Alfabloqueadores como tratamiento expulsivo médico para los cálculos ureterales
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Baseline assessment included non‐contrast spiral CT and furthermore weekly follow‐ups with KUB and US. NCCT was repeated at end of study to confirm stone status. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated randomly in 1:1 ratio into either group." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Using sealed envelopes." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both treating physicians and patients were blinded to randomization." Comment: double‐blind and therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No blinding of outcome assessments described. Comment: No description was available; therefore this method of outcome assessment was considered to have unclear risk of bias. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "17 patients were excluded due to not accepting randomization (6) and loss to follow‐up during study period (4 in group A and 7 in group B)." Comment: Owing to relatively high numbers of exclusions and losses to follow‐up, incomplete outcome data were stated and risk of bias was unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias therefore was considered to be low. |
| Other bias | Low risk | No quotes available. The study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
All groups received 75 mg diclofenac intramuscularly and were advised to drink at least 3 L of fluids. | |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly with KUB and US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized to 3 groups." Comment: Randomisation was stated, but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Spontaneous stone expulsion was observed in 28 of 34 patients (82.3%) in group 1, 24 of 34 (70.5%) in group 2, and 12 of 34 (35.2%) patients in group 3.” Comment: Stone expulsion rate was reported for all participants; therefore, probably none were lost to follow‐up and accordingly risk of bias was considered low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. The study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Placebo group
Both groups were given tab diclofenac sodium 50 mg, 1 tab 8 hourly for pain control on required basis. | |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Participants were evaluated with plain X‐ray KUB after 2 weeks and 4 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned into one of the two groups." Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Three patients lost to follow up, therefore 97 out of 100 patients were evaluated.” Comment: Small number of participants were lost to follow‐up; accordingly, risk of bias was considered low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
All groups received 50 mg diclofenac every 12 hours for 1 week, then 75 mg diclofenac intramuscularly as needed up to 2 times per day. | |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up visits on a weekly basis with urine analysis, serum creatinine measurement, plain X‐ray KUB, and abdominal ultrasonography. Abdominal CT was performed for participants with radiolucent stones if the stone was not expulsed by the end of study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 90 patients with distal ureteral stones ≤10 mm in diameter were randomly divided into 3 equal groups and given medications for 30 days." Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. No intention‐to‐treat analysis. Comment: Owing to lack of intention‐to‐treat analysis, risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Sample size calculated. Weekly follow‐up with KUB, US, and urine analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized between study and placebo medications using a computer‐generated random number assignment, adjusted at a ratio of 1:1." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization data were kept strictly confidential, in sealed envelops, accessible only to the pharmacist at the end of the study." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The investigators and patients were masked to the type of the treatment throughout the study." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “The investigators and patients were masked to the type of the treatment throughout the study.” Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: “Four patients in the placebo group were lost to follow‐up.” Comment: Four participants in the placebo group were lost to follow‐up and were excluded from analysis. It is unclear whether this had a clinically relevant impact on the intervention effect estimate; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias therefore was considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up visits on a weekly basis with urine analysis, serum creatinine measurement, plain X‐ray KUB, and abdominal ultrasonography. Abdominal CT was performed for all participants by end of study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quote available. Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Out of the 120 patients included in the study, patients were divided randomly into three groups of 40 patients." Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Blood pressure was not reported in the Results section (as a secondary outcome measurement); therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
Participants in groups 1 and 2 received diclofenac when needed (100 mg once daily). | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Missing data on stone expulsion rate. Follow‐up was 10 days, then again KUB, US, or CT. All participants were suggested to drink at least 2 L of drinking water daily. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomized in 3 groups.” Comment: Randomisation was stated, but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: No predefined endpoints were mentioned; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered low. |
| Methods |
| |
| Participants |
| |
| Interventions | Control group
Treatment group
Researchers emphasised that all participants should drink 2 L of water daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up was every 2 weeks with KUB, US, or CT. Not all abbreviations were explained in the text. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomly assigned to a control group (n = 46) and study group (n = 50).” Comment: Randomisation was stated but no information on method used was available; therefore selection bias was considered to have unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. No intention‐to‐treat analysis. Comment: Owing to lack of intention‐to‐treat analysis, risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Results on pain episodes were not reported; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore we considered risk of other bias to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Control group
Treatment group
Researchers emphasised that all participants should drink 2 L of water daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Assessment of trial results was conducted at 10 days and 30 days. On day 30, stone expulsion was assessed by plain X‐ray, ultrasonography, or urography. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were divided randomly into 2 treatment groups.” Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Aescin = standard therapy at this centre. Included participants from the study by Autorino et al 2005. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization, not blinded, was performed using a stratified permuted randomization algorithm.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: All participants were included in the analysis; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “were randomly divided into.” Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Unclear risk | No quotes available. Baseline data are missing. Comment: Owing to lack of information at baseline, risk of other bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients satisfying the inclusion criteria were randomly divided in to 2 groups by random number table.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Primary outcomes were not prespecified; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
All participants were encouraged to maintain water intake of 2‐2.5 L/d. | |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed using the Power Analysis & Sample Size Software (PASS_ ) for Windows (NCSS Inc., Kaysville, UT). According to the software, the patients were randomly assigned to one of the following three groups.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "randomly divided into three groups according to the order of arrival (1:1:1 ratio)." Comment: This method of random sequence generation was considered to have high risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "all tomography and direct urinary system graphy of the patients were analyzed and confirmed by a urologist blinded to the group of the patients." Comment: Risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A total of 13 patients, 6 in group 1, 4 in group 2, and 3 in group 3, were excluded from the study because they were lost in follow‐up. 5 patients in group 1 and 3 patients in group 3 were also excluded from the study because they declared more than one sexual intercourse or masturbation per week." Comment: 21/211 participants were lost to follow‐up and were equally divided over the 3 groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | High risk | No quotes available. Only males were included in the study. Comment: Potential selection bias; therefore high risk of other bias. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Placebo group
| |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "using a convenience sample." Comment: This method of random sequence generation was considered to have high risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blinded, placebo‐controlled." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Of the 127 patients enrolled during this study, 15 were lost to follow‐up, and 12 received surgical intervention before the 7‐day mark, leaving 100 patients for analysis." Comment: Owing to a reasonable number of participants lost to follow‐up, risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
All participants were made to keep a drinking regimen of at least 2.5 L of water or weak tea daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Study authors stated the study model was a double blind RCT but described no blinding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "we have applied a double blind randomized study." Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "As two pts. were excluded from group “A” because during treatment they developed acute obstruction pyelonephritis, both groups consisted of a rather unusual identical number of experimental and standardly treated pts. (51)." Comment: In the light of the small number of participants lost to follow‐up, risk of attrition bias was stated as low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | All participants received trospium chloride (50 mg 3 times daily). All participants received initial treatment of 90 mg diclofenac by intramuscular injection and 5 mg cimetropium bromide by intravenous injection, with a second dose after 30 minutes or 1 hour if necessary. Treatment group 1
Treatment group 2
Treatment group 3
Control group
| |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization list was generated by using the permuted block method.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: “... was concealed from the patient‐enrolling investigators (confined with a doctor assisting in the procedure but not participating in the study).” Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Placebo group
| |
| Outcomes | Primary outcome measures
Secondary outcome measures
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Participants were followed up at days 14 (visit 1), 28 (visit 2), 60 (visit 3), and 90 (visit 4) after initiation of medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by the Medical Research Collaboration Center of Seoul National University Bundang Hospital using random permuted blocks of different sizes. The size of the next block was randomly chosen from the available block sizes." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "The size of the next block was randomly chosen from the available block sizes. Randomization was stratified by each recruiting study site." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "One person packed the 14‐day supply of tablets for each patient. All study staff at all hospitals were blinded to treatment allocation and remained blind until the end of the trial." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Figure 1 shows a detailed flow diagram of participant follow‐up, including information regarding loss to follow‐up, exclusion due to adverse events, or need for intervention. Comment: Detailed information on participant follow‐up was available; risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Information on primary and secondary outcomes was presented in detail in the protocol at www.clinicaltrials.gov. Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up visits on a weekly basis with urine analysis, plain X‐ray KUB, and abdominal ultrasonography. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomly divided into 3 groups with the random number table envelope method.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: “The names of the groups were written on small papers with the same size, they were folded, put in an envelope, and drawn by the patients.“ Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quote available. Participants were blinded, and blinding of doctors was not described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “On follow‐up, plain urinary tract x‐ray images of the patients were seen and analyzed by an urologist (MFK) blinded to the group of the patients.“ Comment: Risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) | High risk | No quote available. 13/57 participants lost to follow‐up equally distributed among control and treatment groups. Comment: Owing to a reasonable number of participants lost to follow‐up (> 20%), risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | High risk | No quote available. No number of pain episodes described in the Results section (although this was a secondary outcome measurement). Furthermore adverse effects were predefined in Methods section and were not described in Results section. Comment: Owing to inconsistent reporting, risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group (group 3)
Control group (group 4)
Four treatment arms, of which the first 2 underwent ESWL (groups 1 and 2); groups 3 and 4 did not want to undergo ESWL. | |
| Outcomes |
| |
| Funding sources | Study was supported by research funds from Astellas Phama Korea, Inc. | |
| Declarations of interest | None stated. | |
| Notes | Dong Il Kang; only the abstract was written in English and therefore was interpretable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No quotes available. Comment: Participants were those who did not want to undergo ESWL; therefore risk of selection bias was considered to be high. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group (group 2)
Control group (group 1)
All participants were asked to drink > 2 L of water daily and received diclofenac 75 mg IM on demand. | |
| Outcomes |
| |
| Funding sources | Welcome Trust: Howard Hughes Medical Institute and others. | |
| Declarations of interest | None. | |
| Notes | Article 2015. Conference abstract 2014. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomly assigned to either the control group or the alfuzosin group based on a computer‐generated random table.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “This was a prospective, randomized, open–label, controlled study.” Comment: open label trial; therefore risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “This was a prospective, randomized, open–label, controlled study.“ Comment: open label trial; therefore risk of detection bias was considered to be high. |
| Incomplete outcome data (attrition bias) | Low risk | No quote available. Comment: All participants were included in the analysis; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Group 1: placebo group. Group 2: tamsulosin group. Group 3: Uralyt group. Group 4: Uralyt and tamsulosin group. All participants were advised to increase fluid intake to more than 2 L per day and to receive IM injection of 75 mg of diclofenac sodium. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Non‐enhanced spiral CT was done at the end of study period for all participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were prospectively randomized through a computer generated randomization process into four equal groups.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: “The investigators and the patients were blinded to the treatment given, until the end of the study.” Comment: Randomisation method was described and would not allow investigator/participant to know or influence the intervention group before eligible participant entered into the study. Therefore this method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “The investigators and the patients were blinded to the treatment given, until the end of the study.” Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “5 cases were lost to follow‐up (2 in the control group and one in each of the other three groups).” Comment: Owing to the small number of participants lost to follow‐up, risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Primary outcomes were not prespecified; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Group 1: tamsulosin group. Group 2: corticosteroid group. Group 3: tamsulosin and corticosteroid group. Group 4: control group. Data for groups 1 and 4 were used for the review. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. Follow‐up on weekly basis with imaging (not discussed in detail). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A total of 134 patients with distal ureteral stone of 4‐10 mm were randomized into 4 groups.” Comment: Randomisation stated but no information on method used was available; therefore selection bias was considered to be at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | High risk | No quotes available. 6 patients in control group lost to follow‐up. Comment: losses to follow‐up not balanced between treatment groups; therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1:
Treatment group 2:
Treatment group 3:
Control group:
All participants were treated with prophylactic antibiotic therapy (cefuroxime axetil 250 mg (once a day)) and received 2500 mL hydration daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Weekly checkups and follow‐up with renal ultrasonography, complete urinalysis, serum urea creatinine measurements, and direct urinary system graphics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After randomization to one of four groups, the patients received treatment.” Comment: Owing to insufficient information, random sequence generation was considered to be at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. 5 participants were lost to follow‐up; they were included in the analysis. Comment: Owing to the small number of participants lost to follow‐up, risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Control group
Tamsulosin group
| |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “simple randomization method by generating a random digit (0–60 in each group) has been used within each group.” Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. Comment: Losses to follow‐up were not clearly described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Funding sources | This study was funded by an academic grant from the Maine Medical Center Mentored Research Committee. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up with all participants at 2, 5, and 14 days post discharge from the ED. Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was accomplished by using a table of random numbers.” Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: “The information on group assignment was contained in a sealed envelope within each study packet, and the envelopes were clearly labelled “do not open until informed consent is obtained.” Comment: This method of allocation concealment was considered to be at low risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Participants lost to follow‐up (4), discontinued intervention (2), excluded because the stone was located in the proximal ureter (1). Comment: Losses to follow‐up were balanced across treatment groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Placebo group
Analgesia was given at the discretion of the treating physician. | |
| Outcomes |
| |
| Funding sources | Grant from the Queensland Emergency Medicine Research Foundation. | |
| Declarations of interest | None stated. | |
| Notes | Power calculation was performed. Intention‐to‐treat‐principle. Telephone contacts at 7, 14, 21, and 28 days with pelvic non‐contrast CT at day 28. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was produced with a computer‐generated program in permuted blocks of random lengths stratified by hospital and stone size." Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Once informed consent was obtained and patients were deemed appropriate for discharge, they were allocated to the next sequentially numbered study medication pack." Comment: This method of allocation concealment was considered to be at low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Investigators, the treating physician, and patients were blinded to the allocation for the duration of the study and data analysis." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Investigators, the treating physician, and patients were blinded to the allocation for the duration of the study and data analysis.” Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Similar proportions of patients with missing and nonmissing outcome data." Comment: Missing outcome data were balanced in numbers across both groups, and reasons for missing data were similar; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up at 14 ± 2 days with urinalysis, KUB, and ultrasonography. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization process was achieved by means of sealed envelopes equally nominating one of the three treatment alternative." Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was performed using the SNOSE method (sequentially‐numbered, opaque, sealed envelopes)." Comment: This method of allocation concealment was considered to be at low risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes | Successful results were defined as complete stone passage; failure was considered if:
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly with KUB. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... were divided randomly." Comment: Randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Outcomes were not prespecified; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
All participants were allowed 30 mg ketorolac IM injections on demand. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Only English abstract available for judgement. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. Comment: Losses to follow‐up were not clearly described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled patients underwent randomisation in a 1:1 fashion in blocks of 10 to receive either a daily single dose of tamsulosin (0.4 mg) or placebo. The sequence of randomisation was computer generated and was performed by the university hospital pharmacy using DatInf Randlist software v.1.0 (DatInf GmbH, Tübingen, Germany)." Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation data were kept strictly confidential in sealed envelopes, accessible only to the primary and senior investigator." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patient, the attending urologist were not aware of study arm assignments until the final assessment of outcome." Comment: double‐blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Personnel responsible for outcome assessments were blinded." Comment: double‐blind; therefore low risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Patients were included in the final analysis on an intention‐to‐treat basis. Patients who experienced stone expulsion before first medication, who withdrew their consent, or who were lost to follow‐up were excluded from the analysis." Comment: missing outcome data and losses to follow‐up balanced in numbers across both groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. Follow‐up time: 2 weeks, visits at 1st and 2nd weeks after medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | High risk | No quotes available. 61 patients excluded from the study; no details available. Comment: large number of patients excluded from the study without clarification. Therefore, risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Unclear risk | No quotes available. No information on baseline data. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Control group/Study group I
Treatment group 1/Study group II
Treatment group 2/Study group III
| |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly for 4 weeks; every visit focussed history, physical examination, and urinary US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised systematically at a ratio of 1:1." Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | High risk | No quotes available. Comment: Numbers of participants lost to follow‐up in the different study groups were not equally distributed; therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
All participants received 2500 mL hydration daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up weekly with renal ultrasonography, X‐ray KUB, urinalysis, and serum creatinine measurements. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: Low percentage of loss to follow‐up in each group (< 5%); therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Mean diclofenac sodium dosage and drug adverse events were not described as outcome parameters in the Methods section; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Control group
Treatment group
All participants were instructed to drink 2 L of water daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. Comment: Loss to follow‐up was not described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | High risk | No quotes available. Need for analgesics was one of the outcome parameters but was not described in Results section. Adverse events were described in Results section but were not stated as one of the outcome parameters. Comment: Owing to inconsistency in describing outcome parameters, risk of reporting bias was considered to be high. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Study group A/Control group
Study group B/Treatment group
All participants were instructed to drink 2 L of water daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Diagnostics for follow‐up were not described. Conference abstract in 2015. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided into two groups by using a random number table envelope method." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: 1 participant lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | High risk | No quotes available. Stone size was significantly different between the 2 study groups. Furthermore, only men were included in the study. Comment: Owing to potential selection bias, we considered judgement as having high risk of bias. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
50 mg diclofenac suppository on demand for all participants. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up: maximum of 4 weeks, weekly or biweekly X‐ray and US. Sample size was arbitrarily determined and was not based on statistical calculation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated into two treatment groups by using a random number table." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: 6 participants were excluded from the study and were balanced between intervention groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
All participants were instructed to ingest at least 2 L of fluids daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. Comment: Loss to follow‐up was not described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group (group 2)
Control group (group 1)
Four‐arm study, of which groups 3 and 4 underwent SWL. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up only at the 15th day (last day of study) with plain abdominal X‐ray and helical CT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the coin method." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patient follow‐up examinations were performed by two of us who were unaware of the treatment received." Comment: Investigators evaluating follow‐up examinations were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: No participants were lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Study group A/Control group
Study group B/Treatment group
All participants were asked to drink 2 L water daily. | |
| Outcomes |
| |
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Sample size calculation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A predefined randomization sequence was created by a computer random number generator using a block size of 4." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This prospective, randomized, open‐label, multicenter trial." Comment: Open‐label trial is considered to have high risk of bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | High risk | No quotes available. Comment: significant number of participants lost to follow‐up. Loss to follow‐up was not balanced between intervention groups; therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Control group 1
Treatment group 2
Control group 2
All participants were advised to consume at least 2 L of water daily and were allowed to use symptomatic therapy of 75 mg of diclofenac on demand. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The type of therapy (doxazosin or not) was determined according to the day the patient presented to the hospital, with every patient who presented on odd‐numbered days being assigned to take doxazosin." Comment: This method of random sequence generation was considered to have high risk of bias. |
| Allocation concealment (selection bias) | High risk | No quotes available. Open random allocation schedule. Comment: This method of allocation concealment was considered to have high risk of bias. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "unblinded." Comment: no blinding; therefore risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Although mentioned as outcome measurement in the Methods section, total dosage of diclofenac use was not described in the Results section; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
If participants developed renal colic during treatment, they were given an IM injection of 75 mg diclofenac in the emergency department. | |
| Outcomes |
| |
| Funding sources | Astellas Pharma (Thailand). | |
| Declarations of interest | None. | |
| Notes | Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomized in three groups by the assistant nurse." Comment: This method of random sequence generation was considered to have unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | High risk | No quote available. Comment: no blinding; therefore risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Control group/Study group I
Tamsulosin group/Study group 2
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. Data on stone clearance and stone relocation rate at 4 weeks were not described separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. Comment: Loss to follow‐up was not described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1/tamsulosin group
Treatment group 2/tamsulosin + nifedipine group
Placebo group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly with KUB and US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Occurrence of adverse effects was not predefined as outcome measurement. Time to stone expulsion was not measured with SDs. Number of colic episodes was not specified. Analgesic use was not described in detail (no SDs given). Comment: inconsistency in describing outcome measurements; therefore risk of bias was considered to be high. |
| Other bias | High risk | No quotes available. Comment: questionable whether this study was placebo‐controlled based on information provided in the Methods section; therefore risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1/tamsulosin group
Placebo group. | |
| Outcomes |
| |
| Funding sources | Research reported in this abstract was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number 3 U01 DK 096037. Content is solely the responsibility of the trial authors and does not necessarily represent the official views of the National Institutes of Health. | |
| Declarations of interest | None. | |
| Notes | 5 telephone calls during trial period (day 2, day 7, day 15, day 20, day 29). Follow‐up CT in a certain group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | No quotes available. Blinding of both participants and personnel. Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: Only a small number of participants were lost to follow‐up with clear description; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1/tamsulosin group
Treatment group 2/doxazosin group
Control group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up weekly with urine analysis, serum creatinine measurement, KUB, and US NCCT at the end of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. Blinding was not described in detail. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | High risk | No quotes available. Comment: no intention‐to‐treat analysis used and 5 participants lost to follow‐up; therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | High risk | No quotes available. Comment: Owing to incorrect data for stone clearance stratified for stone size, information is not interpretable and risk of other sources of bias was considered to be high. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Control group 1
Treatment group 2
Control group 2
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. Maximum follow‐up in group 1: 28 days. Weekly re‐evaluation with X‐ray and US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Study group A/Treatment group
Study group B/placebo group
All participants were instructed to drink at least 2 L of water per day and to carry out normal activities. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up every 14 days with plain abdominal film and abdominal ultrasonogram. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | No quotes available. Blinding of both participants and personnel. Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. No imaging at the end of follow‐up was done to evaluate stone clearance. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Study group A/control group
Study group B/treatment group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up weekly with KUB or CT. Primary endpoint was cumulative stone passage rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Not all participants received CT scan at the end of the trial period. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | High risk | No quotes available. Comment: a significant number of participants lost to follow‐up (20%‐30%); therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Funding sources | Sanofi‐Aventis Pharmaceuticals. | |
| Declarations of interest | None stated. | |
| Notes | Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... using a random number assignment. A computerized random number generator was used." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | No quotes available. Comment: Allocation was concealed; therefore the allocation method was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Investigators were blinded to the randomization scheme and patients and investigators were blinded to medication until termination of the study.” Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. 7 patients excluded: stone passage before first dose in 4. and withdrawal of consent in 3. Comment: small number of participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1/tamsulosin group
Treatment group 2/nifedipine group
Control group
Standard pain medication ‐ not described in detail. | |
| Outcomes | Primary outcome measurements
Secondary outcome measurements
| |
| Funding sources | UK National Institute for Health Research Health Technology Assessment Programme. | |
| Declarations of interest | Trial author JN is a member of the HTA commissioning board and the NIHR HTA and Efficacy and Mechanism Evaluation editorial board. All other trial authors declare no competing interests. | |
| Notes | Sample size calculation. Follow‐up at 4 and 12 weeks with questionnaires, case report forms during clinical visits, or telephone contact. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... by a remote randomisation system." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "... supplied by an independent Source." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Participants, clinicians, and trial personnel remained unaware of the allocated group." Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) | Low risk | No quotes available. Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: Small number of participants lost to follow‐up equally balanced between treatment groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Unclear risk | No quotes available. Comment: Time to stone passage was evaluated in only a small portion of the study group (potential selection bias); therefore risk of other bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
All participants received first treatment with 500 mL saline and analgesic (diclofenac or tramadol). All participants were allowed to use symptomatic therapy with IM injections of diclofenac (on demand) and were instructed to drink a minimum of 2 L of water daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Treatment on an "intention‐to‐treat" basis. Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Not all participants received CT scan at the end of the trial period. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: small number (3) of participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
All participants were allowed to use therapy to alleviate symptoms, that is, IM injections with 75 mg diclofenac on demand, and were required to drink ≥ 2 L of water daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Sample size calculated. Follow‐up only at the end of the study (11th day). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Follow‐up weekly with US. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using random number table." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Total dosage of diclofenac used was not described in Results section, although it was mentioned as an outcome measurement in the Methods section. Owing to this inconsistency in describing outcome measurements, this type of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Every week, a clinical examination was performed and KUB X‐rays and abdominal US were obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Weekly follow‐up urine analysis and radiological assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed with the MedCalc statistical software." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Stone clearance rate and stone clearance time were not predefined explicitly in the Methods section. Owing to this inconsistency in describing outcome measurements, this type of reporting bias was considered to be unclear. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
All participants were instructed to drink a minimum of 2 L water daily. An indomethacin suppository was used to control acute episodes of ureteral colic, if present. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Naftopidil = selective alpha‐1D. Ultrasonography and KUB were performed on days 7 and 14. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Number of pain episodes and requirement for pain medication were not reported explicitly. Owing to this inconsistency in describing outcome measurements, risk of this type of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Placebo group
All participants received pain medication (oxycodone 5 mg). Other medication that would not confound study results was permitted and documented. | |
| Outcomes | Primary outcome variables
Secondary outcome variables
| |
| Funding sources | Actavis Inc. supported the study. | |
| Declarations of interest | None stated. | |
| Notes | Power calculation, intention‐to‐treat‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... using a randomization schedule prepared by a nonstudy statistician." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Each kit had a unique sequential number." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The investigator, patient, and study team were blinded to treatment assignment throughout the study." Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) | Low risk | No quotes available. Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: Numbers of participants lost to follow‐up in the different study groups were equally distributed; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
| |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No quotes available. Comment: Loss to follow‐up was not described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Both groups received ketoprofen 50 mg, 3 capsules daily, and phloroglucinol 80 mg, 6 tablets daily, for 5 days. All participants were told to drink at least 2 L water daily and to filter urine. | |
| Outcomes |
| |
| Funding sources | Financed by the French Ministry of Health and Yamanouchi Pharmaceutical Co Ltd. | |
| Declarations of interest | Dr. Vincendeau is an investigator for Astellas Pharma Inc, AstraZeneca, Beckman‐Coulter/Hybritech Inc, and Pfizer Incorporated. | |
| Notes | Follow‐up of 42 days. Evaluation with X‐ray and US on days 1, 14, 28, and 42. Telephone contact on days 21 and 35. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was centrally performed, concealed, and stratified by center in blocks of 4 according to a computer generated random number table." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "... sequentially numbered boxes containing the whole treatment for each patient following the order of the randomization list." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All patients, health medical and nursing staffs, and pharmacists remained masked throughout the study period." Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) | Low risk | No quotes available. Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: missing outcome data balanced in numbers across both groups, with similar reasons for missing data across groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions |
All participants were prescribed ketorolac 10 mg, 3 times/d, allowed to use sublingual buprenorphine 0.3 mg as needed, and were required to drink a minimum of 2 L water/d. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up with KUB and abdominal US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... randomly divided into three groups using the statistical software programs Plus 1.0 and Plus 2.10." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Placebo group
| |
| Outcomes | Primary endpoints
Secondary endpoints
| |
| Funding sources | Supported by health industry special scientific research projects, Ministry of Health of China (201002010). Astellas Pharma supported this study and was involved in preparation of the manuscript. No financial disclosures. | |
| Declarations of interest | None. | |
| Notes | Weekly follow‐up with CT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization and double blinding were performed according to the randomization sequence, which was produced with a computer‐generated program." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered study e‐packs were securely stored at each study center using a password‐protected computer database and were known only to the trial designer and statistician." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The investigator, participants, care providers, and those assessing outcomes were blinded to treatment assignment throughout the trial, as the randomization list was generated by an independent statistician and was not available until the study analysis." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The investigator, participants, care providers, and those assessing outcomes were blinded to treatment assignment throughout the trial, as the randomization list was generated by an independent statistician and was not available until the study analysis." Comment: blinding of outcome assessment; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Figure 1 shows the trial profile with a detailed schedule of participant follow‐up including information regarding loss to follow‐up, withdrawal percentage, etc. Intention‐to‐treat analysis was used. Comment: Detailed information on participant follow‐up was available; risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group/group 1
All participants were instructed for adequate hydration. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up of 4 weeks. Evaluation with X‐ray, US, or NCCT when stone passage occurred. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
All participants were allowed to use symptomatic therapy with injections of 75 mg diclofenac on demand and were required to consume a minimum of 2 L of water daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Each participant was controlled with KUB and US every week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Both groups were also advised to remain active and to drink at least 2 L of water daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly visits with X‐ray or low‐dose CT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore selection bias was considered to have unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Adverse effects were not described in the Results section, although they were stated as secondary outcomes in the Methods section; therefore risk of reporting bias was considered to be unclear. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Both groups received 50 mg diclofenac twice daily for a maximum of 10 days and were allowed to drink 2 L of fluids daily. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Weekly follow‐up with KUB or US. Sample size was calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Randomization was done by assigning consecutive patients to alternate groups." Comment: This method of random sequence generation was considered to have high risk of bias. |
| Allocation concealment (selection bias) | High risk | No quotes available. Comment: The type of allocation concealment used in this study was considered to have high risk of bias. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open‐label." No blinding performed. Comment: Risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: one exclusion; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group 1
Treatment group 2
Control group
Both groups were also advised to drink at least 2 L of fluids daily. An indomethacin suppository was recommended for routine use during pain episodes. | |
| Outcomes |
| |
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly with KUB and ultrasonography. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
AKI: acute kidney injury; CCB: calcium channel blocker; CKD: chronic kidney disease; CT: computed tomography; DB: double‐blind; ED: emergency department; eGFR: estimated glomerular filtration rate; ESWL: extracorporeal shockwave lithotripsy; EuroQOL: EuroQOL Group quaity of life questionnaire; GFR: glomerular filtration rate: HRQOL: health‐related quality of life; HTA: health technology assessment; HU: Hounsfield unit; IM: intramuscular; IQR: interquartile ratio; IV: intravenous; IVU: Intravenous urography; KUB: kidney, ureter, and bladder plain x‐ray; MET: medical expulsive therapy; NCCT: non‐contrast computed tomography; NHS: National Health Service; NIHR: National Institute for Health Research; NS: not stated; NSAID: non‐steroidal anti‐inflammatory drug; PDE5: phosphodiesterase type 5; QALY: quality‐adjusted life‐year; QOL: quality of life; RCT: randomised controlled trial; SCr: serum creatinine; SD: standard deviation; SWL: shockwave lithotripsy; US: ultrasound; UTI: urinary tract infection; VAS: visual analogue scale; WBC: white blood cell.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| MET adjunct therapy to ESWL. | |
| Stones larger than 10 mm were included, and data could not be separated for adequate analysis of stones measuring 10 mm or less. | |
| Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. | |
| Participants were children. | |
| No extracted data were available. | |
| Prospective study, but unclear whether this study was randomised. | |
| Intervention was calcium channel blocker. | |
| No randomisation was performed. | |
| Patients with pyelonephritis were included. | |
| Intervention was calcium channel blocker. | |
| Use of Rowatinex/Terpenes vs tamsulosin without control or placebo group. | |
| Participants were patients who had undergone ureteral stent positioning. | |
| Participants were patients with stone‐related hydronephrosis who had opted for conservative management with insertion of a double‐J ureteral stent. | |
| Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. | |
| Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. | |
| The intervention comprised corticosteroids added to 1 of 2 tamsulosin groups. | |
| Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. | |
| Tamsulosin vs silodosin. No placebo or control group available for comparison. | |
| Tamsulosin vs herbal preparation. No placebo or control group available for comparison. | |
| Hyoscine‐N‐butylbromide (antispasmodic drug) vs alfuzosin vs doxazosin vs terazosin. HBB was not given to alpha‐blocker groups. No placebo or control group available for comparison. | |
| Unclear Results section. Data not interpretable. | |
| Retrospective study. | |
| Tamsulosin vs silodosin. No placebo or control group available for comparison. | |
| Study closed in January 2011 owing to insufficient recruitment. | |
| Retrospective study. | |
| Tamsulosin vs tamsulosin and tadalafil. No placebo or control group available for comparison. | |
| Participants were patients with large renal or ureteral calculi who underwent ureteroscopic laser lithotripsy. | |
| Patients with multiple stones were included. | |
| Tamsulosin vs tamsulosin and tadalafil. No placebo or control group available for comparison. | |
| Tamsulosin and prednisolone vs naftopidil and prednisolone vs watchful waiting (no prednisolone). Therapy groups cannot be compared with watchful waiting group because prednisolone was used by treatment groups. Every positive effect reported in the therapy group could be due to use of prednisolone instead of the alpha‐blocker. | |
| Retrospective study. | |
| Naftopidil vs naftopidil and tolterodine vs tolterodine. No placebo or control group available for comparison. | |
| Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. | |
| Retrospective study. | |
| Naftopidil vs buscopan group. No placebo or control group available for comparison. | |
| Retrospective study. Not an RCT. | |
| Retrospective study. | |
| Data on primary and secondary outcomes not available. | |
| Intervention was calcium channel blocker. | |
| No information on inclusion and exclusion criteria available. Stones measuring > 7 mm were included; maximum diameter was unknown. | |
| Study method is cohort; not an RCT. | |
| Stones larger than 10 mm were included; separate data were not available for stones measuring 10 mm or less. | |
| Investigators included no control group. | |
| Silodosin vs tamsulosin. No placebo or control group was available for comparison. | |
| Children were included in the study (8 years old). | |
| Tamsulosin vs alfuzosin. No placebo or control group was available for comparison. | |
| "Random base"; not an RCT. | |
| Intervention was calcium channel blocker. | |
| Data were not consistent throughout the article. Participant numbers were different in tables as compared with the flow chart and text. | |
| Primary outcome was resolution of colicky pain. Stone clearance rate was not reported. | |
| Retrospective study. | |
| Naftopidil vs silodosin. No placebo or control group available for comparison. | |
| No randomisation performed. | |
| Patients underwent ESWL before study entry. |
ESWL: extracorporeal shockwave lithotripsy; HBB: hyoscine‐N‐butylbromide; MET: medical expulsive therapy; RCT: randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 67 | 10509 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.36, 1.55] |
| Analysis 1.1  Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 1 Stone clearance. | ||||
| 2 Major adverse events Show forest plot | 18 | 3124 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.80, 1.96] |
| Analysis 1.2 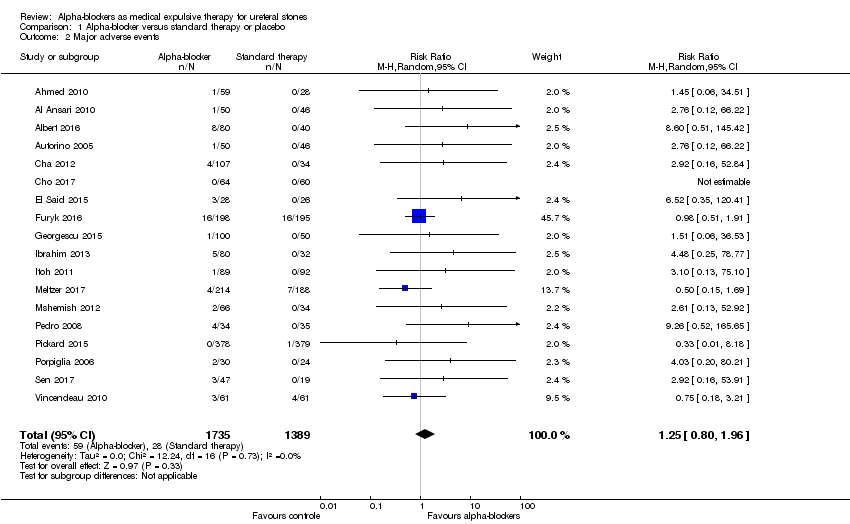 Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 2 Major adverse events. | ||||
| 3 Stone expulsion time Show forest plot | 37 | 6031 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐4.17, ‐2.63] |
| Analysis 1.3  Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 3 Stone expulsion time. | ||||
| 4 Pain episodes Show forest plot | 15 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.91, ‐0.42] |
| Analysis 1.4 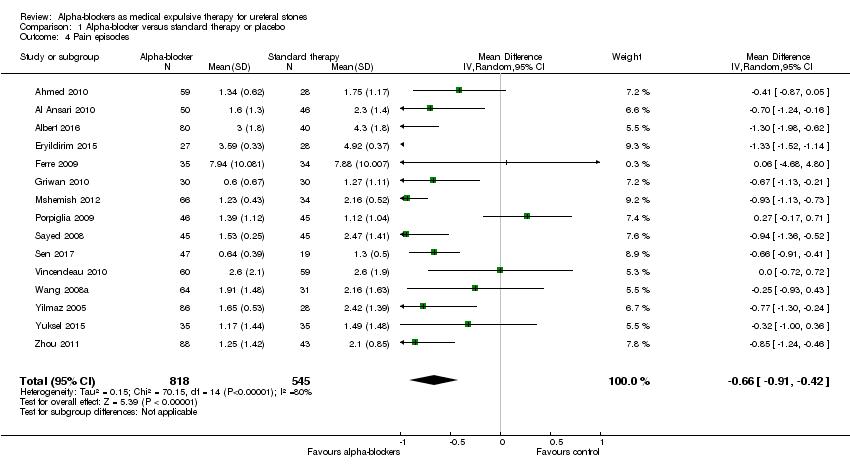 Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 4 Pain episodes. | ||||
| 5 Dose of diclofenac Show forest plot | 14 | 4373 | Mean Difference (IV, Random, 95% CI) | ‐82.41 [‐122.51, ‐42.31] |
| Analysis 1.5  Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 5 Dose of diclofenac. | ||||
| 6 Hospitalisation Show forest plot | 13 | 1876 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.77] |
| Analysis 1.6  Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 6 Hospitalisation. | ||||
| 7 Surgical intervention Show forest plot | 19 | 3292 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.02] |
| Analysis 1.7  Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 7 Surgical intervention. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 16 | 5509 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.08, 1.27] |
| Analysis 2.1  Comparison 2 Subgroup analysis 1: stones measuring 5 mm or less versus stones measuring 6 to 10 mm, Outcome 1 Stone clearance. | ||||
| 1.1 Stone size ≤ 5 mm | 14 | 2622 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 1.2 Stone size 6‐10 mm | 10 | 2887 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.22, 1.72] |
| 2 Stone expulsion time Show forest plot | 6 | 3148 | Mean Difference (IV, Random, 95% CI) | ‐3.39 [‐5.82, ‐0.97] |
| Analysis 2.2  Comparison 2 Subgroup analysis 1: stones measuring 5 mm or less versus stones measuring 6 to 10 mm, Outcome 2 Stone expulsion time. | ||||
| 2.1 Stone size ≤ 5 mm | 6 | 1264 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.99, ‐0.15] |
| 2.2 Stone size 6‐10 mm | 4 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐5.99 [‐7.16, ‐4.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 60 | 9370 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.34, 1.54] |
| Analysis 3.1  Comparison 3 Subgroup analysis 2: distal ureter versus mid or proximal ureter stones, Outcome 1 Stone clearance. | ||||
| 1.1 Distal ureter | 57 | 8576 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.36, 1.57] |
| 1.2 Mid or proximal ureter | 9 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.99, 1.66] |
| 2 Stone expulsion time Show forest plot | 32 | 5599 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐4.50, ‐2.85] |
| Analysis 3.2  Comparison 3 Subgroup analysis 2: distal ureter versus mid or proximal ureter stones, Outcome 2 Stone expulsion time. | ||||
| 2.1 Distal ureter | 32 | 5453 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐4.26, ‐2.60] |
| 2.2 Mid or proximal ureter | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐8.64 [‐19.75, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 67 | 10897 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.39, 1.58] |
| Analysis 4.1 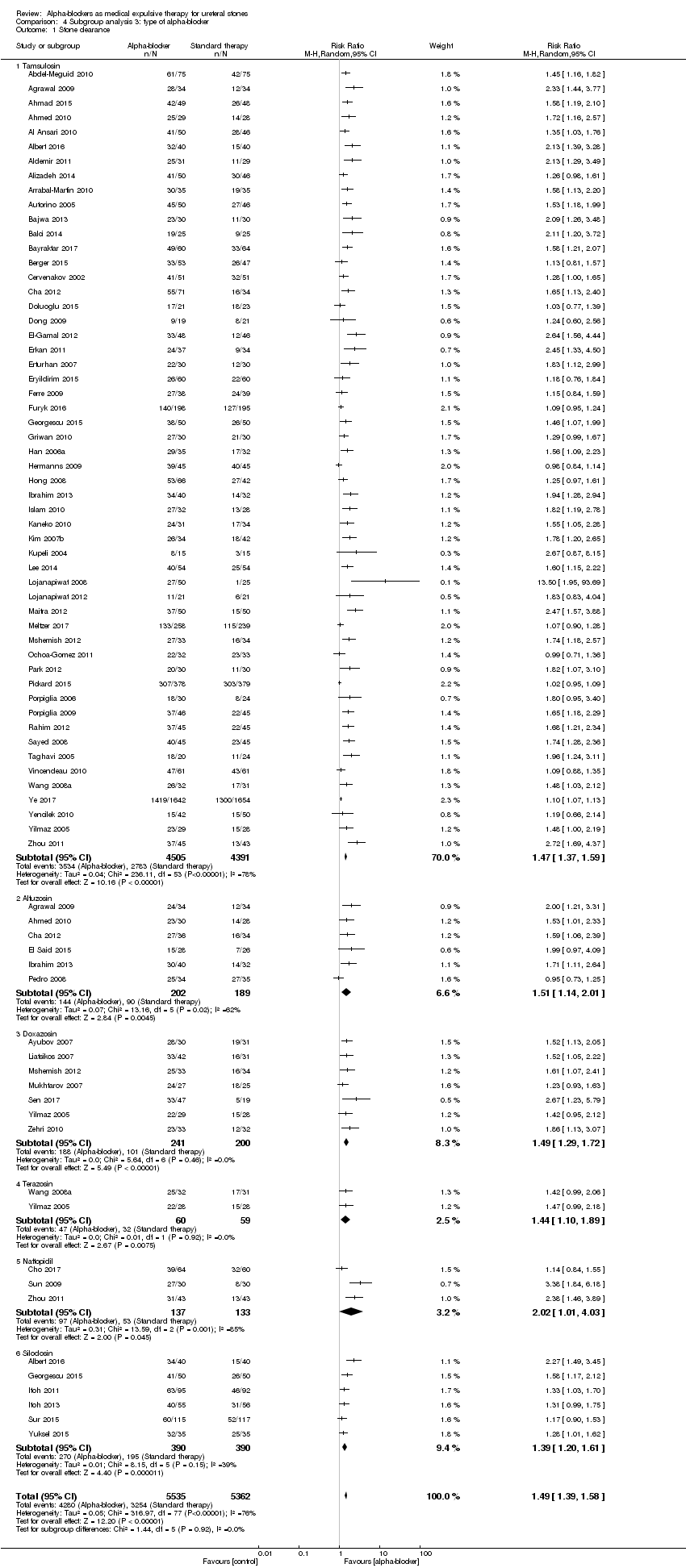 Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 1 Stone clearance. | ||||
| 1.1 Tamsulosin | 54 | 8896 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.37, 1.59] |
| 1.2 Alfuzosin | 6 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.14, 2.01] |
| 1.3 Doxazosin | 7 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.29, 1.72] |
| 1.4 Terazosin | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.10, 1.89] |
| 1.5 Naftopidil | 3 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.01, 4.03] |
| 1.6 Silodosin | 6 | 780 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.20, 1.61] |
| 2 Major adverse events Show forest plot | 18 | 3003 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.12, 3.48] |
| Analysis 4.2 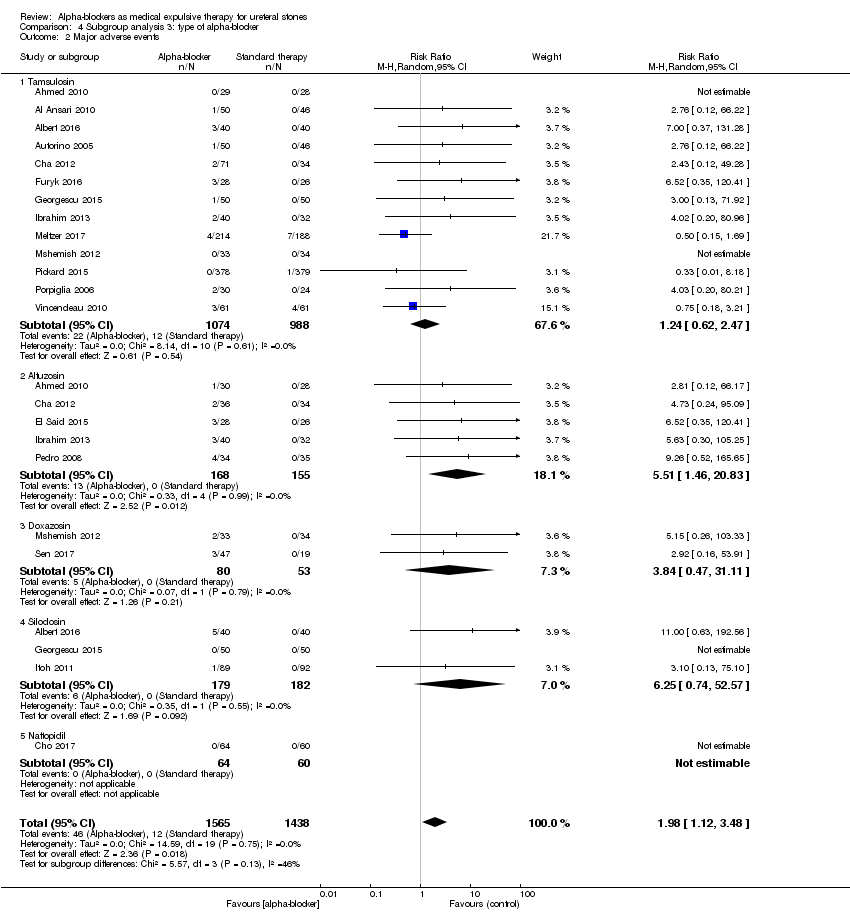 Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 2 Major adverse events. | ||||
| 2.1 Tamsulosin | 13 | 2062 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.62, 2.47] |
| 2.2 Alfuzosin | 5 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 5.51 [1.46, 20.83] |
| 2.3 Doxazosin | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 3.84 [0.47, 31.11] |
| 2.4 Silodosin | 3 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 6.25 [0.74, 52.57] |
| 2.5 Naftopidil | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Stone expulsion time Show forest plot | 35 | 6212 | Mean Difference (IV, Random, 95% CI) | ‐3.56 [‐4.25, ‐2.87] |
| Analysis 4.3  Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 3 Stone expulsion time. | ||||
| 3.1 Tamsulosin | 30 | 5052 | Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.04, ‐2.09] |
| 3.2 Alfuzosin | 3 | 180 | Mean Difference (IV, Random, 95% CI) | ‐4.75 [‐6.63, ‐2.88] |
| 3.3 Doxazosin | 4 | 263 | Mean Difference (IV, Random, 95% CI) | ‐4.73 [‐6.15, ‐3.32] |
| 3.4 Terazosin | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐4.42 [‐5.36, ‐3.48] |
| 3.5 Naftopidil | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐2.80, ‐0.80] |
| 3.6 Silodosin | 4 | 512 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐6.43, ‐3.52] |
| 4 Pain episodes Show forest plot | 15 | 1595 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐0.89, ‐0.52] |
| Analysis 4.4  Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 4 Pain episodes. | ||||
| 4.1 Tamsulosin | 13 | 992 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.98, ‐0.41] |
| 4.2 Alfuzosin | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.82, 0.18] |
| 4.3 Doxazosin | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.92, ‐0.60] |
| 4.4 Terazosin | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.16, ‐0.17] |
| 4.5 Naftopidil | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.23, ‐0.37] |
| 4.6 Silodosin | 2 | 150 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐2.05, 0.26] |
| 5 Dose of diclofenac [mg] Show forest plot | 14 | 4429 | Mean Difference (IV, Random, 95% CI) | ‐78.11 [‐113.25, ‐42.97] |
| Analysis 4.5 ![Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 5 Dose of diclofenac [mg].](/cdsr/doi/10.1002/14651858.CD008509.pub3/media/CDSR/CD008509/image_n/nCD008509-CMP-004-05.png) Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 5 Dose of diclofenac [mg]. | ||||
| 5.1 Tamsulosin | 12 | 4135 | Mean Difference (IV, Random, 95% CI) | ‐87.60 [‐131.98, ‐43.21] |
| 5.2 Doxazosin | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐63.46 [‐72.88, ‐54.04] |
| 5.3 Terazosin | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐64.29 [‐74.17, ‐54.41] |
| 5.4 Silodosin | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐54.72 [‐89.91, ‐19.53] |
| 6 Hospitalisation Show forest plot | 13 | 2028 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.66] |
| Analysis 4.6  Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 6 Hospitalisation. | ||||
| 6.1 Tamsulosin | 11 | 1606 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.86] |
| 6.2 Alfuzosin | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.09, 1.45] |
| 6.3 Doxazosin | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.05] |
| 6.4 Silodosin | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.12, 0.55] |
| 7 Surgical intervention Show forest plot | 19 | 3404 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| Analysis 4.7  Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 7 Surgical intervention. | ||||
| 7.1 Tamsulosin | 16 | 2820 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.59, 1.13] |
| 7.2 Alfuzosin | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.09, 2.35] |
| 7.3 Doxazosin | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.15, 1.11] |
| 7.4 Silodosin | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 15 | 5787 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.07, 1.25] |
| Analysis 5.1  Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 1 Stone clearance. | ||||
| 2 Major adverse events Show forest plot | 10 | 1650 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.13, 3.86] |
| Analysis 5.2 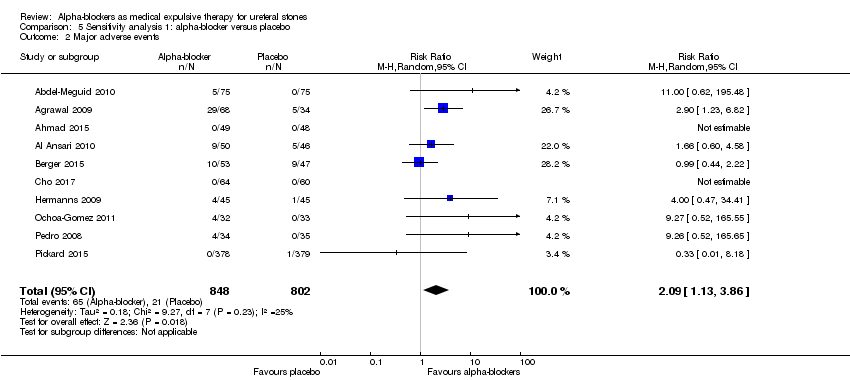 Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 2 Major adverse events. | ||||
| 3 Stone expulsion time Show forest plot | 7 | 3240 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐3.71, ‐0.24] |
| Analysis 5.3 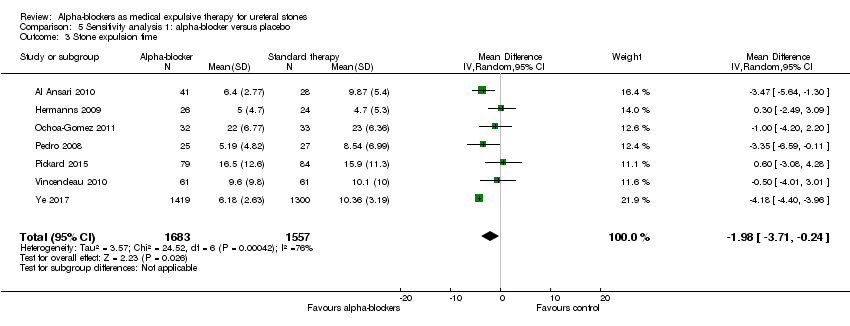 Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 3 Stone expulsion time. | ||||
| 4 Pain episodes Show forest plot | 2 | 215 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐1.07, 0.29] |
| Analysis 5.4 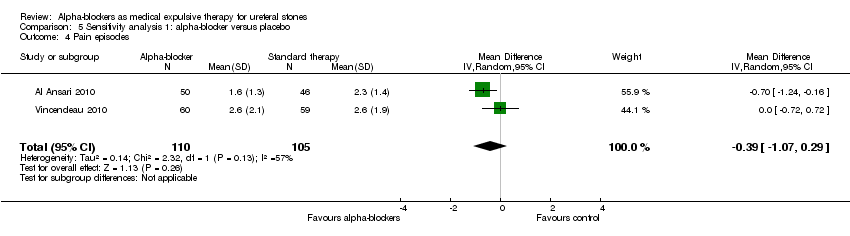 Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 4 Pain episodes. | ||||
| 5 Dose of diclofenac Show forest plot | 4 | 3576 | Mean Difference (IV, Random, 95% CI) | ‐126.32 [‐181.73, ‐70.90] |
| Analysis 5.5 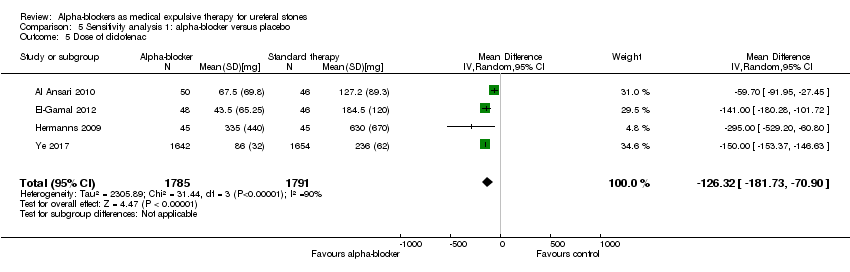 Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 5 Dose of diclofenac. | ||||
| 6 Hospitalisation Show forest plot | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.47] |
| Analysis 5.6  Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 6 Hospitalisation. | ||||
| 7 Surgical intervention Show forest plot | 5 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.24] |
| Analysis 5.7 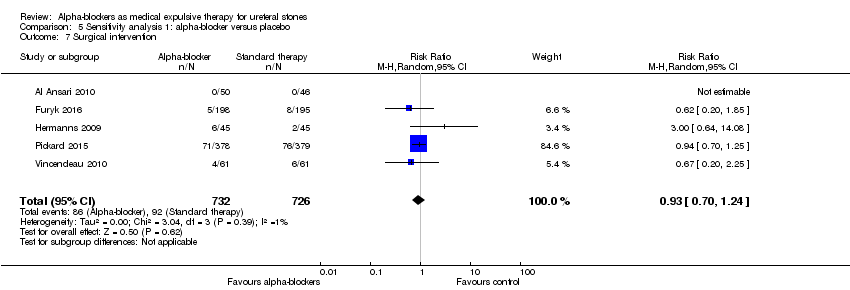 Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 7 Surgical intervention. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 5 | 4133 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.06, 1.13] |
| Analysis 6.1  Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 1 Stone clearance. | ||||
| 2 Major adverse events Show forest plot | 2 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.51, 1.72] |
| Analysis 6.2  Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 2 Major adverse events. | ||||
| 3 Stone expulsion time Show forest plot | 3 | 2891 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐5.13, 1.68] |
| Analysis 6.3 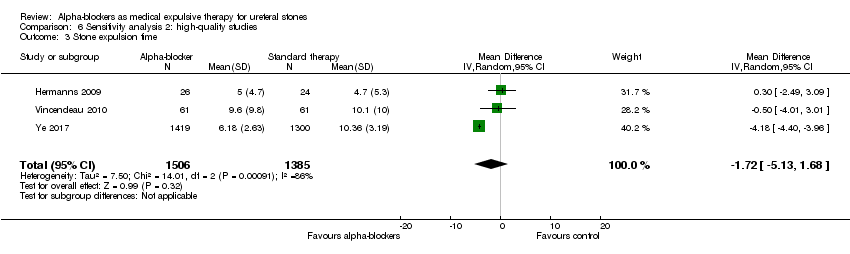 Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 3 Stone expulsion time. | ||||
| 4 Pain episodes Show forest plot | 1 | 119 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.72, 0.72] |
| Analysis 6.4  Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 4 Pain episodes. | ||||
| 5 Dose of diclofenac Show forest plot | 2 | 3386 | Mean Difference (IV, Random, 95% CI) | ‐173.28 [‐277.60, ‐68.95] |
| Analysis 6.5  Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 5 Dose of diclofenac. | ||||
| 6 Hospitalisation Show forest plot | 1 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.49, 1.52] |
| Analysis 6.6  Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 6 Hospitalisation. | ||||
| 7 Surgical intervention Show forest plot | 3 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.38, 2.32] |
| Analysis 6.7 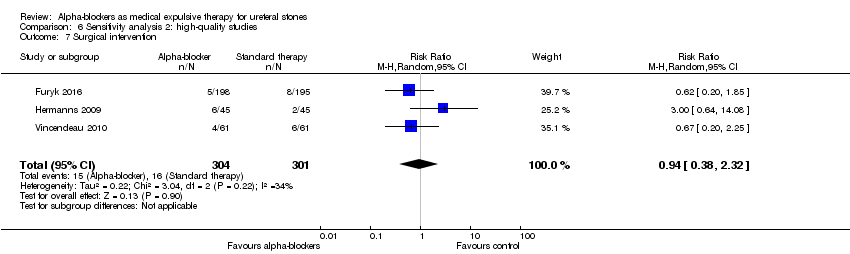 Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 7 Surgical intervention. | ||||

Study flow diagram.
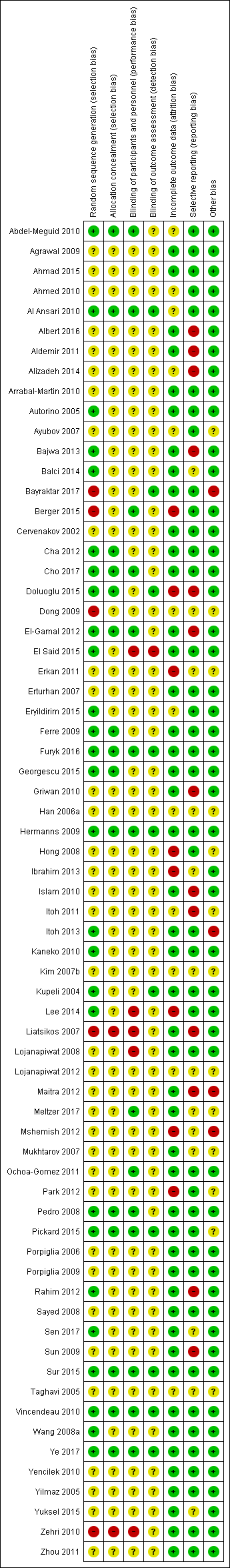
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
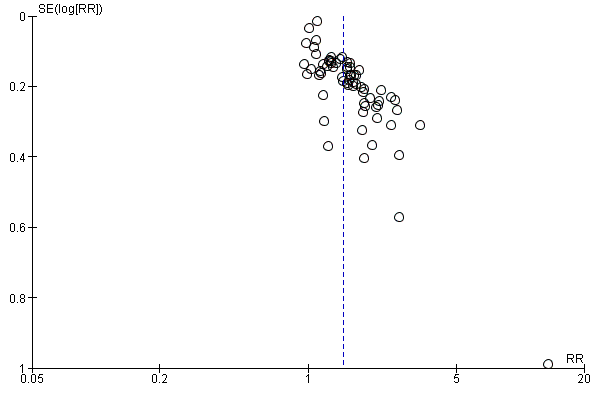
Funnel plot of comparison: 1 Alpha‐blocker versus standard therapy or placebo, outcome: 1.1 Stone clearance.

Funnel plot of comparison: 1 Alpha‐blocker versus standard therapy or placebo, outcome: 1.2 Major adverse events.
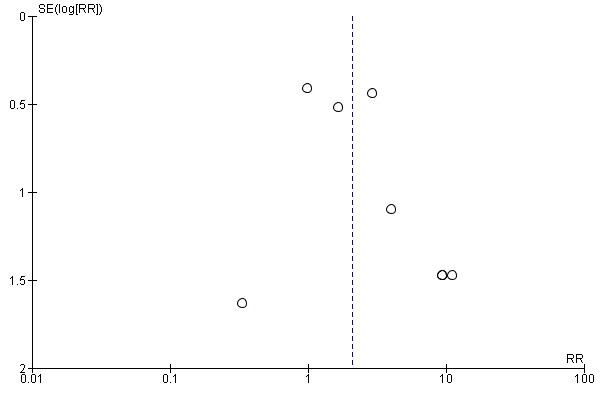
Funnel plot of comparison 2: Alpha‐blocker versus placebo, outcome: 2.2 Major adverse events.
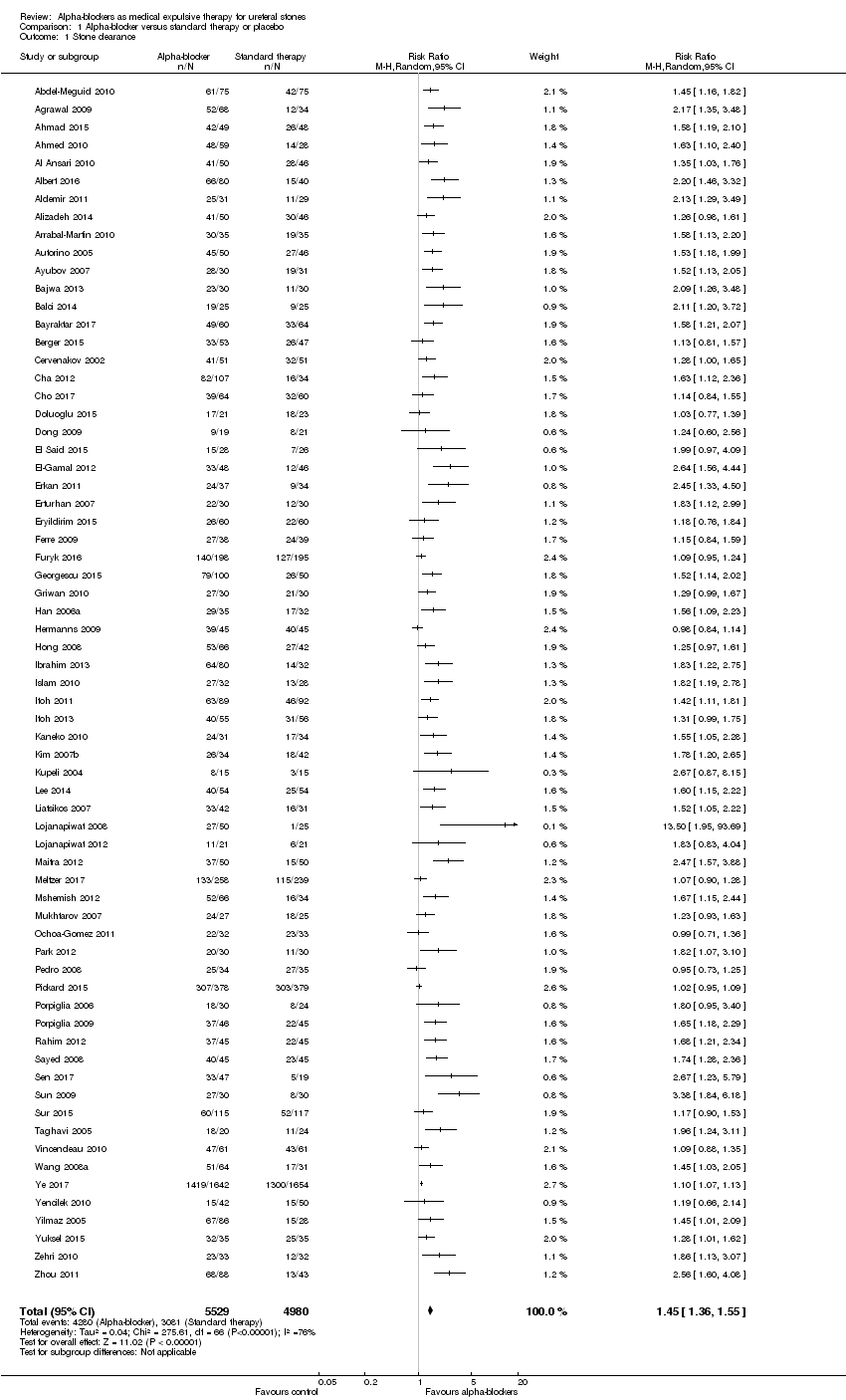
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 1 Stone clearance.
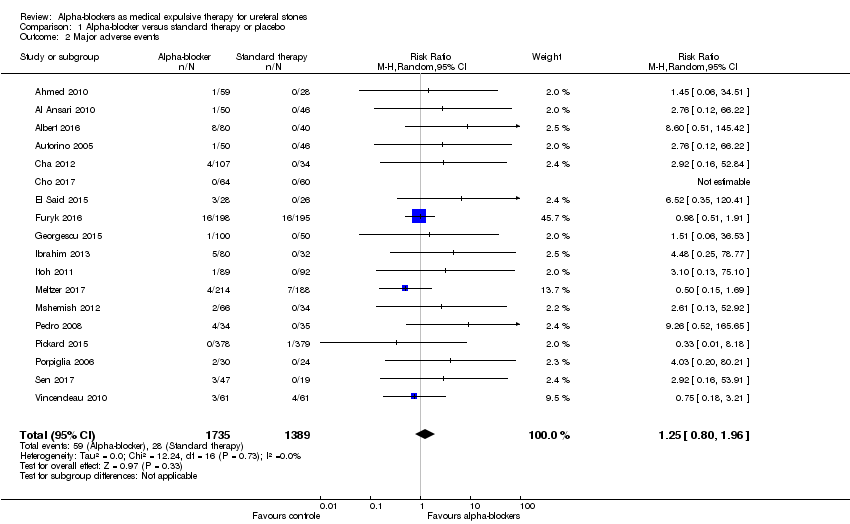
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 2 Major adverse events.
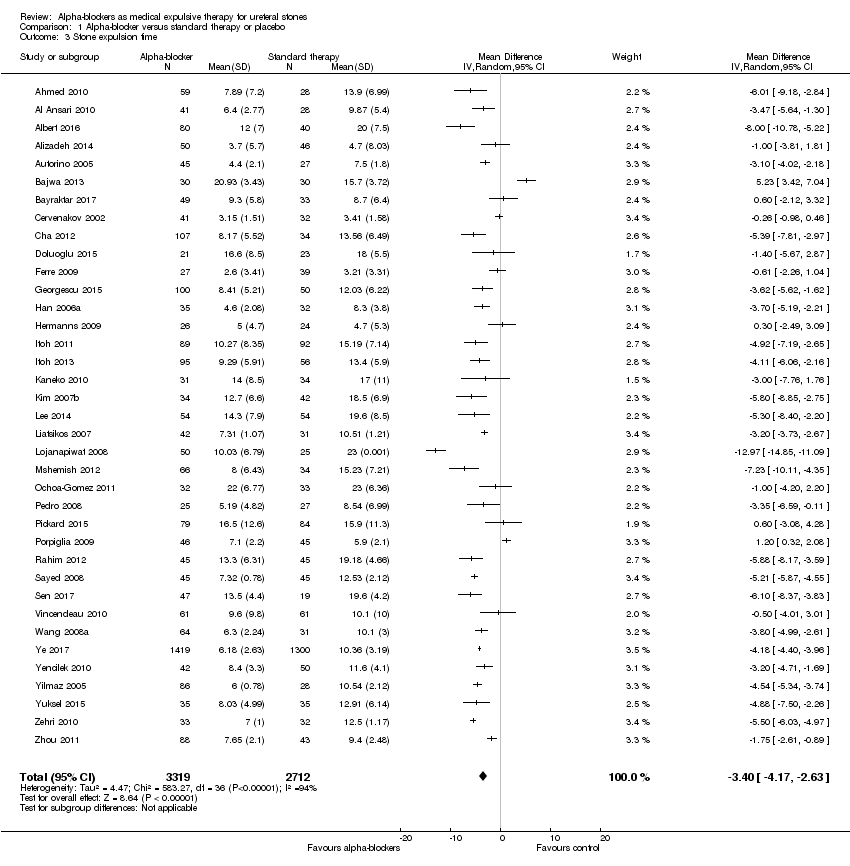
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 3 Stone expulsion time.
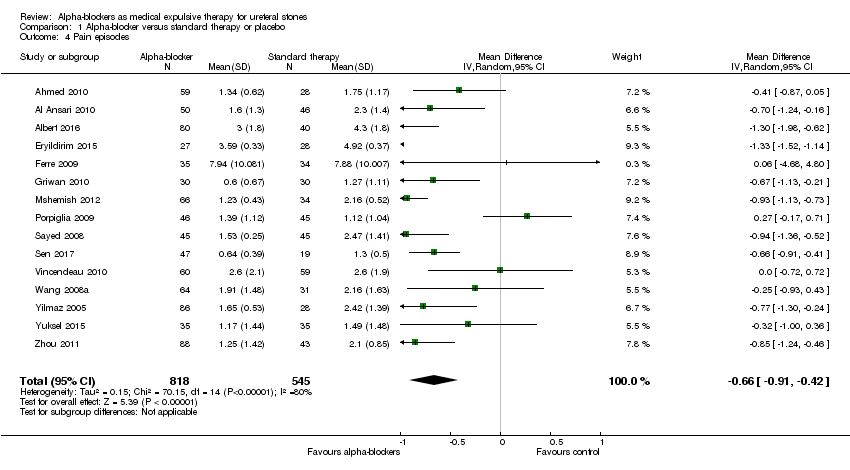
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 4 Pain episodes.
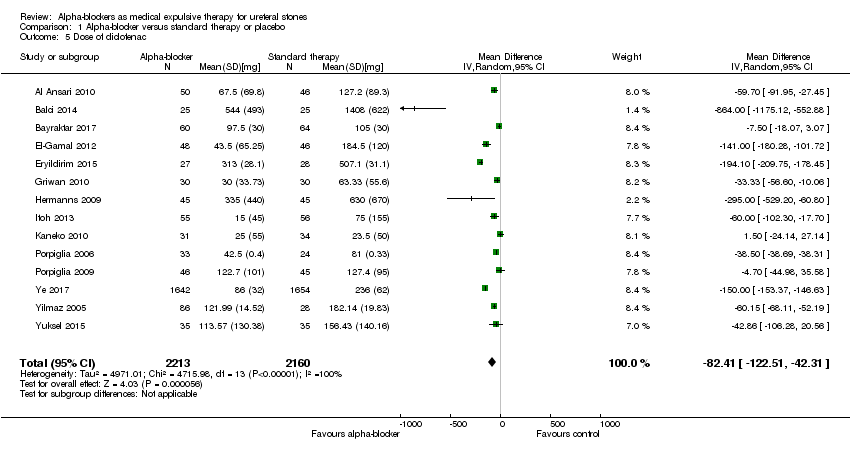
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 5 Dose of diclofenac.

Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 6 Hospitalisation.
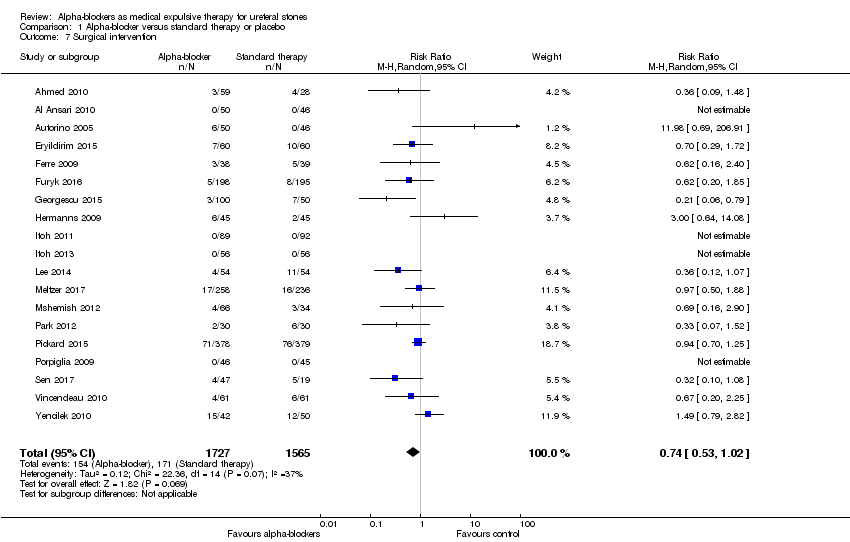
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 7 Surgical intervention.
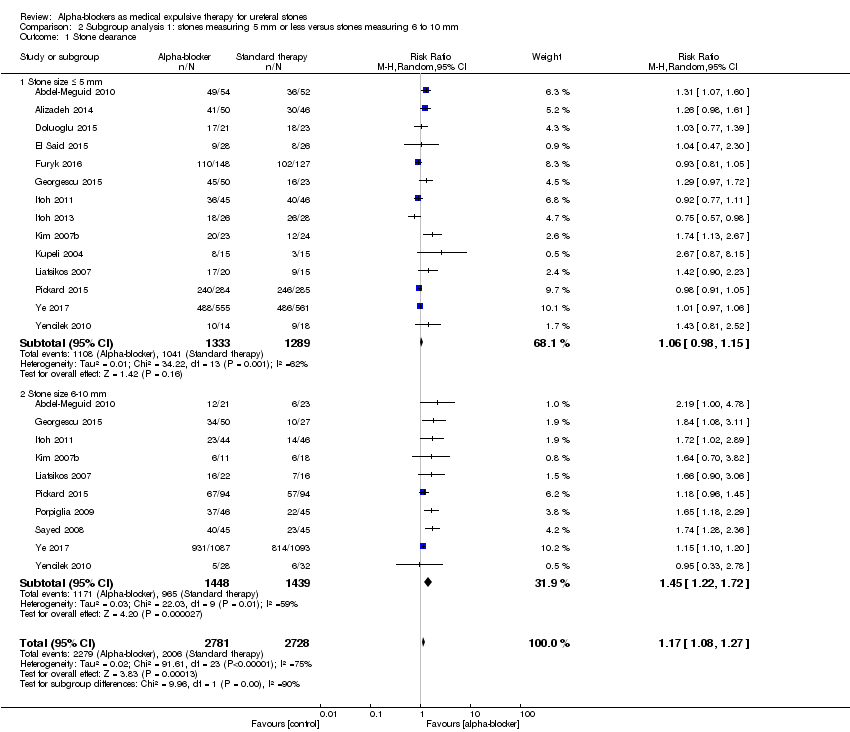
Comparison 2 Subgroup analysis 1: stones measuring 5 mm or less versus stones measuring 6 to 10 mm, Outcome 1 Stone clearance.

Comparison 2 Subgroup analysis 1: stones measuring 5 mm or less versus stones measuring 6 to 10 mm, Outcome 2 Stone expulsion time.
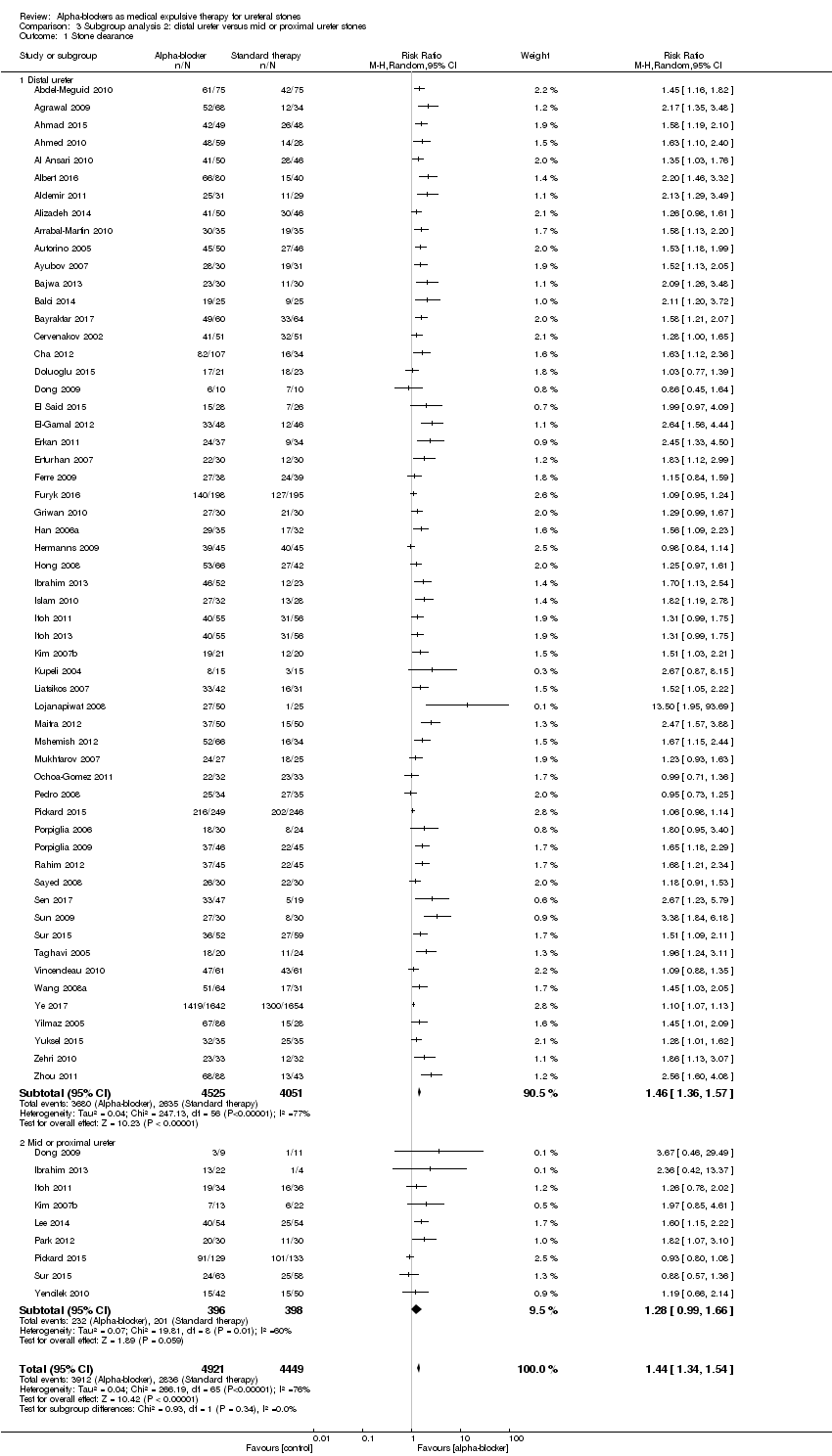
Comparison 3 Subgroup analysis 2: distal ureter versus mid or proximal ureter stones, Outcome 1 Stone clearance.

Comparison 3 Subgroup analysis 2: distal ureter versus mid or proximal ureter stones, Outcome 2 Stone expulsion time.
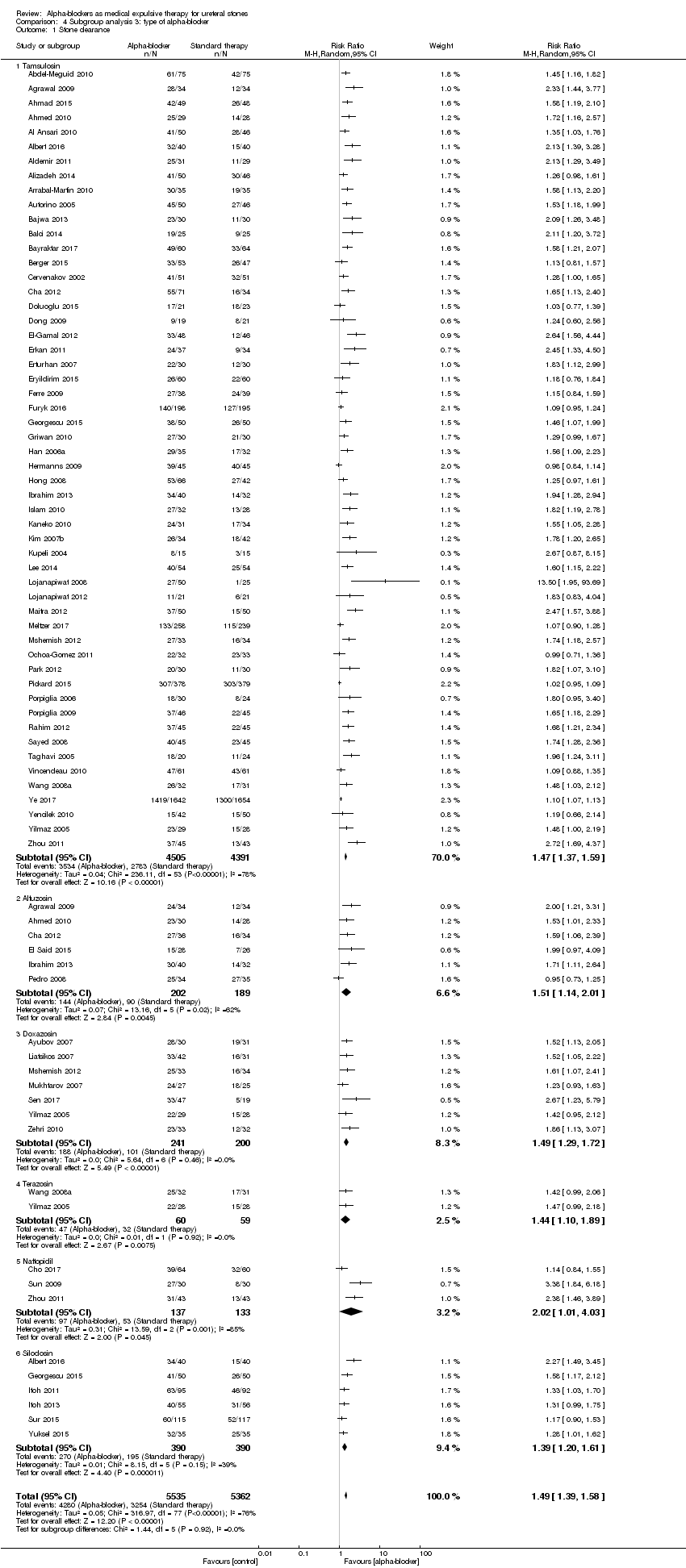
Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 1 Stone clearance.

Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 2 Major adverse events.

Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 3 Stone expulsion time.

Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 4 Pain episodes.
![Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 5 Dose of diclofenac [mg].](/es/cdsr/doi/10.1002/14651858.CD008509.pub3/media/CDSR/CD008509/image_n/nCD008509-CMP-004-05.png)
Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 5 Dose of diclofenac [mg].

Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 6 Hospitalisation.
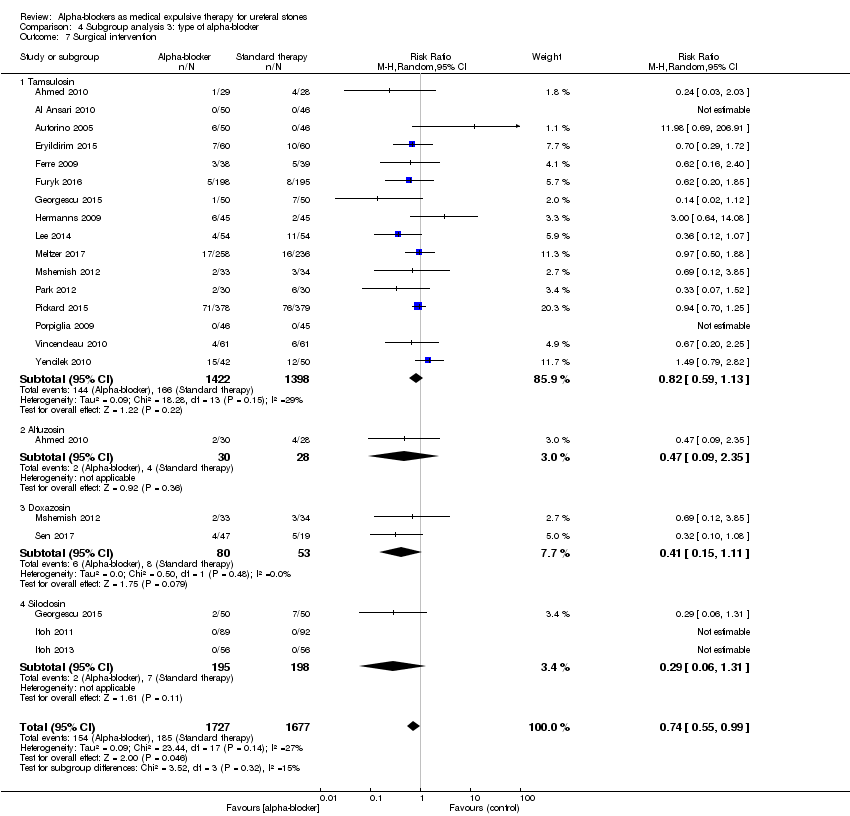
Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 7 Surgical intervention.
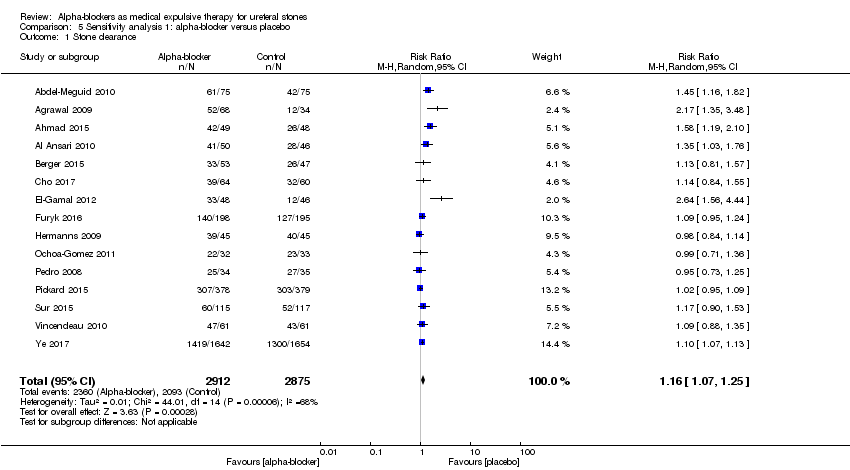
Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 1 Stone clearance.

Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 2 Major adverse events.

Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 3 Stone expulsion time.

Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 4 Pain episodes.

Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 5 Dose of diclofenac.
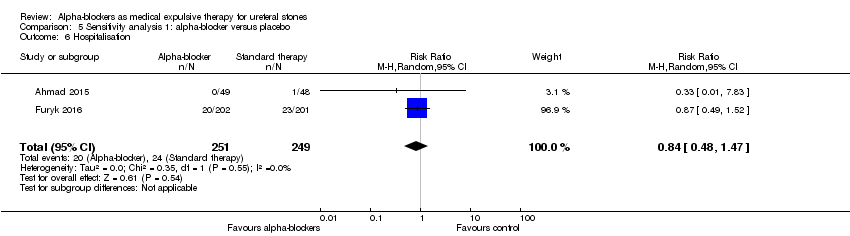
Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 6 Hospitalisation.

Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 7 Surgical intervention.
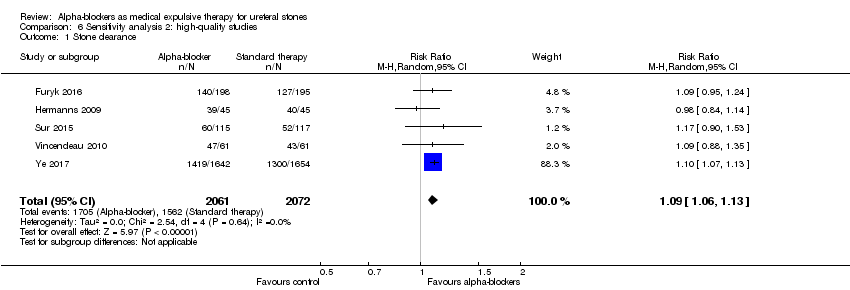
Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 1 Stone clearance.

Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 2 Major adverse events.

Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 3 Stone expulsion time.
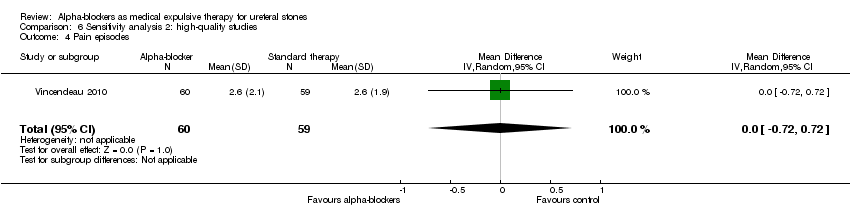
Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 4 Pain episodes.

Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 5 Dose of diclofenac.
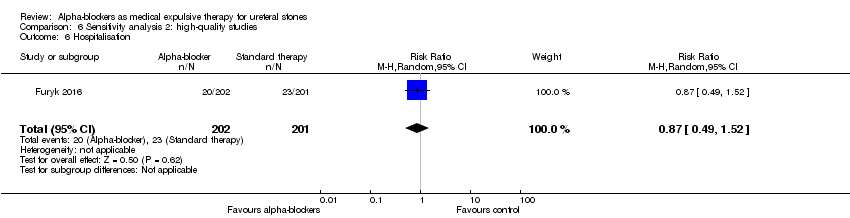
Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 6 Hospitalisation.

Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 7 Surgical intervention.
| Alpha‐blockers compared with standard therapy for ureteral stones | |||||
| Patient or population: adult patients presenting with symptoms of ureteral stone disease Setting: single or multicenter Intervention: alpha‐blocker Comparison: standard therapy | |||||
| Outcomes | No. of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with standard therapy | Risk difference with alpha‐blockers | ||||
| Stone clearance | 10509 | ⊕⊕⊝⊝ | RR 1.45 | Study population | |
| 619 per 1000 | 278 more per 1000 | ||||
| Major adverse events | 3124 | ⊕⊕⊝⊝ | RR 1.25 | Study population | |
| 20 per 1000 | 5 more per 1000 | ||||
| Stone expulsion time | 6031 | ⊕⊕⊝⊝ | ‐ | MD 3.4 lower | |
| Pain episodes | 1363 | ⊕⊕⊝⊝ | ‐ | MD 0.66 lower | |
| Dose of diclofenac | 4373 | ⊕⊕⊝⊝ | ‐ | MD 82.41 mg lower | |
| Hospitalisation | 1876 | ⊕⊕⊕⊝ | RR 0.51 | Study population | |
| 141 per 1000 | 69 fewer per 1000 | ||||
| Surgical intervention | 3292 | ⊕⊕⊝⊝ | RR 0.74 | Study population | |
| 109 per 1000 | 28 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aMost studies were rated as having high or unclear risk of bias. bClinically important heterogeneity with I² of 76%; provided rationale for downgrading together with suspected publication bias. cPublication bias suspected given funnel plot asymmetry. dConfidence interval consistent; no effect and clinically important harm. eClinically important heterogeneity with I² of 94%; provided rationale for downgrading together with suspected publication bias. fClinically important heterogeneity with I² of 80%; provided rationale for downgrading together with suspected publication bias. gClinically important heterogeneity with I² of 100%; provided rationale for downgrading together with suspected publication bias. | |||||
| Alpha‐blockers compared with placebo for ureteral stones | |||||
| Patient or population: adult patients presenting with symptoms of ureteral stone disease Setting: single or multicenter Intervention: alpha‐blocker Comparison: placebo | |||||
| Outcomes | No. of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with alpha‐blockers | ||||
| Stone clearance | 5787 | ⊕⊕⊕⊝ | RR 1.16 | Study population | |
| 728 per 1000 | 116 more per 1000 | ||||
| Major adverse events | 1650 | ⊕⊕⊕⊝ | RR 2.09 | Study population | |
| 26 per 1000 | 29 more per 1000 | ||||
| Stone expulsion time | 3240 | ⊕⊕⊝⊝ | ‐ | MD 1.98 lower | |
| Pain episodes | 215 | ⊕⊕⊝⊝ | ‐ | MD 0.39 lower | |
| Dose of diclofenac (mg) | 3576 | ⊕⊕⊝⊝ | ‐ | MD 126.32 lower | |
| Hospitalisation | 500 | ⊕⊕⊕⊝ | RR 0.84 | Study population | |
| 96 per 1000 | 15 fewer per 1000 | ||||
| Surgical intervention | 1458 | ⊕⊕⊕⊕ | RR 0.93 | Study population | |
| 127 per 1000 | 9 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aDowngraded owing to inconsistency (high heterogeneity with I² of 68%). bDowngraded owing to imprecision (wide confidence interval consistent with negligible to substantial harm). cDowngraded owing to inconsistency (heterogeneity with I² of 57%). dDowngraded owing to imprecision (wide confidence interval; wide confidence interval consistent with large to negligible benefit). eDowngraded owing to imprecision (wide confidence interval consistent with no effect and small benefit). fDowngraded owing to inconsistency (high heterogeneity with I² of 90%). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 67 | 10509 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.36, 1.55] |
| 2 Major adverse events Show forest plot | 18 | 3124 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.80, 1.96] |
| 3 Stone expulsion time Show forest plot | 37 | 6031 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐4.17, ‐2.63] |
| 4 Pain episodes Show forest plot | 15 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.91, ‐0.42] |
| 5 Dose of diclofenac Show forest plot | 14 | 4373 | Mean Difference (IV, Random, 95% CI) | ‐82.41 [‐122.51, ‐42.31] |
| 6 Hospitalisation Show forest plot | 13 | 1876 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.77] |
| 7 Surgical intervention Show forest plot | 19 | 3292 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 16 | 5509 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.08, 1.27] |
| 1.1 Stone size ≤ 5 mm | 14 | 2622 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 1.2 Stone size 6‐10 mm | 10 | 2887 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.22, 1.72] |
| 2 Stone expulsion time Show forest plot | 6 | 3148 | Mean Difference (IV, Random, 95% CI) | ‐3.39 [‐5.82, ‐0.97] |
| 2.1 Stone size ≤ 5 mm | 6 | 1264 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.99, ‐0.15] |
| 2.2 Stone size 6‐10 mm | 4 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐5.99 [‐7.16, ‐4.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 60 | 9370 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.34, 1.54] |
| 1.1 Distal ureter | 57 | 8576 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.36, 1.57] |
| 1.2 Mid or proximal ureter | 9 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.99, 1.66] |
| 2 Stone expulsion time Show forest plot | 32 | 5599 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐4.50, ‐2.85] |
| 2.1 Distal ureter | 32 | 5453 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐4.26, ‐2.60] |
| 2.2 Mid or proximal ureter | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐8.64 [‐19.75, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 67 | 10897 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.39, 1.58] |
| 1.1 Tamsulosin | 54 | 8896 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.37, 1.59] |
| 1.2 Alfuzosin | 6 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.14, 2.01] |
| 1.3 Doxazosin | 7 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.29, 1.72] |
| 1.4 Terazosin | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.10, 1.89] |
| 1.5 Naftopidil | 3 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.01, 4.03] |
| 1.6 Silodosin | 6 | 780 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.20, 1.61] |
| 2 Major adverse events Show forest plot | 18 | 3003 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.12, 3.48] |
| 2.1 Tamsulosin | 13 | 2062 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.62, 2.47] |
| 2.2 Alfuzosin | 5 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 5.51 [1.46, 20.83] |
| 2.3 Doxazosin | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 3.84 [0.47, 31.11] |
| 2.4 Silodosin | 3 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 6.25 [0.74, 52.57] |
| 2.5 Naftopidil | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Stone expulsion time Show forest plot | 35 | 6212 | Mean Difference (IV, Random, 95% CI) | ‐3.56 [‐4.25, ‐2.87] |
| 3.1 Tamsulosin | 30 | 5052 | Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.04, ‐2.09] |
| 3.2 Alfuzosin | 3 | 180 | Mean Difference (IV, Random, 95% CI) | ‐4.75 [‐6.63, ‐2.88] |
| 3.3 Doxazosin | 4 | 263 | Mean Difference (IV, Random, 95% CI) | ‐4.73 [‐6.15, ‐3.32] |
| 3.4 Terazosin | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐4.42 [‐5.36, ‐3.48] |
| 3.5 Naftopidil | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐2.80, ‐0.80] |
| 3.6 Silodosin | 4 | 512 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐6.43, ‐3.52] |
| 4 Pain episodes Show forest plot | 15 | 1595 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐0.89, ‐0.52] |
| 4.1 Tamsulosin | 13 | 992 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.98, ‐0.41] |
| 4.2 Alfuzosin | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.82, 0.18] |
| 4.3 Doxazosin | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.92, ‐0.60] |
| 4.4 Terazosin | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.16, ‐0.17] |
| 4.5 Naftopidil | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.23, ‐0.37] |
| 4.6 Silodosin | 2 | 150 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐2.05, 0.26] |
| 5 Dose of diclofenac [mg] Show forest plot | 14 | 4429 | Mean Difference (IV, Random, 95% CI) | ‐78.11 [‐113.25, ‐42.97] |
| 5.1 Tamsulosin | 12 | 4135 | Mean Difference (IV, Random, 95% CI) | ‐87.60 [‐131.98, ‐43.21] |
| 5.2 Doxazosin | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐63.46 [‐72.88, ‐54.04] |
| 5.3 Terazosin | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐64.29 [‐74.17, ‐54.41] |
| 5.4 Silodosin | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐54.72 [‐89.91, ‐19.53] |
| 6 Hospitalisation Show forest plot | 13 | 2028 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.66] |
| 6.1 Tamsulosin | 11 | 1606 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.86] |
| 6.2 Alfuzosin | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.09, 1.45] |
| 6.3 Doxazosin | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.05] |
| 6.4 Silodosin | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.12, 0.55] |
| 7 Surgical intervention Show forest plot | 19 | 3404 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| 7.1 Tamsulosin | 16 | 2820 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.59, 1.13] |
| 7.2 Alfuzosin | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.09, 2.35] |
| 7.3 Doxazosin | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.15, 1.11] |
| 7.4 Silodosin | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 15 | 5787 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.07, 1.25] |
| 2 Major adverse events Show forest plot | 10 | 1650 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.13, 3.86] |
| 3 Stone expulsion time Show forest plot | 7 | 3240 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐3.71, ‐0.24] |
| 4 Pain episodes Show forest plot | 2 | 215 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐1.07, 0.29] |
| 5 Dose of diclofenac Show forest plot | 4 | 3576 | Mean Difference (IV, Random, 95% CI) | ‐126.32 [‐181.73, ‐70.90] |
| 6 Hospitalisation Show forest plot | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.47] |
| 7 Surgical intervention Show forest plot | 5 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone clearance Show forest plot | 5 | 4133 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.06, 1.13] |
| 2 Major adverse events Show forest plot | 2 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.51, 1.72] |
| 3 Stone expulsion time Show forest plot | 3 | 2891 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐5.13, 1.68] |
| 4 Pain episodes Show forest plot | 1 | 119 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.72, 0.72] |
| 5 Dose of diclofenac Show forest plot | 2 | 3386 | Mean Difference (IV, Random, 95% CI) | ‐173.28 [‐277.60, ‐68.95] |
| 6 Hospitalisation Show forest plot | 1 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.49, 1.52] |
| 7 Surgical intervention Show forest plot | 3 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.38, 2.32] |

