess |
| Methods | Factorial design, individually randomised trial conducted in India | |
| Participants | Eligibility: children aged 1‐5 years were included in the study. Those without parental consent were excluded. Sample: 487 children were randomised to 4 intervention groups. Mean age and proportion of boys not described | |
| Interventions | The 4 intervention groups were as follows:
| |
| Outcomes | Mean vitamin A serum levels | |
| Notes | Data have been included in 2 sets | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the baseline survey, the children were assigned, randomly, into four groups, matched for age, anthropometry, serum vitamin A, and worm infestation and the following treatment was given." Comment: insufficient details provided to make judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: insufficient information to permit judgment |
| Selective reporting (reporting bias) | Unclear risk | Quote: "After 6 months and 12 months, heights and weights were measured, clinical status was assessed and morbidity for the preceding one month was recorded. Finger‐prick blood samples were collected and serum vitamin A levels were estimated, stool samples were examined for the presence of ascaris ova and other parasites." Comment: authors do not report height or weights, or detailed data on clinical status or morbidity |
| Other bias | Unclear risk | Comment: insufficient information to permit judgment |
| Methods | — | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | As Reddy 1986a above | |
| Methods | Randomised, double‐blind controlled trial conducted in Guinea savannah area of Ghana | |
| Participants | Eligibility: children aged 6‐59 months were included. Those with active xerophthalmia or measles were excluded from the trial the moment they were confirmed. Sample: 1455 children were included. The proportion of male children was 49.5%. | |
| Interventions | Children in vitamin A group received either 200,000 IU retinol equivalent for participants aged > 12 months or 100,000 IU for children aged 6‐12 months. The control group received placebo. Interventions were given every 4 months for 12 months. | |
| Outcomes | All‐cause mortality; mean daily prevalence of respiratory tract disease, diarrhoea, measles, malaria; mean vitamin A serum levels; all‐cause hospitalisations | |
| Notes | The study populations were rural and their main staple foods are deficient in carotenoids and vitamin A. Vitamin A deficiency and xerophthalmia were recognised as problems locally. Children were visited weekly for 1 year. Children in the Health Study were followed up 596 child‐years for vitamin A group and 589 for control group. According to WHO, Ghana is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was blocked in both studies to ensure similar numbers of children in each group in each part of the study area." Comment: explicit methods for generating allocation sequence not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was carried out in London by an independent statistician, who held the randomisation code and who also did an interim analysis of the mortality results from the Survival Study for the trial's data‐monitoring committee after a year of follow‐up." Comment: code was protected for the duration of the trial |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Vitamin A and placebo were supplied by Hoffmann‐La‐Roche's Sight and Life Programme, and were similar in taste and colour. In the Survival Study, liquid vitamin A or placebo was supplied in opaque 150 mL bottles containing 20 IU/mL vitamin E alone (placebo) or plus 100,000 IU/mL retinol equivalent as retinyl palmitate (vitamin A) in purified peanut oil. Each bottle had a unique number, and was labelled with a cluster code before despatch to Ghana." |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: in view of the blinding procedures in place elsewhere in the study, this was probably adequate |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: morbidity information was missing for 5% to 7% of the weekly follow‐up visits, owing to temporary absences of the study children or their mothers, but the missing data were equally distributed between the treatment groups. |
| Selective reporting (reporting bias) | High risk | Comment: there was an indication that xerophthalmia data were measured, but none are reported. No protocol is available. |
| Other bias | Low risk | Comment: no other apparent bias |
| Methods | Cluster‐randomised trial conducted in Ghana | |
| Participants | Eligibility: children aged 6‐90 months were eligible for inclusion in the trial. Xeropthalmic children were excluded. Sample: study involved 185 clusters that included 21,906 children. Proportion of boys was 51.5%. | |
| Interventions | The experimental group received vitamin A supplementation in a dose of 100,000 IU for children aged 6‐11 months and 200,000 IU for older children. The comparison group received placebo. Vitamin E in a dose of 20 IU was given to both the groups. Intervention were delivered every 4 months for 24 months. | |
| Outcomes | All‐cause mortality and cause‐specific mortality due to diarrhoea, respiratory disease, measles, and meningitis; mean vitamin A serum levels; malaria prevalence | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was blocked in both studies to ensure similar numbers of children in each group in each part of the study area." Comment: explicit methods for generating allocation sequence not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was carried out in London by an independent statistician, who held the randomisation code and who also did an interim analysis of the mortality results from the Survival Study for the trial's data‐monitoring committee after a year of follow‐up." Comment: code was protected for the duration of the trial. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Vitamin A and placebo were supplied by Hoffmann‐La‐Roche's Sight and Life Programme, and were similar in taste and colour. In the Survival Study, liquid vitamin A or placebo was supplied in opaque 150 mL bottles containing 20 IU/mL vitamin E alone (placebo) or plus 100,000 IU/mL retinol equivalent as retinyl palmitate (vitamin A) in purified peanut oil. Each bottle had a unique number, and was labelled with a cluster code before despatch to Ghana." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: in view of the blinding procedures in place elsewhere in the study, this was probably adequate. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 8.4% (1847) children lost to follow‐up and similar between treatment groups. The reasons for losses to follow‐up are not provided. |
| Selective reporting (reporting bias) | High risk | Comment: authors collected data on night blindness, Bitot's spots, and xerophthalmia, but do not report them. |
| Other bias | Unclear risk | Comment: the method for inflating the CIs is not well‐described. No ICC reported |
| Methods | Individually randomised trial conducted in Indonesia | |
| Participants | Eligibility: children aged 3‐6 years were eligible for inclusion in the study. Those who had median weight for age < 80% of the National Center for Health Statistics were excluded from the study. Children with serious illness were also excluded from the study and treated appropriately. Sample: 236 children were randomised to 4 intervention groups. Mean age of participants was 58.9 months, and proportion of boys was 71.6% | |
| Interventions | There were 4 intervention groups. 2 groups (vitamin A and placebo) had clinical signs of vitamin A deficiency, while 2 groups were clinically normal. Participants in vitamin A groups received a single dose of 60 000 microgram of retinol equivalent. Children were followed for 1 month. | |
| Outcomes | Mean vitamin A serum levels | |
| Notes | The 2 vitamin A and 2 placebo groups were combined, respectively, for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A double‐masked, randomised, placebo‐controlled, clinical trial involving 236 preschool children, age 3‐6 years, was carried out at the outpatient clinic of the Cicendo Eye Hospital in Bandung, West Java, Indonesia." Comment: details of sequence generation not provided |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment code was broken after the conclusion of the study." Comment: allocation sequence appears to have been protected |
| Blinding (performance bias and detection bias) | Low risk | Quote: "A double‐masked, randomised, placebo‐controlled, clinical trial involving 236 preschool children." Quote: "The vitamin A and placebo solutions were supplied in coded containers, and the identity of the solutions was known only to the manufacturer . . . The solutions were identical in colour, taste, smell and consistency." |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; providers likely to have been adequately blinded. |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: the provider administering vitamin A and the outcome assessor appear to be different individuals, and it is not clearly stated if the outcome assessors were also blinded to group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 232/236 children enrolled at baseline completed the study protocol (p 102) |
| Selective reporting (reporting bias) | Unclear risk | Comment: does not reference a protocol or trial registration number and does not state that all measured outcomes are reported |
| Other bias | Unclear risk | Comment: insufficient information to permit judgment |
| Methods | Individually randomised study in rural Indonesia | |
| Participants | Eligibility: children aged 6 months at vaccination against measles were included. Children who had measles previously were excluded. Sample: 336 children were randomised to the 2 treatment groups. Baseline details on age and sex were not provided. | |
| Interventions | Vitamin A given as a single dose (100,000 IU) versus placebo Vitamin A or placebo given with measles vaccine Study duration: 6 months | |
| Outcomes | Measles | |
| Notes | The primary objective of the study was to measure the antibody response to measles vaccine when given along with vitamin A or placebo. Trialists found a significant decrease in seroconversion of measles vaccine in the intervention group compared to placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment was assigned by random number table in blocks of ten." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Infants received identification numbers as they were enrolled in the study, and each identification number had an envelope with an identical capsule containing either vitamin A or placebo." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Vitamin A, 100,000 IU, or placebo in identical capsules." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Infants received identification numbers as they were enrolled in the study, and each identification number had an envelope with an identical capsule containing either vitamin A or placebo." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; probably done |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Follow‐up rates were 93% and 90% at one and six months post immunisation, respectively." Comment: the reasons for lost to follow‐up not given; only available case data given |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol was not available |
| Other bias | Unclear risk | Comment: inadequate information presented to assess this formally |
| Methods | Individually randomised trial conducted in the northwestern region of the Quito, Ecuador | |
| Participants | Eligibility: children aged 6‐36 months were eligible for inclusion in the review. Those children who had clinical vitamin A deficiency, who did not reliably stay at home or at day care centres during weekdays or who had been given multivitamins in the last 3 months, were excluded. Sample: 400 children were randomised either to vitamin A or placebo group with equal (200 each) in both the groups. Mean age of participants was 21.1 months, and half the participants were boys | |
| Interventions | Children in the supplement‐treated group received a weekly dose of 10,000 IU of vitamin A for 40 weeks, and children in the non‐supplement group received a weekly placebo for the same period. | |
| Outcomes | Incidence of diarrhoea and respiratory disease morbidity, mean vitamin A serum levels | |
| Notes | The baseline study characteristics were comparable in both the groups. The study was conducted in a slum with substantial rates of malnutrition and subclinical vitamin A deficiency. Morbidity surveillance was done weekly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For random allocation of each child to treatment or placebo group the following procedure was performed. Identical flasks containing vitamin A or placebo were numbered from 1 to 400 by members of the study team in Boston, Massachusetts. The local Ethical Committee of the Ecuadorian Biotechnology Corporation in Quito did not know the identity of the active or placebo flasks, because they did not have the code. Then, this committee assigned each flask to a specific child from a random list by using a table of random numbers. After randomisation, the ethical committee received the confidential code from Boston." |
| Allocation concealment (selection bias) | Low risk | Quote: "After randomisation, the ethical committee received the confidential code from Boston and kept it for the remainder of the study, when it was revealed." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Identical flasks containing vitamin A or placebo were numbered from 1 to 400 by members of the study team in Boston, Massachusetts." Comment: trial described as double blind; given procedures used for ensuring that intervention and placebo were identical, it is very likely that blinding of children was maintained. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The syrups were administered at home and at day care centres by study researchers who were blinded to the presence or absence of active drug." |
| Blinding (performance bias and detection bias) | Low risk | Comment: outcome assessors were the same as the providers, therefore blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A total of 306 children finished the study, because 50 children from the supplement‐treated group and 44 from the non‐supplemented group were lost to follow‐up when their families moved to other neighbourhoods. Of all children, 70%, including those lost to follow‐up, accumulated > 30 weeks of observation . . . Children with incomplete follow‐up were distributed evenly in relation to the baseline variables" Comment: loss to follow‐up similar in magnitude in both groups and for similar reasons. Some lost still contributed data |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol referred to but not referenced. Not explicitly stated if all measured outcomes were reported. |
| Other bias | Low risk | Comment: no other apparent bias was noted |
| Methods | Individually randomised trial conducted in Guinea Bissau | |
| Participants | Eligibility: children aged 6‐60 months and those who planned to reside within the study area for at least 1 year were eligible for inclusion in the trial. Those with ocular signs of vitamin A deficiency or history of night blindness were excluded. Sample: 480 children were randomised either to vitamin A or placebo group. The vitamin A group had 239 participants and the placebo group had 241. Proportion of boys in the study population was 51% | |
| Interventions | The experimental group received vitamin A supplementation in a dose of 100,000 IU for children aged < 1 year and 200,000 IU for older children. The comparison group received placebo. Both the groups received 20 IU of vitamin E. Intervention was given every 4 months for 13 months | |
| Outcomes | Incidence of diarrhoea and malaria morbidity, mean vitamin A serum levels | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Within these strata, children were individually allocated vitamin A or placebo in blocks of four (two vitamin A, two placebo) by computer generated randomly permutated codes." |
| Allocation concealment (selection bias) | Low risk | Quote: "Capsules were encoded into four groups; two placebo and two vitamin A, and the code was kept offsite by personnel who were not involved in the study." |
| Blinding (performance bias and detection bias) | Low risk | Comment: identical capsules, and allocation was concealed and code kept off site; described as double‐blind |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: unlikely that the trained village‐based morbidity worker knew the assignments, however, this is never stated explicitly. Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Cross sectional follow‐up rates for mid‐study and end of study were 428 of 480 (89%) and 410 of 480 (85%), respectively, and similar for vitamin A and placebo groups. During the trial two children dropped out, 66 moved out of the study area, and two died." Comment: intention‐to‐treat used. Missing outcome data balanced in numbers across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not referenced and not stated that all measured outcomes were reported. Data at 7 months not completely reported |
| Other bias | Low risk | Comment: no other apparent bias |
| Methods | Individually randomised trial conducted in India | |
| Participants | Eligibility: children aged 2 months to 4.5 years were eligible for inclusion in the trial. No exclusion criteria was described. Sample: 306 children were randomised either to vitamin A or placebo group in equal numbers (153 in each group) | |
| Interventions | Children in experimental group received vitamin A in a dose of 200,000 IU every 4 months for 12 months. The comparison group received placebo only. | |
| Outcomes | Bitot spots, side effects (vomiting) | |
| Notes | The people in the study population were extremely poor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children were divided in two groups of 153 each (two of the children died in the 1st year and two left the village) and were matched for age, sex, socioeconomic status, and playmate contacts. One of the children of each matched pair was selected randomly for receiving vitamin A and the other child received a placebo." Comment: no detail about randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "In a separate laboratory, the designated 2‐ml dose of vitamin A or placebo for each child was put into a vial labelled with the child's number and the vials were then shipped to the field station for distribution. Neither the clinician nor the paramedical workers, who personally fed vitamin A and placebo or examined the children for the signs and symptoms of vitamin A deficiency, knew which children received vitamin A." Comment: insufficient details provided |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the clinician nor the paramedical workers, who personally fed vitamin A and placebo or examined the children for the signs and symptoms of vitamin A deficiency, knew which children received vitamin A." Comment: probably done. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the clinician nor the paramedical workers, who personally fed vitamin A and placebo or examined the children for the signs and symptoms of vitamin A deficiency, knew which children received vitamin A." Quote: "The placebo consisted of deodorized arachis oil which was coloured and favoured with orange to match exactly the vitamin A preparation." Comment: provider blinded |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the clinician nor the paramedical workers, who personally fed vitamin A and placebo or examined the children for the signs and symptoms of vitamin A deficiency, knew which children received vitamin A." |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: based on the outcome data reported, it does not seem that any children dropped out (i.e. there were no losses); however, this could be because the authors are conducting an intention‐to‐treat analysis but never say so. They are not explicit in this regard, as such the risk of bias due to incomplete outcome data is unclear. |
| Selective reporting (reporting bias) | Unclear risk | Comment: does not reference a protocol or trial registration number and does not state that all measured outcomes are reported. |
| Other bias | Low risk | Comment: no other apparent bias |
| Methods | Factorial design, individually randomised trial conducted in Belize | |
| Participants | Eligibility: children aged 2.2‐5.5 years were eligible for inclusion in the trial. Those with fever or serious respiratory illness were excluded. Sample: 51 children were randomised to 4 intervention groups. Mean age of the children was 46.3 months | |
| Interventions | The 4 intervention groups were:
Study duration: 6 months | |
| Outcomes | Vitamin A serum level | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children selected were randomly assigned to receive one of the following supplements once per week: placebo; Zn, 70 mg as Zn gluconate; vitamin A, 3030 RE as retinyl palmitate; or a combination of vitamin A and Zn." Comment: stated to be randomised, but no further data reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient details provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "Supplements were ingested orally in an orange flavoured powder (10 g), Tangt (Kraft General Foods Inc, White Plains, NY 10625) prepared as a beverage dissolved in approximately 120 mL of water." Comment: stated to be "double‐blind" in the article keywords, but there appear to be no details about blinding methods in the text. The intervention (or no intervention in the placebo group) were diluted in the same solution, so presumably all groups were identical. |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: not adequately reported |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: not adequately reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: insufficient details provided; losses not accounted for by group and small sample size makes this especially relevant |
| Selective reporting (reporting bias) | Unclear risk | Comment: does not reference a protocol or trial registration number and does not state that all measured outcomes are reported |
| Other bias | Unclear risk | Comment: insufficient details provided |
| Methods | Cluster‐randomised trial conducted in a rural area of Indonesia | |
| Participants | Eligibility: children aged 0‐5 years were included. Children with active xerophthalmia were excluded from the study. Sample: 29,236 children from 450 villages (cluster sites) in Java. 50% of the participants were boys | |
| Interventions | Vitamin A (capsules administered twice over the course of the study: 200,000 IU of vitamin A) was compared with a no treatment control group that served as a waiting list control. 40 IU of vitamin E was also administered with vitamin A. Study duration: 9‐13 months | |
| Outcomes | Mortality, diarrhoea, Bitot's spots, night blindness, xerophthalmia | |
| Notes | ICC not reported (CIs from analyses reported to have been adjusted for design effect). TJL back‐calculated an ICC of 0.008307 from effect estimate provided in paper. Vitamin A was not intended to have been distributed to children under the age of 12 months, but it would appear that some 0‐12 month‐old children received the vitamin A capsule. Outcome data were reported on a cohort of 0‐12 month‐old children. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "From a random start, 450 villages were systematically selected for the study; these were then randomised for capsule distribution after the baseline examination . . ." Comment: inadequate information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: inadequate information was presented in order to assess this item in relation to timing of recruitment into the study |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "The Government of Indonesia would not condone the use of placebos but field‐workers collecting demographic data were unaware that mortality was a research issue." Comment: described as a controlled study, without adequate description of what the control group received |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "The Government of Indonesia would not condone the use of placebos but field‐workers collecting demographic data were unaware that mortality was a research issue." Comment: described as a controlled study, without adequate description of what the control group received |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "The Government of Indonesia would not condone the use of placebos but field‐workers collecting demographic data were unaware that mortality was a research issue." Comment: described as a controlled study, without adequate description of what the control group received |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Follow‐up information was available on 89% of the programme children and 88.4% of the controls." Comment: authors indicate percentage remaining per group at follow‐up, but nothing more detailed |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial protocol not available |
| Other bias | Unclear risk | Comment: insufficient information to permit judgement |
| Methods | Individually randomised trial conducted in Guinea Bissau | |
| Participants | Eligibility: children aged 6 months of age were eligible for inclusion in the trial. Sample: 68 children were included: 32 in vitamin A group and 36 in placebo | |
| Interventions | Children in the intervention group received vitamin A in a dose of 100,000 IU at the time of measles vaccination at 6 and 9 months of age. The comparison group received placebo only. | |
| Outcomes | Side effects (bulging fontanelle) | |
| Notes | Denominator data not entirely clear in Table 1 of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Carrying out a double‐blinded, randomised, placebo‐controlled trial." Comment: sequence generation not mentioned in the paper |
| Allocation concealment (selection bias) | Unclear risk | Comment: nothing mentioned regarding allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: claimed it was blinded but no detail provided |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: claimed it was blinded but no detail provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote from author: "Children were examined by one of us (CS) to see if their fontanelle was normal, sunken or bulging" Comment: appears outcome assessors were the same individuals as the investigators |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: losses to follow‐up by group indicated but no detail provided. Unclear what losses actually occurred in Table 1. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol referenced, nor statement that all measured outcomes were reported |
| Other bias | Unclear risk | Comment: short communication, insufficient detail to make an informed judgment |
| Methods | Randomised, placebo‐controlled trial conducted in north west of Haiti | |
| Participants | Eligibility: children aged 6‐83 months were included in the study. Those with corneal changes consistent with vitamin A deficiency, with measles, and those who had received vitamin A within the past 4 months were excluded. Sample: 13,651 children were found to be eligible for inclusion in the trial. The proportion of boys in the study population was 49%. | |
| Interventions | The vitamin A group received 100,000 IU supplements every 4 months for 3 distribution cycles for those aged 6‐11 months and 200,000 IU for the older children, while the other group only received placebo. | |
| Outcomes | 2‐week prevalence of signs of respiratory tract infections (cold, cough and rapid breathing, and diarrhoea) | |
| Notes | A slightly larger number of children (55%) were assigned to vitamin A group. There was a significant difference between 2 study groups with respect to age. Study area had a high prevalence of malnutrition and xerophthalmia in the study population. Children were visited every 2 weeks for 12 months. The respiratory disease morbidity was reported with respect to cold, cough, and rapid breathing, which were too non‐specific for inclusion under umbrella of pneumonia or lower respiratory tract infection morbidity in our review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from the author: "A random number generator was used to number the first household and the households were numbered sequentially thereafter. Every other household was given a green capsule, while the rest were given red capsules." Comment: alternate allocation |
| Allocation concealment (selection bias) | Low risk | Quote from the author: "The manufacturer (Roche) held the code until the study was completed." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The colour code was held only by the manufacturer until the study was completed." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Before the study inquiries among health workers and community members had indicated no symbolism associated with or preference for either green or red." Comment: highly unlikely that providers would be biased about a single intervention |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The colour code was held only by the manufacturer until the study was completed." Comment: probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The frequency of non‐participation was essentially identical among children from even and odd‐numbered households." Comment: probably done |
| Selective reporting (reporting bias) | High risk | Quote: "We did not collect data on the impact of supplementation on vitamin A status, or on the incidence, duration, or severity of symptoms of infection." Comment: only mortality and morbidity outcomes given. Protocol not available |
| Other bias | Low risk | Comment: this study appears to be free of other bias |
| Methods | Individually randomised, non‐placebo trial conducted in Thailand | |
| Participants | Eligibility: no exclusion criteria were described Sample: study included 30 children in which 14 were in vitamin A group and 21 in control group. Participants had a mean age of 3.1 years | |
| Interventions | Children in experimental group received a single dose vitamin A in a dose of 200,000 IU. Study participants were followed for 4 months | |
| Outcomes | Mean vitamin A serum levels | |
| Notes | Children were recruited from 3 rural day care centres | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After selection, 14 children were randomly supplemented with a single, oral dose of vitamin A (110 mg retinyl palmitate, 200,000 IU),according to WHO recommendations (9), and 21 children served as a control group." Comment: inadequate information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: inadequate information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: inadequate information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: inadequate information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Comment: inadequate information provided |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Due to the absence of some children at the different time points the number of data available for statistical analysis was less than the total number of children involved in this study . . . the number of children from whom complete data sets could be collected was rather low." Comment: no comprehensive data given on lost to follow‐up nor reasons for loss |
| Selective reporting (reporting bias) | High risk | Comment: does not report data on serum retinol levels, which were collected/measured |
| Other bias | Unclear risk | Comment: inadequate information provided |
| Methods | Individually randomised trial conducted in India | |
| Participants | Eligibility: infants aged 6 months were included Sample: 909 infants were randomised to 3 intervention groups. Proportion of boys in the study was 50% | |
| Interventions | The 3 intervention groups were as follows:
Dose of vitamin A for infant was 200,000 IU | |
| Outcomes | All‐cause mortality and cause‐specific mortality due to diarrhoea and respiratory disease. Incidence of diarrhoea and respiratory disease morbidity | |
| Notes | We have included the data for groups AA vs AP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each pair of subjects enrolled for the study was randomly allocated to one of the following three groups: (i) AA‐Both mother and infant received Vitamin A, the former soon after delivery and the latter at 6 months; (ii) AP: mother received Vitamin A but her infant received a placebo (Sesame oil); and (iii) PP: both mother and infant received placebo, the former Vitamin E and the latter Sesame oil." Comment: insufficient detail to form judgment |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail to form judgment |
| Blinding (performance bias and detection bias) | Low risk | Quote: "At the age of 6 to 6Vi months, the infant was weighed again and given the appropriate syrup by the Medical Officer from coded bottles, supplied again by the Statistical Section at the Camp Office." Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; probably done |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "4 each in the AA and AP groups and 5 in the PP group were withdrawn from the trial on medical grounds such as congenital abnormalities, epileptic fits or jaundice. Migration accounted for the loss of 34 infants in the AA group, 25 in the AP group and 20 in the PP group while 7, 9 and 7 were excluded due to other miscellaneous reasons. Of the remaining 263, 255 and 256 infants in the three group, 233 in the AA and 228 each in the AP and PP groups were followed‐up very regularly and form the basis for analyses in this report." Comment: they provided specific information about losses by group. However, it is unclear why 263, 255 and 256 infants that remain in the 3 groups after attrition is described in the Results as only 233 in the AA and 228 each in the AP and PP groups being used as the basis for analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: does not reference a protocol or trial registration number and does not state that all measured outcomes are reported |
| Other bias | Low risk | Quote: "Quality control of the morbidity data collected by the field investigators was undertaken throughout. As long recall periods pose problems, the collection of morbidity data was intensified from once a fortnight to once a week when the study had been in progress for 9 months." Comment: authors attempted to minimise other biases such as recall bias, though specific details of "quality control" are not provided. |
| Methods | Cluster‐randomised study in rural India | |
| Participants | Eligibility: children aged 1‐5 years were eligible for entry in the study. Children with corneal involvement were excluded from the review. Sample: 15,775 children in 84 clusters were randomised to the treatment groups. 50.4% participants were boys | |
| Interventions | Vitamin A given twice (200,000 IU) versus placebo (arachis oil) Study duration: not clear | |
| Outcomes | Mortality, diarrhoea, acute respiratory infections, measles | |
| Notes | Respiratory infection has non‐specific definition of "clinically significant cough" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The villages were allocated randomly into two groups‐treatment and control." Comment: insufficient detail to form judgment |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail provided |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The trial was double blind: the investigators and medical officers did not know which were the treatment and which were the control areas. They were not aware whether the dose they were distributing was vitamin A or placebo. Decoding was done only after data had been collected." |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above probably done |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: insufficient detail provided |
| Selective reporting (reporting bias) | High risk | Comment: incidence of infections outcome not given with respect to vitamin A and control groups. Given according to the clinical vitamin A status of all the study children |
| Other bias | Low risk | Comment: this study appears to be free of other bias |
| Methods | Cluster‐randomised study in rural Nepal | |
| Participants | Eligibility: children aged between 0 and 5 years were eligible for the study. Children with xerophthalmia were included. Children who had recently participated in a vitamin A programme were excluded from the study. Sample: 28,630 children in 261 clusters were recruited. 51.3% of participants were boys | |
| Interventions | Vitamin A (100,000 IU for 6‐11 months and 200,000 IU for children 12 months and older) administered 1‐3 times was compared with a very low dose of vitamin A (1000 IU). Both supplements contained 40 IU vitamin E. Study duration: 16 months | |
| Outcomes | Mortality, cause‐specific mortality, Bitot's spots, night blindness, xerophthalmia | |
| Notes | ICC not disclosed, although study estimates reported to have been adjusted for the unit of allocation. Study had additional recruitment phases in second and third treatment cycles. 1807 and 2018 children entered at 4 and 8 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After blocking on the local development area, the 261 wards were randomly assigned to receive vitamin A supplementation or placebos at 4‐month intervals." Comment: inadequately described to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Both the investigators and communities were masked to the random assignment." Comment: the study was a cluster‐designed trial and there was insufficient information to determine whether allocation took place before or after treatment group assignment was known. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The supplements were given as single‐dose gelatin capsules of identical taste and appearance." |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; probably done |
| Blinding (performance bias and detection bias) | Low risk | Comment: as above; probably done |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "All analyses were carried out on an intention‐to‐treat basis. Computed mortality rates were based on child‐years of observation." Comment: the rates of withdrawal were balanced between the treatment groups and the data were analysed based on patient years of observation. The unclear reasons for withdrawals, variable duration of follow‐up due to more than recruitment cycle and the low rate of mortality in relation to the withdrawal rates mean that it is uncertain whether the study is at risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: complete data for all time points were available for the review. The last available observation reported in a follow‐up article gave a RR for mortality slightly higher than that for the 12‐month data given in the primary study report (0.74 versus 0.7). |
| Other bias | Low risk | Comment: a method for estimating the ICCs was reported in Katz 1995. |
ALRI: acute lower respiratory illnesses;BMI: body mass index; CENSIA: Centro Nacional para la Salud de la Infancia y la Adolescencia; CI: confidence interval; CMCH: Christian Medication College & Hosptal; HPLC: high performance liquid chromatography; ICC: intra‐cluster correlation coefficient; RAND: a function in Excel, which is used to generate a random number; RDA: recommended dietary allowance; RR: risk ratio; RSV: respiratory syncytial virus; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Included children aged 7‐12 years | |
| Included children currently having diarrhoea | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial. The mean age of children was 6.6 years (range 3‐9 years) | |
| All groups received vitamin A supplementation | |
| All groups received vitamin A supplementation | |
| Maternal vitamin A supplementation | |
| Included children aged 3‐6 months | |
| Vitamin A given with maize | |
| Vitamin A given to both groups | |
| Not a randomised controlled trial | |
| Vitamin A supplementation not randomized | |
| Vitamin A given to both groups | |
| Vitamin A given as a therapeutic intervention for Bitot's spots | |
| Study population consisted of children infected with HIV | |
| Not a randomised controlled trial | |
| Vitamin A given to both groups Ineligible control | |
| Ineligible intervention. Other micronutrients were supplemented with vitamin A and these supplements were not balanced out in the control group. It was difficult to disaggregate the effect of vitamin A. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Individually randomised, placebo‐controlled trial conducted in Zambia, Africa |
| Participants | Boys aged 3‐4 years were eligible for inclusion in the trial. A total of 36 children were included in the trial in which 19 were in the vitamin A group and 17 in the placebo group |
| Interventions | The intervention group received a single dose of 60 mg vitamin A and the control group received the same amount of placebo |
| Outcomes | Mean plasma retinol levels, prevalence of fever, diarrhoea, rhinorrhoea, cough, and malaria |
| Notes | Data were available only in the form of abstract, and the numbers do not match those given in the Results section of abstract. It was decided among the group to wait for publication of this study before we include it in the review |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality at longest follow‐up Show forest plot | 19 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.83, 0.93] | |
| Analysis 1.1  Comparison 1 Vitamin A versus Control, Outcome 1 All‐cause mortality at longest follow‐up. | ||||
| 2 All‐cause mortality at longest follow‐up (subgroup analysis): age Show forest plot | 5 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Vitamin A versus Control, Outcome 2 All‐cause mortality at longest follow‐up (subgroup analysis): age. | ||||
| 2.1 6 to 12 months of age | 4 | Risk Ratio (Fixed, 95% CI) | 0.59 [0.43, 0.82] | |
| 2.2 1 to 5 years of age | 4 | Risk Ratio (Fixed, 95% CI) | 0.68 [0.57, 0.81] | |
| 3 All‐cause mortality at longest follow‐up (subgroup analysis): sex Show forest plot | 7 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 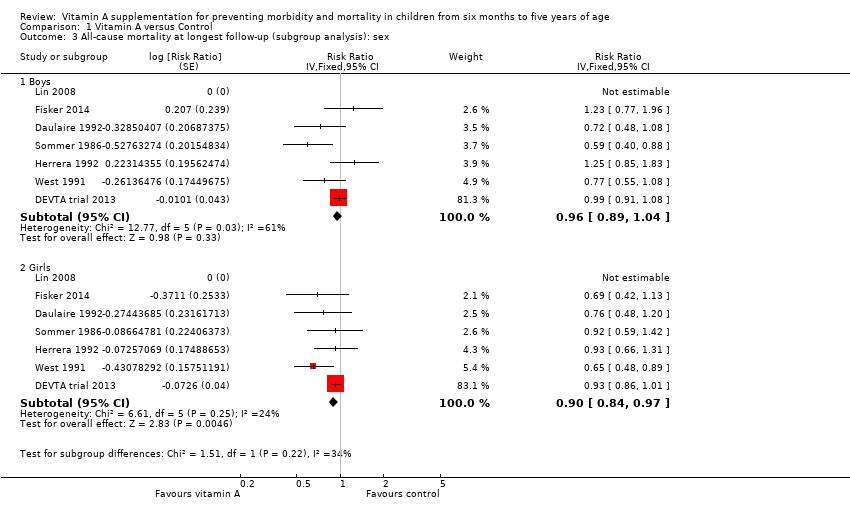 Comparison 1 Vitamin A versus Control, Outcome 3 All‐cause mortality at longest follow‐up (subgroup analysis): sex. | ||||
| 3.1 Boys | 7 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.89, 1.04] | |
| 3.2 Girls | 7 | Risk Ratio (Fixed, 95% CI) | 0.90 [0.84, 0.97] | |
| 4 Mortality due to diarrhoea at longest follow‐up Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.79, 0.98] | |
| Analysis 1.4  Comparison 1 Vitamin A versus Control, Outcome 4 Mortality due to diarrhoea at longest follow‐up. | ||||
| 5 Mortality due to measles at longest follow‐up Show forest plot | 6 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.69, 1.11] | |
| Analysis 1.5  Comparison 1 Vitamin A versus Control, Outcome 5 Mortality due to measles at longest follow‐up. | ||||
| 6 Mortality due to meningitis at longest follow‐up Show forest plot | 3 | Risk Ratio (Random, 95% CI) | 0.57 [0.17, 1.88] | |
| Analysis 1.6  Comparison 1 Vitamin A versus Control, Outcome 6 Mortality due to meningitis at longest follow‐up. | ||||
| 7 Mortality due to lower respiratory tract infection (LRTI) at longest follow‐up Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.86, 1.12] | |
| Analysis 1.7  Comparison 1 Vitamin A versus Control, Outcome 7 Mortality due to lower respiratory tract infection (LRTI) at longest follow‐up. | ||||
| 8 Diarrhoea incidence at longest follow‐up Show forest plot | 16 | Risk Ratio (Fixed, 95% CI) | 0.85 [0.82, 0.87] | |
| Analysis 1.8  Comparison 1 Vitamin A versus Control, Outcome 8 Diarrhoea incidence at longest follow‐up. | ||||
| 9 Diarrhoea prevalence at longest follow‐up Show forest plot | 4 | Risk Ratio (Fixed, 95% CI) | 1.06 [1.03, 1.10] | |
| Analysis 1.9  Comparison 1 Vitamin A versus Control, Outcome 9 Diarrhoea prevalence at longest follow‐up. | ||||
| 10 Measles incidence at longest follow‐up Show forest plot | 6 | Risk Ratio (Fixed, 95% CI) | 0.50 [0.37, 0.67] | |
| Analysis 1.10  Comparison 1 Vitamin A versus Control, Outcome 10 Measles incidence at longest follow‐up. | ||||
| 11 Malaria incidence at longest follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11 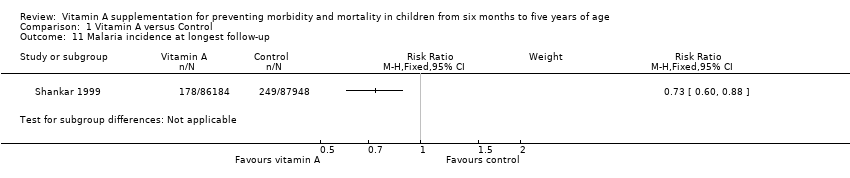 Comparison 1 Vitamin A versus Control, Outcome 11 Malaria incidence at longest follow‐up. | ||||
| 12 Malaria prevalence at longest follow‐up Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.73 [0.41, 1.28] | |
| Analysis 1.12  Comparison 1 Vitamin A versus Control, Outcome 12 Malaria prevalence at longest follow‐up. | ||||
| 13 LRTI incidence at longest follow‐up Show forest plot | 12 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.92, 1.06] | |
| Analysis 1.13  Comparison 1 Vitamin A versus Control, Outcome 13 LRTI incidence at longest follow‐up. | ||||
| 14 LRTI prevalence at longest follow‐up Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.60 [0.45, 0.81] | |
| Analysis 1.14  Comparison 1 Vitamin A versus Control, Outcome 14 LRTI prevalence at longest follow‐up. | ||||
| 15 Bitot's spots prevalence at longest follow‐up Show forest plot | 5 | Risk Ratio (Fixed, 95% CI) | 0.42 [0.33, 0.53] | |
| Analysis 1.15  Comparison 1 Vitamin A versus Control, Outcome 15 Bitot's spots prevalence at longest follow‐up. | ||||
| 16 Night blindness incidence at longest follow‐up Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 Vitamin A versus Control, Outcome 16 Night blindness incidence at longest follow‐up. | ||||
| 17 Night blindness prevalence at longest follow‐up Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.32 [0.21, 0.50] | |
| Analysis 1.17  Comparison 1 Vitamin A versus Control, Outcome 17 Night blindness prevalence at longest follow‐up. | ||||
| 18 Xerophthalmia incidence at longest follow‐up Show forest plot | 3 | Risk Ratio (Fixed, 95% CI) | 0.85 [0.70, 1.03] | |
| Analysis 1.18  Comparison 1 Vitamin A versus Control, Outcome 18 Xerophthalmia incidence at longest follow‐up. | ||||
| 19 Xerophthalmia prevalence at longest follow‐up Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.31 [0.22, 0.45] | |
| Analysis 1.19  Comparison 1 Vitamin A versus Control, Outcome 19 Xerophthalmia prevalence at longest follow‐up. | ||||
| 20 Hospitalisation: number of children hospitalised once or more at longest follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.20  Comparison 1 Vitamin A versus Control, Outcome 20 Hospitalisation: number of children hospitalised once or more at longest follow‐up. | ||||
| 21 Hospitalisation due to diarrhoea at longest follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.21  Comparison 1 Vitamin A versus Control, Outcome 21 Hospitalisation due to diarrhoea at longest follow‐up. | ||||
| 22 Hospitalisation due to LRTI at longest follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.22  Comparison 1 Vitamin A versus Control, Outcome 22 Hospitalisation due to LRTI at longest follow‐up. | ||||
| 23 Side effect: vomiting Show forest plot | 4 | 4427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.44, 2.69] |
| Analysis 1.23  Comparison 1 Vitamin A versus Control, Outcome 23 Side effect: vomiting. | ||||
| 24 Side effect: bulging fontanelle Show forest plot | 4 | 2318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.74, 2.08] |
| Analysis 1.24  Comparison 1 Vitamin A versus Control, Outcome 24 Side effect: bulging fontanelle. | ||||
| 25 Vitamin A deficiency status: number deficient at longest follow‐up Show forest plot | 4 | 2262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| Analysis 1.25  Comparison 1 Vitamin A versus Control, Outcome 25 Vitamin A deficiency status: number deficient at longest follow‐up. | ||||
| 26 Vitamin A deficiency status: vitamin A serum retinol level at longest follow‐up Show forest plot | 15 | 11788 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.22, 0.30] |
| Analysis 1.26  Comparison 1 Vitamin A versus Control, Outcome 26 Vitamin A deficiency status: vitamin A serum retinol level at longest follow‐up. | ||||
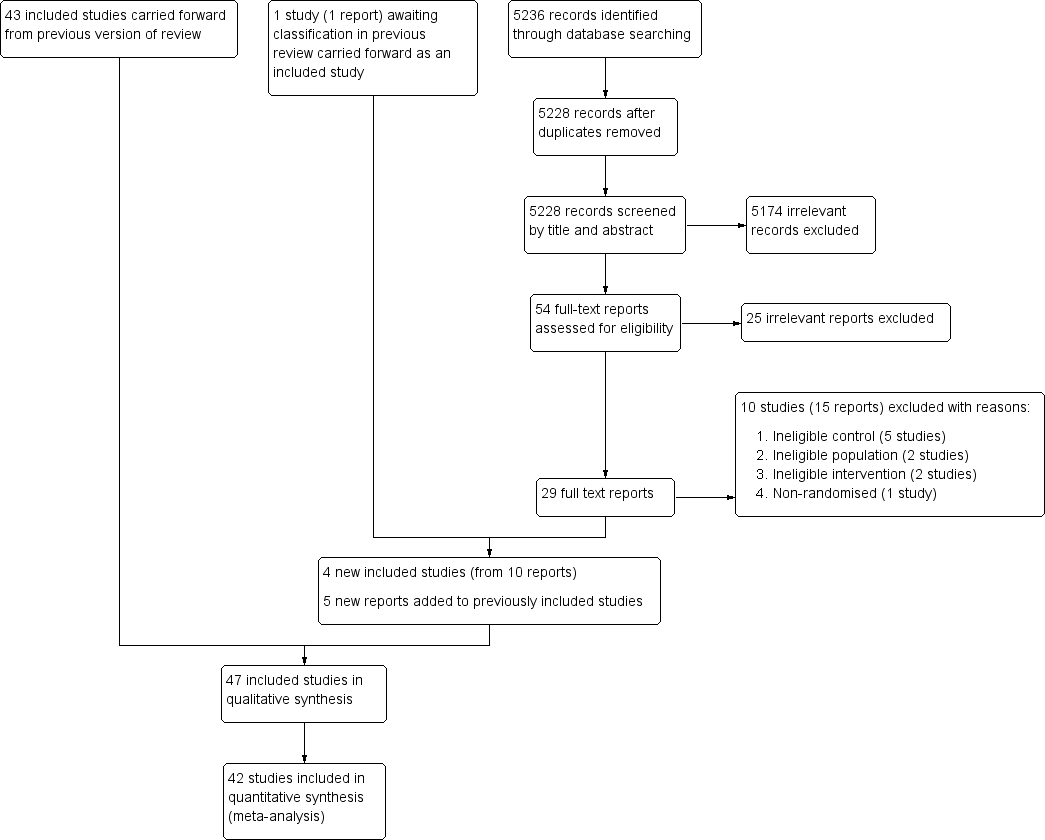
Study flow diagram.
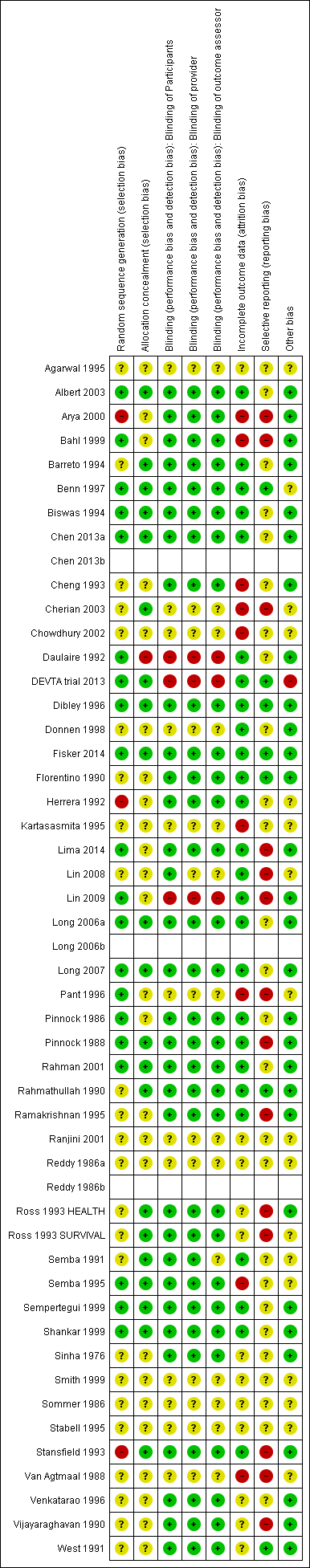
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
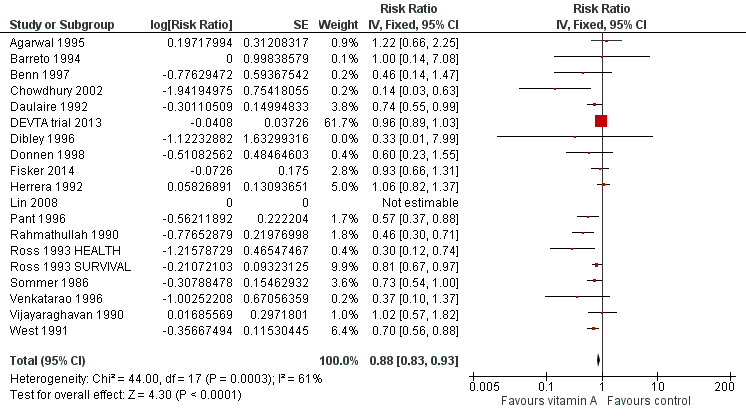
Forest plot of comparison: 1 Vitamin A versus Control, outcome: 1.1 All‐cause mortality at longest follow‐up.
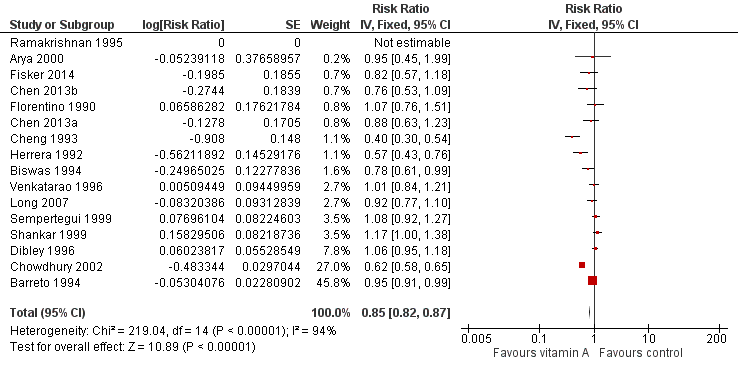
Forest plot of comparison: 1 Vitamin A versus Control, outcome: 1.8 Diarrhoea incidence at longest follow‐up.
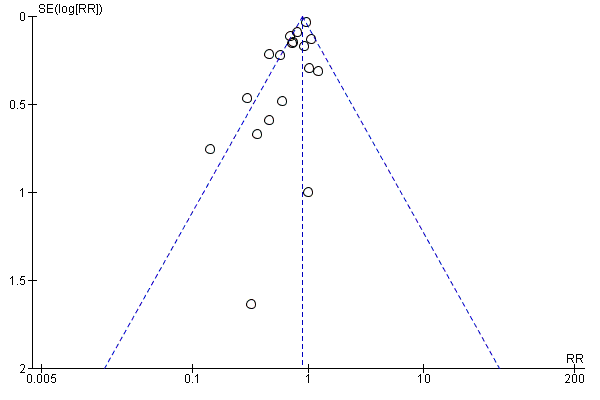
Funnel plot of comparison: 1 Vitamin A versus Control, outcome: 1.1 All‐cause mortality at longest follow‐up.
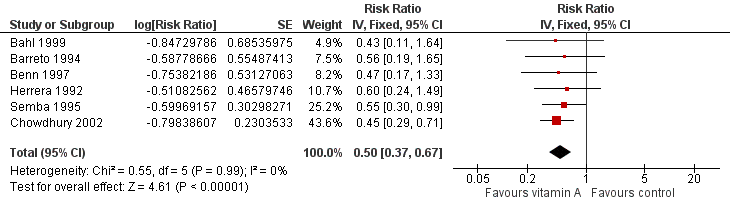
Forest plot of comparison: 1 Vitamin A versus Control, outcome: 1.12 Measles Incidence at Longest Follow‐up.
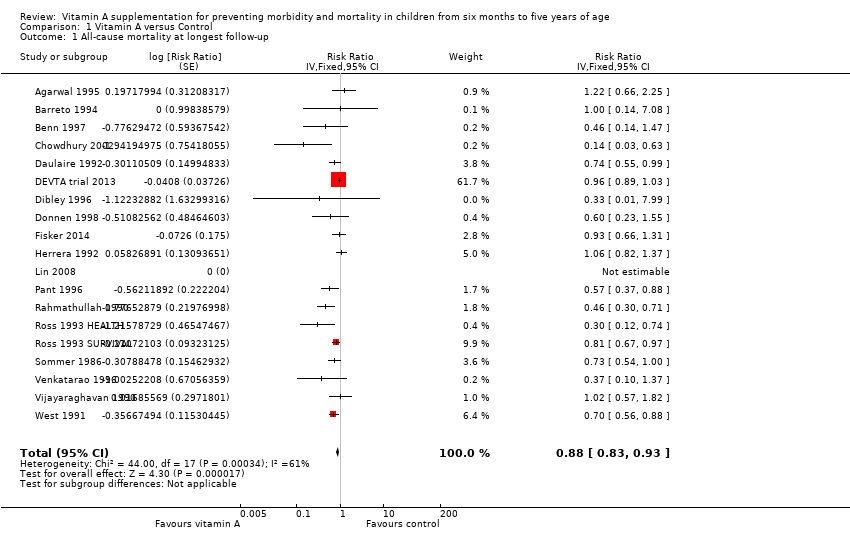
Comparison 1 Vitamin A versus Control, Outcome 1 All‐cause mortality at longest follow‐up.
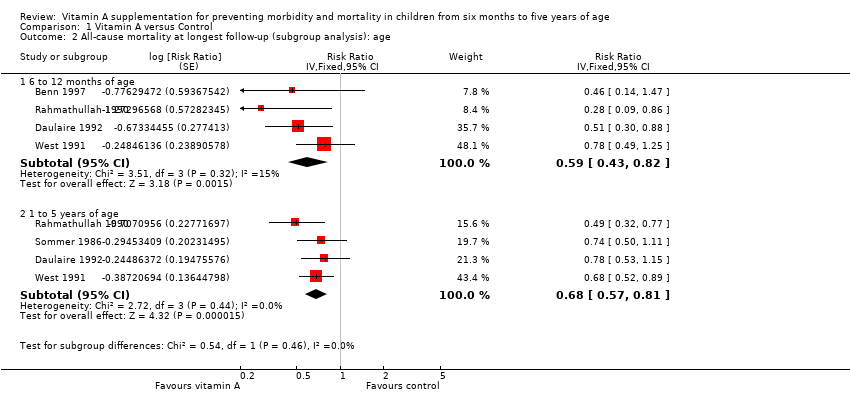
Comparison 1 Vitamin A versus Control, Outcome 2 All‐cause mortality at longest follow‐up (subgroup analysis): age.
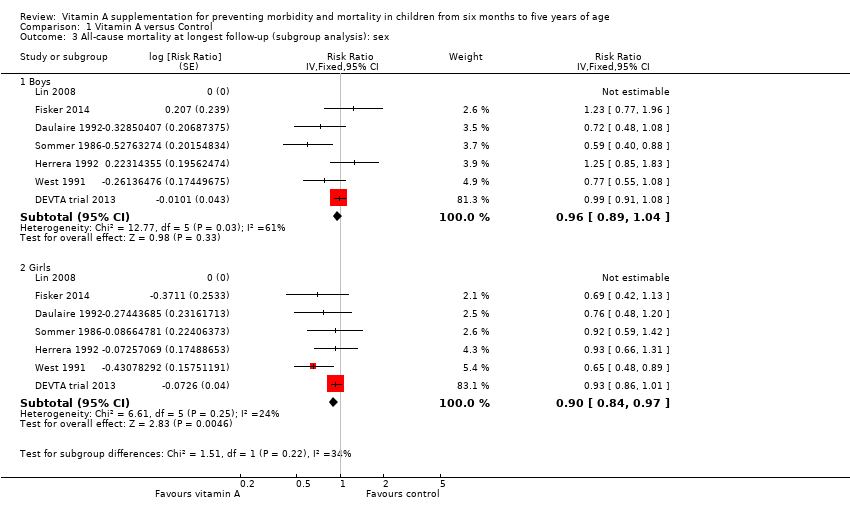
Comparison 1 Vitamin A versus Control, Outcome 3 All‐cause mortality at longest follow‐up (subgroup analysis): sex.
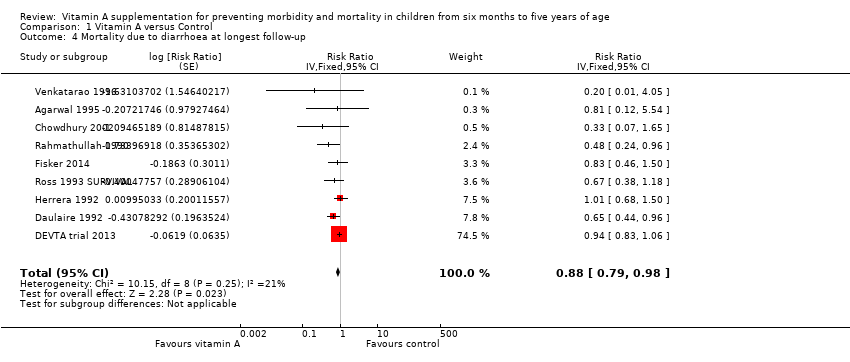
Comparison 1 Vitamin A versus Control, Outcome 4 Mortality due to diarrhoea at longest follow‐up.
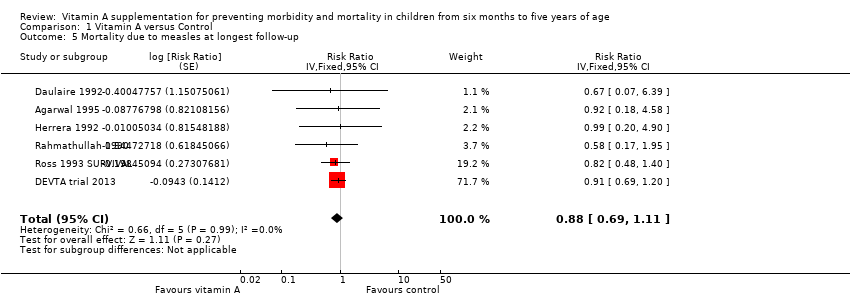
Comparison 1 Vitamin A versus Control, Outcome 5 Mortality due to measles at longest follow‐up.
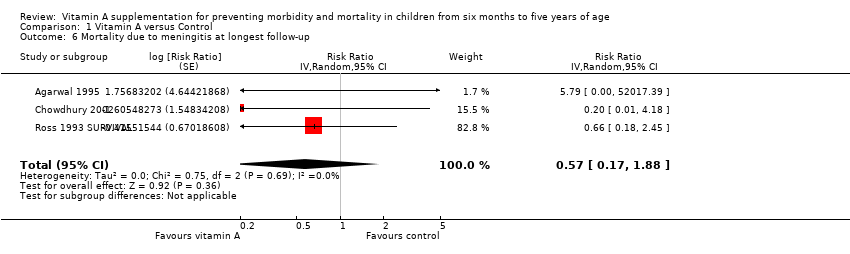
Comparison 1 Vitamin A versus Control, Outcome 6 Mortality due to meningitis at longest follow‐up.
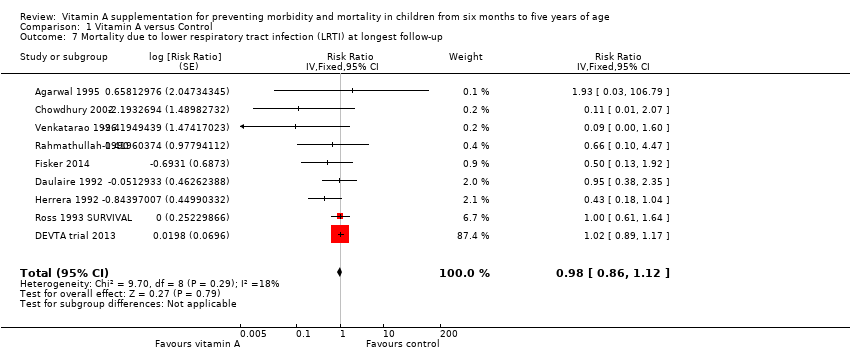
Comparison 1 Vitamin A versus Control, Outcome 7 Mortality due to lower respiratory tract infection (LRTI) at longest follow‐up.
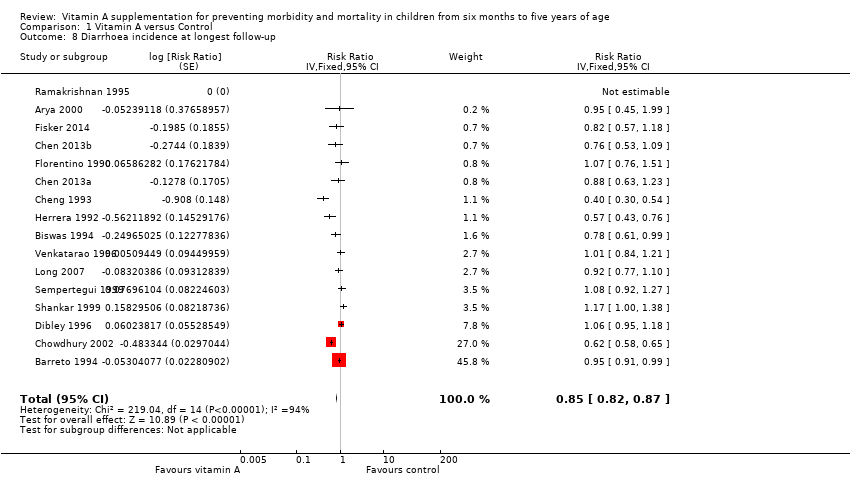
Comparison 1 Vitamin A versus Control, Outcome 8 Diarrhoea incidence at longest follow‐up.
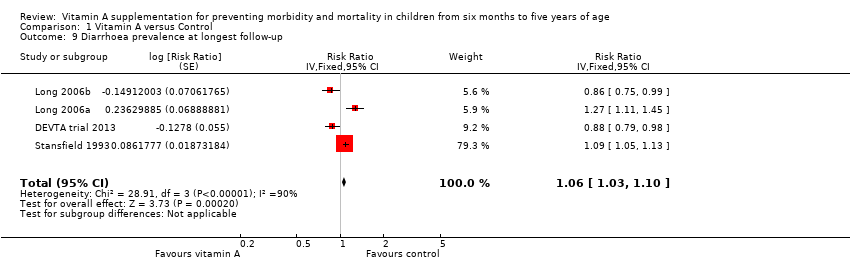
Comparison 1 Vitamin A versus Control, Outcome 9 Diarrhoea prevalence at longest follow‐up.
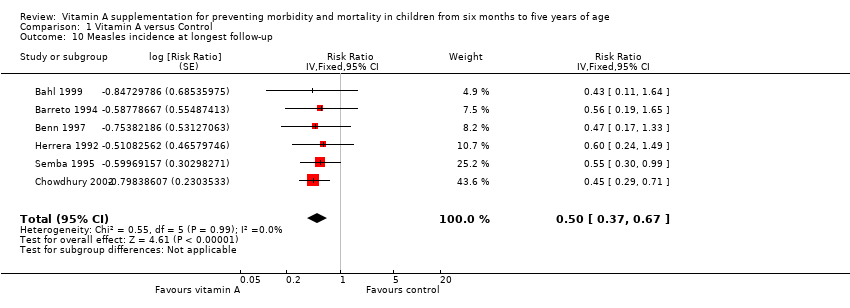
Comparison 1 Vitamin A versus Control, Outcome 10 Measles incidence at longest follow‐up.
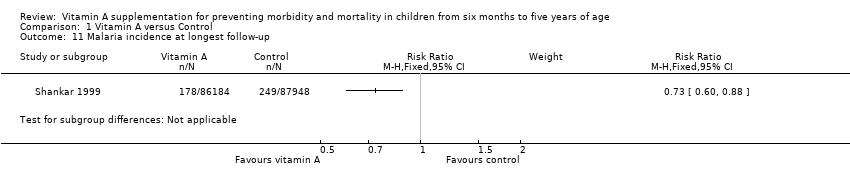
Comparison 1 Vitamin A versus Control, Outcome 11 Malaria incidence at longest follow‐up.
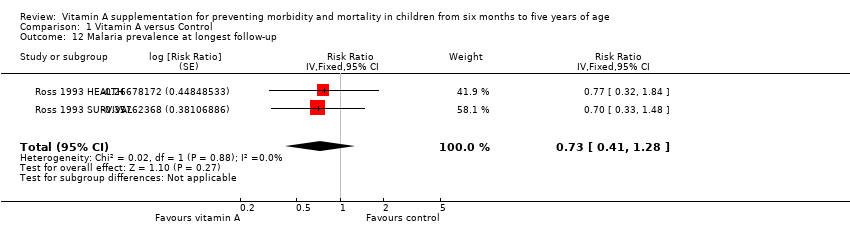
Comparison 1 Vitamin A versus Control, Outcome 12 Malaria prevalence at longest follow‐up.
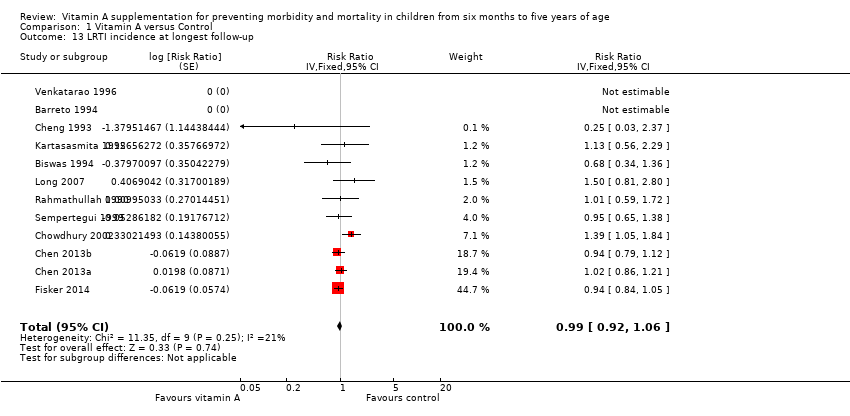
Comparison 1 Vitamin A versus Control, Outcome 13 LRTI incidence at longest follow‐up.
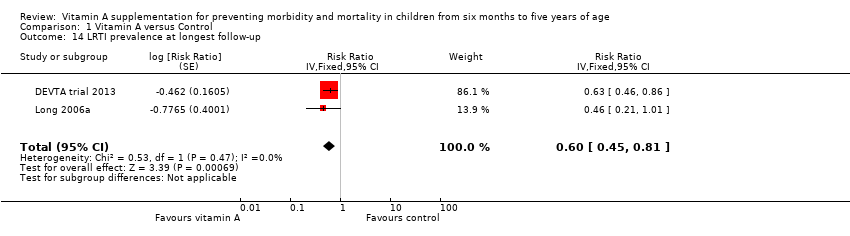
Comparison 1 Vitamin A versus Control, Outcome 14 LRTI prevalence at longest follow‐up.
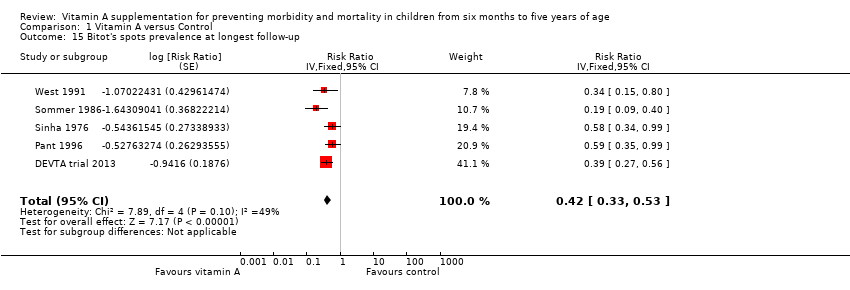
Comparison 1 Vitamin A versus Control, Outcome 15 Bitot's spots prevalence at longest follow‐up.
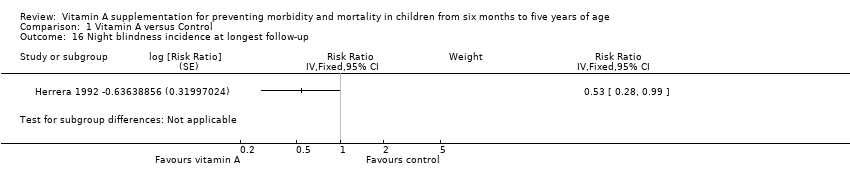
Comparison 1 Vitamin A versus Control, Outcome 16 Night blindness incidence at longest follow‐up.
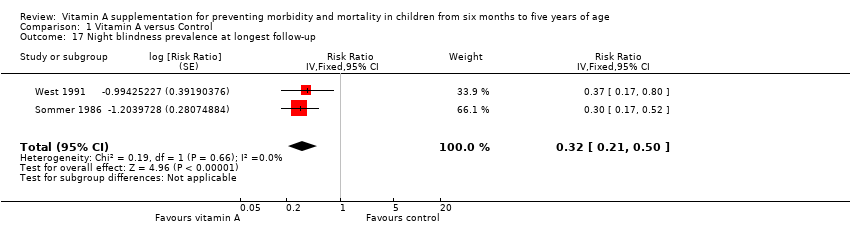
Comparison 1 Vitamin A versus Control, Outcome 17 Night blindness prevalence at longest follow‐up.
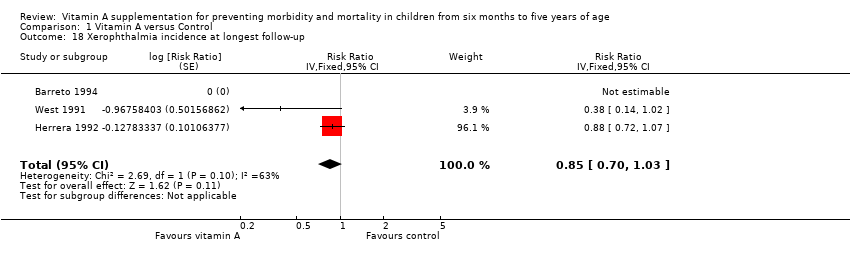
Comparison 1 Vitamin A versus Control, Outcome 18 Xerophthalmia incidence at longest follow‐up.
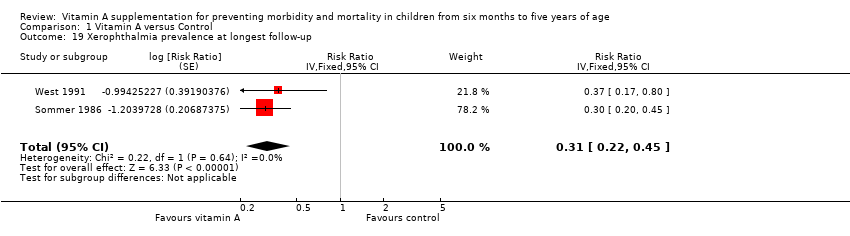
Comparison 1 Vitamin A versus Control, Outcome 19 Xerophthalmia prevalence at longest follow‐up.
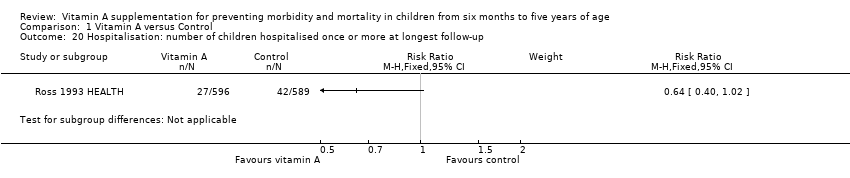
Comparison 1 Vitamin A versus Control, Outcome 20 Hospitalisation: number of children hospitalised once or more at longest follow‐up.
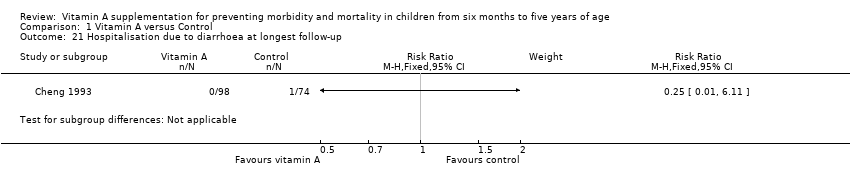
Comparison 1 Vitamin A versus Control, Outcome 21 Hospitalisation due to diarrhoea at longest follow‐up.
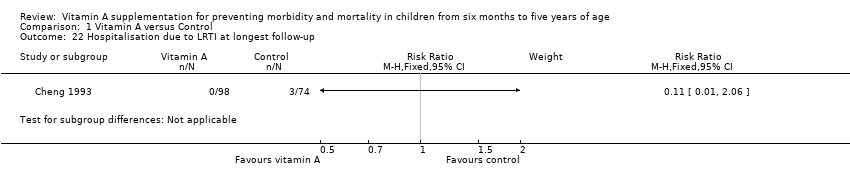
Comparison 1 Vitamin A versus Control, Outcome 22 Hospitalisation due to LRTI at longest follow‐up.
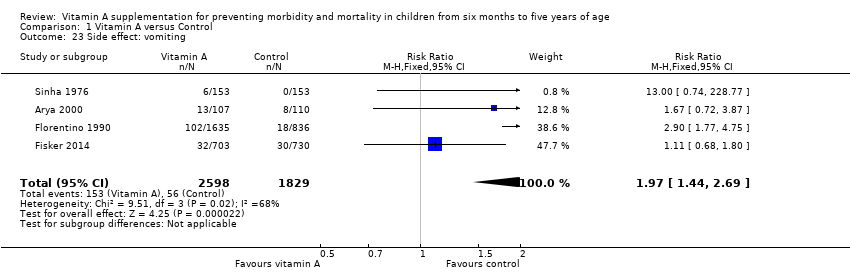
Comparison 1 Vitamin A versus Control, Outcome 23 Side effect: vomiting.
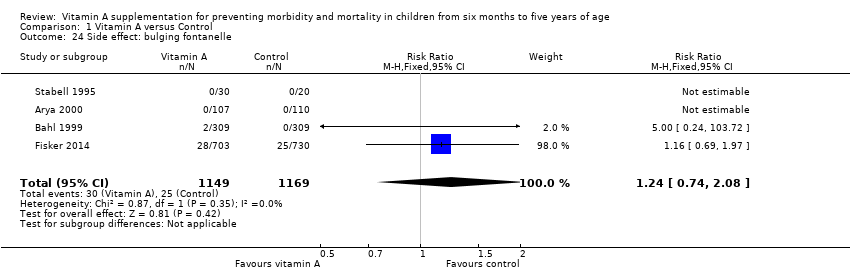
Comparison 1 Vitamin A versus Control, Outcome 24 Side effect: bulging fontanelle.
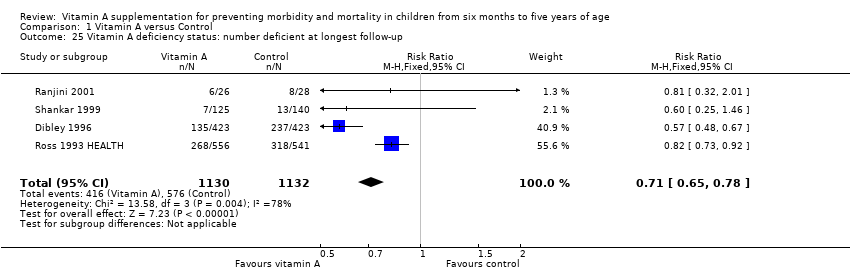
Comparison 1 Vitamin A versus Control, Outcome 25 Vitamin A deficiency status: number deficient at longest follow‐up.
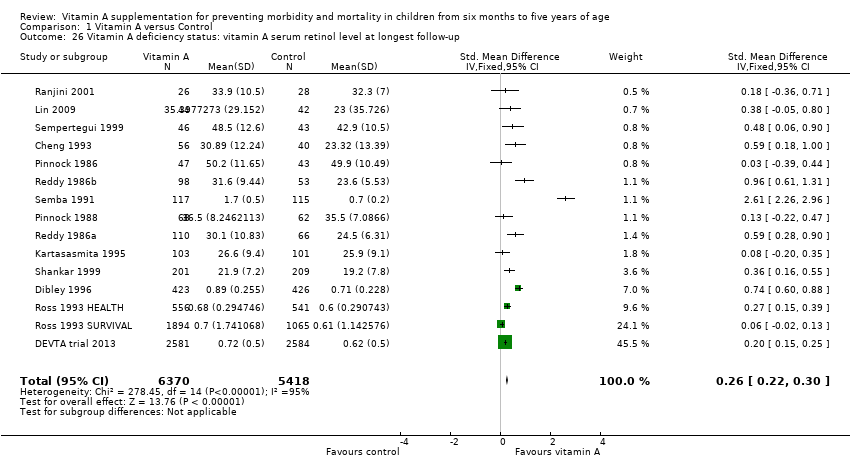
Comparison 1 Vitamin A versus Control, Outcome 26 Vitamin A deficiency status: vitamin A serum retinol level at longest follow‐up.
| Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age | ||||||
| Patient or population: children aged between 6 months and 5 years Intervention: vitamin A supplementation Comparison: placebo or usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vitamin A | |||||
| All‐cause mortality Follow‐up: 12‐96 weeks | Study population | RR 0.88 (0.83 to 0.93) | 1,202,382 (19 studies) | ++++ | Random‐effects RR 0.76 (95% CI 0.66 to 0.88) | |
| 26 per 1000a | 23 per 1000 (22 to 24) | |||||
| Mortality due to diarrhoea Follow‐up: 48‐104 weeks | Study population | RR 0.88, (0.79 to 0.98) | 1,098,538 | ++++ | Total number of participants reflects number randomised to studies. The analysis combined cumulative risk and risk per 1000 years follow‐up | |
| 8 per 1000a | 7 per 1000 (6 to 8) | |||||
| Mortality due to measles Follow‐up: 52 to 104 weeks | Study population | RR 0.88, (0.69 to 1.11) | 1,088,261 | ++OO Lowc,d | Total number of participants reflects number randomised to studies. The analysis combined cumulative risk and risk per 1000 years follow‐up | |
| 2 per 10,000a | 2 per 1000 (1 to 2) | |||||
| Mortality due to LRTI Follow‐up: 48‐104 weeks | Study population | RR 0.98, (0.86 to 1.12) | 1,098,538 | ++OO | Total number of participants reflects number randomised to studies. The analysis combined cumulative risk and risk per 1000 years follow‐up | |
| 4 per 10,000a | 4 per 1000 (3 to 5) | |||||
| Diarrhoea incidence Mean episodes per child per year | Study population | Rate ratio 0.85, 95% CI 0.82 to 0.87 | 77,946 | ++OO | — | |
| Mean episodes of diarrhoea in control group: 4.0 per child per yeare | VAS led to 3 fewer episodes of diarrhoea per child per year (3 to 4 fewer episodes) | |||||
| Measles incidence Mean episodes of measles per child per year | Study population | Rate ratio 0.50, 95% CI 0.37 to 0.67 | 19,566 | ++O Moderatec | — | |
| Mean episodes of measles in control group: 0.2 per child per yeare | VAS led to 0.015 fewer episodes per child per year (0.019 events fewer per child to 0.01 events fewer per child) | |||||
| LRTI incidence Mean episodes per child per year | Study population | Rate ratio 0.99, 95% CI 0.92 to 1.06 | 27, 540 | ++OO Lowc,d | — | |
| Mean episodes of LRTI in control group: 0.1 episodes per child per yeare | VAS led to 0.1 more episodes of LRTI per child per year (0.1 fewer episodes to 0.1 more episodes) | |||||
| Bitot's spots incidence Follow‐up: mean 80.72 weeks | Study population | RR 0.42, 95% CI 0.33 to 0.53 | 1,063,278 | +++O Moderatec | — | |
| 35 per 1000a | 15 per 1000 (12 to 19) | |||||
| Night blindness incidence Follow‐up: 52 to 68 weeks | Study population | RR 0.32, 95% CI 0.21 to 0.50 | 22,972 (2 studies) | +++O Moderatec | — | |
| 4 per 1000g | 1 per 1000 (1 to 2) | |||||
| Vitamin A deficiency Follow‐up: mean 54.5 weeks | Study population | RR 0.71, 95% CI 0.65 to 0.78 | 2262 | +++O | — | |
| 509 per 1000g | 361 per 1000 (331 to 397) | |||||
| Vomiting Follow‐up: 0.14 to 52 weeks | Study population | RR 1.97, 95% CI 1.44 to 2.69 | 10541 | +++O | — | |
| 31 per 1000g | 61 per 1000 (45 to 83) | |||||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aBased on control group mortality risk in DEVTA trial 2013. | ||||||
| Outcome or subgroup | Studies | Heterogeneity | Statistical Method | Effect estimate | Test for subgroup differences (P value) |
| All‐cause mortality, outcomes < 1 year since randomisation | 13 | Chi2 = 34.29, df = 12; P < 0.001; I2 = 65% | Risk ratio (GIV, fixed, 95% CI) | 0.83 (0.75 to 0.92) | NA |
| All‐cause mortality, outcomes 13 months to 59 months since randomisation | 6 | Chi2 = 15.75, df = 5; P < 0.001; I2 = 68% | Risk ratio (GIV, fixed, 95% CI) | 0.88 (0.81 to 0.97) | NA |
| All‐cause mortality at longest follow‐up (subgroup analysis): Asia | 12 | Chi2 = 42.65, df = 10; P < 0.001; I2 = 77% | Risk ratio (GIV, fixed, 95% CI) | 0.90 (0.84 to 0.96) | 0.83 |
| All‐cause mortality at longest follow‐up (subgroup analysis): Africa | 6 | Chi2 = 10.06, df = 5; P = 0.07; I2 = 50% | Risk ratio (GIV, fixed, 95% CI) | 0.86 (0.75 to 0.98) | |
| All‐cause mortality at longest follow‐up (subgroup analysis): Latin America | 1 | NA | Risk ratio (GIV, fixed, 95% CI) | 1.00 (0.14 to 7.08) | |
| All‐cause mortality at longest follow‐up, by national child mortality rate (subgroup analysis): high (> 40/1000) | 17 | Chi2 = 53.07, df = 16 (P < 0.001; I2 = 70% | Risk ratio (GIV, fixed, 95% CI) | 0.89 (0.84 to 0.94) | 0.9 |
| All‐cause mortality at longest follow‐up, by national child mortality rate (subgroup analysis): low (< 40/1000) | 2 | NA | Risk ratio (GIV, fixed, 95% CI) | 1.00 (0.14 to 7.08) | |
| All‐cause mortality at longest follow‐up (sensitivity analysis): random‐effects model | 19 | Tau2 = 0.04; Chi2 = 44.00, df = 17; P = 0.001; I2 = 61% | Risk ratio (GIV, fixed, 95% CI) | 0.76 (0.66 to 0.88) | NA |
| All‐cause mortality at longest follow‐up (sensitivity analysis): without DEVTA trial | 18 | Chi2 = 30.38, df = 16; P = 0.02; I2 = 47% | Risk ratio (GIV, fixed, 95% CI) | 0.77 (0.70 to 0.84) | NA |
| All‐cause mortality at longest follow‐up (sensitivity analysis): ICC = 0.002 (assumes no impact of clustering for studies with unknown ICC) | 19 | Chi2 = 57.02, df = 16; P < 0.001; I2 = 72% | Risk ratio (GIV, fixed, 95% CI) | 0.89 (0.84, 0.94) | NA |
| All‐cause mortality at longest follow‐up (sensitivity analysis): ICC = 0.010 (assumes high impact of clustering for studies with unknown ICC) | 19 | Chi2 = 47.87, df = 16; P < 0.001; I2 = 67% | Risk ratio (GIV, fixed, 95% CI) | 0.89 (0.84 to 0.94) | NA |
| Mortality due to diarrhoea, outcomes < 1 year since randomisation | 6 | Chi2 = 5.23, df = 5; P = 0.39; I2 = 4% | Risk ratio (GIV, fixed, 95% CI) | 0.76 (0.61 to 0.95) | NA |
| Mortality due to measles, outcomes < 1 year since randomisation | 4 | Chi2 = 0.52, df = 3; P = 0.91; I2 = 0% | Risk ratio (GIV, fixed, 95% CI) | 0.85 (0.52 to 1.37) | NA |
| Mortality due to meningitis, outcomes < 1 year since randomisation | 1 | NA | Risk ratio (GIV, fixed, 95% CI) | 5.79 (0.22 to 153.24) | NA |
| Mortality due to LRTI, outcomes < 1 year since randomisation | 6 | Chi2 = 5.66, df = 5; P = 0.34; I2 = 12% | Risk ratio (GIV, fixed, 95% CI) | 0.66 (0.40 to 1.10) | NA |
| Diarrhoea incidence at longest follow‐up (sensitivity analysis): analysis without studies Cheng 1993; Chowdhury 2002 | 13 | Heterogeneity: chi2 = 30.71, df = 12; P = 0.002; I2 = 61% | Risk ratio (GIV, fixed, 95% CI) | 0.96 (0.93 to 1.00) | NA |
| Diarrhoea incidence, outcomes < 1 year since randomisation | 13 | Chi2 = 51.64, df = 11; P < 0.001; I2 = 79% | Risk ratio (GIV, fixed, 95% CI) | 0.93 (0.89 to 0.96) | NA |
| Diarrhoea incidence at longest follow‐up (sensitivity analysis): random‐effects model | 15 | Tau2 = 0.07; Chi2 = 219.04, df = 14; P < 0.001; I2 = 94% | Risk ratio (GIV, random, 95% CI) | 0.84 (0.73, 0.98) | NA |
| Measles incidence, outcomes < 1 year since randomisation | 5 | Chi2 = 0.24, df = 4; P = 0.99; I2 = 0% | Risk ratio (GIV, fixed, 95% CI) | 0.54 (0.36 to 0.80) | NA |
| Malaria incidence, outcomes 1 + years since randomisation (subgroup analysis): age | 1 | NA | Risk ratio (M‐H, fixed, 95% CI) | 0.73 (0.60 to 0.88) | NA |
| LRTI Incidence, outcomes < 1 year since randomisation | 11 | Chi2 = 5.23, df = 8; P = 0.73; I2 = 0% | Risk ratio (GIV, fixed, 95% CI) | 0.96 (0.89 to 1.04) | NA |
| Bitot's spots incidence, outcomes < 1 year since randomisation | 1 | NA | Risk ratio (GIV, fixed, 95% CI) | 0.93 (0.76 to 1.14) | NA |
| Bitot's spots prevalence, outcomes < 1 year since randomisation | 3 | Chi2 = 6.06, df = 2; P = 0.05; I2 = 67% | Risk ratio (GIV, fixed, 95% CI) | 0.43 (0.33 to 0.56) | NA |
| Night blindness prevalence, outcomes < 1 year since randomisation | 1 | NA | Risk ratio (GIV, fixed, 95% CI) | 0.30 (0.17 to 0.52) | NA |
| Xerophthalmia incidence, outcomes < 1 year since randomisation | 2 | NA | Risk ratio (GIV, fixed, 95% CI) | 0.88 (0.72 to 1.07) | NA |
| Vitamin A serum retinol level, outcomes < 1 year since randomisation | 11 | Chi2 = 178.42, df = 10; P < 0.001; I2 = 94% | Standardised mean difference (GIV, fixed, 95% CI) | 0.45 (0.37 to 0.53) | NA |
| Vitamin A serum retinol level at longest follow‐up (sensitivity analysis): random‐effects model | 14 | Tau2 = 0.13; Chi2 = 278.45, df = 14; P < 0.001; I2 = 95% | Standardised mean difference (GIV, random, 95% CI) | 0.50 (0.30 to 0.70) | NA |
| CI: confidence interval; GIV: Generic inverse variance; LRTI: lower respiratory tract infection;M‐H: mantel Haenszel method;NA: not applicable. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality at longest follow‐up Show forest plot | 19 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.83, 0.93] | |
| 2 All‐cause mortality at longest follow‐up (subgroup analysis): age Show forest plot | 5 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 6 to 12 months of age | 4 | Risk Ratio (Fixed, 95% CI) | 0.59 [0.43, 0.82] | |
| 2.2 1 to 5 years of age | 4 | Risk Ratio (Fixed, 95% CI) | 0.68 [0.57, 0.81] | |
| 3 All‐cause mortality at longest follow‐up (subgroup analysis): sex Show forest plot | 7 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Boys | 7 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.89, 1.04] | |
| 3.2 Girls | 7 | Risk Ratio (Fixed, 95% CI) | 0.90 [0.84, 0.97] | |
| 4 Mortality due to diarrhoea at longest follow‐up Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.79, 0.98] | |
| 5 Mortality due to measles at longest follow‐up Show forest plot | 6 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.69, 1.11] | |
| 6 Mortality due to meningitis at longest follow‐up Show forest plot | 3 | Risk Ratio (Random, 95% CI) | 0.57 [0.17, 1.88] | |
| 7 Mortality due to lower respiratory tract infection (LRTI) at longest follow‐up Show forest plot | 9 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.86, 1.12] | |
| 8 Diarrhoea incidence at longest follow‐up Show forest plot | 16 | Risk Ratio (Fixed, 95% CI) | 0.85 [0.82, 0.87] | |
| 9 Diarrhoea prevalence at longest follow‐up Show forest plot | 4 | Risk Ratio (Fixed, 95% CI) | 1.06 [1.03, 1.10] | |
| 10 Measles incidence at longest follow‐up Show forest plot | 6 | Risk Ratio (Fixed, 95% CI) | 0.50 [0.37, 0.67] | |
| 11 Malaria incidence at longest follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12 Malaria prevalence at longest follow‐up Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.73 [0.41, 1.28] | |
| 13 LRTI incidence at longest follow‐up Show forest plot | 12 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.92, 1.06] | |
| 14 LRTI prevalence at longest follow‐up Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.60 [0.45, 0.81] | |
| 15 Bitot's spots prevalence at longest follow‐up Show forest plot | 5 | Risk Ratio (Fixed, 95% CI) | 0.42 [0.33, 0.53] | |
| 16 Night blindness incidence at longest follow‐up Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 17 Night blindness prevalence at longest follow‐up Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.32 [0.21, 0.50] | |
| 18 Xerophthalmia incidence at longest follow‐up Show forest plot | 3 | Risk Ratio (Fixed, 95% CI) | 0.85 [0.70, 1.03] | |
| 19 Xerophthalmia prevalence at longest follow‐up Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.31 [0.22, 0.45] | |
| 20 Hospitalisation: number of children hospitalised once or more at longest follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21 Hospitalisation due to diarrhoea at longest follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22 Hospitalisation due to LRTI at longest follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23 Side effect: vomiting Show forest plot | 4 | 4427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.44, 2.69] |
| 24 Side effect: bulging fontanelle Show forest plot | 4 | 2318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.74, 2.08] |
| 25 Vitamin A deficiency status: number deficient at longest follow‐up Show forest plot | 4 | 2262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 26 Vitamin A deficiency status: vitamin A serum retinol level at longest follow‐up Show forest plot | 15 | 11788 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.22, 0.30] |

