Terapia del espejo para mejorar la motricidad después de un accidente cerebrovascular
Resumen
Antecedentes
La terapia del espejo se usa para mejorar la motricidad después de un accidente cerebrovascular. En la terapia del espejo, se coloca un espejo en el plano mediosagital del paciente, de forma tal que se reflejen los movimientos del lado no parético como si fuera el lado afectado.
Objetivos
Resumir la efectividad de la terapia del espejo comparada con ningún tratamiento, placebo o tratamiento inactivo, u otros tratamientos para mejorar la motricidad y la deficiencia motora después del accidente cerebrovascular. También se intentó evaluar los efectos de la terapia del espejo sobre las actividades cotidianas, el dolor y la inatención visuoespacial.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Accidentes Cerebrales Vasculares (Cochrane Stroke Group's Trials Register), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, Embase, CINAHL, AMED, PsycINFO y en PEDro (última búsqueda 16 agosto 2017). También se realizaron búsquedas manuales en actas de congresos relevantes, en registros de ensayos e investigación, se verificaron las listas de referencias y se estableció contacto con autores de ensayos, investigadores y expertos en la especialidad.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios (ECA) y los ensayos aleatorios cruzados que compararon la terapia del espejo con cualquier intervención control en pacientes después de un accidente cerebrovascular.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, seleccionaron los ensayos según los criterios de inclusión, documentaron la calidad metodológica, evaluaron los riesgos de sesgo en los estudios incluidos y extrajeron los datos. La calidad de la evidencia se evaluó mediante el enfoque GRADE. Los resultados se analizaron como diferencias de medias estandarizadas (DME) y diferencias de medias (DM) para las variables continuas y los odds ratios (OR) para las variables dicotómicas.
Resultados principales
Se incluyeron 62 estudios con un total de 1982 participantes que compararon la terapia del espejo con otras intervenciones. De estos, 57 fueron ensayos controlados aleatorios y cinco fueron ensayos aleatorios cruzados. La edad media de los pacientes fue de 59 años (30 a 73 años). La terapia del espejo se proporcionó de tres a siete veces a la semana, entre 15 y 60 minutos para cada sesión y durante dos a ocho semanas (como promedio cinco veces a la semana, en una sesión de 30 minutos, durante cuatro semanas). Cuando se comparó con todas las otras intervenciones, se encontró evidencia de calidad moderada de que la terapia del espejo tiene un efecto significativo positivo sobre la motricidad (DME 0,47; IC del 95%: 0,27 a 0,67 ; 1173 participantes; 36 estudios) y la deficiencia motora (DME 0,49; IC del 95%: 0,32 a 0,66; 1292 participantes; 39 estudios). Sin embargo, los efectos sobre la motricidad están influenciados por el tipo de intervención de control. Además, según la evidencia de calidad moderada, la terapia del espejo puede mejorar las actividades cotidianas (DME 0,48; IC del 95%: 0,30 a 0,65; 622 participantes; 19 estudios). Se encontró evidencia de baja calidad de un efecto positivo significativo sobre el dolor (DME −0,89; IC del 95%: −1,67 a −0,11; 248 participantes; seis estudios) y ningún efecto claro para la mejoría de la inatención visuoespacial (DME 1,06; IC del 95%: −0,10 a 2,23; 175 participantes; cinco estudios). No se informaron efectos adversos.
Conclusiones de los autores
Los resultados muestran evidencia de efectividad de la terapia del espejo para mejorar la motricidad del miembro superior, el déficit motor, las actividades cotidianas y el dolor, al menos como complemento de la rehabilitación normal para los pacientes que sufrieron un accidente cerebrovascular. Las limitaciones principales son los tamaños de la muestra pequeños y la falta de información sobre los detalles metodológicos, lo que da lugar a que la calidad de la evidencia sea incierta.
PICO
Resumen en términos sencillos
Terapia del espejo para mejorar el movimiento después de un accidente cerebrovascular
Pregunta de la revisión
¿La terapia del espejo mejora el movimiento, el rendimiento de las actividades diarias, el dolor y la falta de atención, así como la conciencia con respecto al campo de visión afectado (inatención visuoespacial) después de un accidente cerebrovascular?.
Antecedentes
La parálisis del brazo o la pierna es frecuente después de un accidente cerebrovascular y suele causar problemas con las actividades cotidianas como caminar, vestirse o comer. La terapia del espejo (TE) es una terapia de rehabilitación en la que se coloca un espejo entre los brazos o las piernas del paciente para que la imagen del miembro no afectado proporcione la ilusión de un movimiento normal en el miembro afectado. Mediante esta configuración se estimulan diferentes regiones cerebrales para el movimiento, la sensación y el dolor. Sin embargo, los mecanismos concretos por los que funciona la terapia del espejo aún no están claros. Se realizó una búsqueda de la bibliografía en diversas bases de datos y se extrajeron los datos de los estudios relevantes.
Fecha de la búsqueda
Esta revisión identificó estudios hasta el 16 de agosto de 2017.
Características de los estudios
Se encontraron 62 estudios relevantes, de los que 57 asignaron de manera aleatoria a los participantes a recibir TE o un tratamiento control (ensayos controlados aleatorios) y cinco proporcionaron ambas terapias a todos los participantes, pero en orden aleatorio (ensayos cruzados). Los estudios incorporaron a 1982 participantes con una edad promedio de 59 años (de 30 a 73 años) después de un accidente cerebrovascular. La terapia del espejo se proporcionó de tres a siete veces a la semana, entre 15 y 60 minutos para cada sesión y durante dos a ocho semanas (como promedio cinco veces a la semana, una sesión de 30 minutos durante cuatro semanas).
Resultados clave
Al finalizar el tratamiento, la terapia del espejo mejoró de manera moderada el movimiento del miembro superior e inferior afectados y la capacidad de los pacientes de realizar las actividades diarias en el transcurso y más allá de seis meses después del accidente cerebrovascular. La terapia del espejo redujo el dolor después del accidente cerebrovascular, aunque principalmente en los pacientes con un síndrome de dolor regional complejo. No se encontró un efecto claro para la inatención visuoespacial. Los efectos beneficiosos en el movimiento se mantuvieron durante seis meses, aunque no en todos los grupos de estudio. No se informaron efectos adversos.
Calidad de la evidencia
Los estudios proporcionan evidencia moderadamente fiable de que la TE mejora el movimiento (motricidad, deficiencia motora) y el rendimiento de las actividades diarias. Sin embargo solo hubo fiabilidad baja en que la TE disminuye el dolor y la inatención visuoespacial. Esto puede deberse al pequeño número de estudios. Se necesitan estudios de investigación adicionales más amplios y metodológicamente sólidos.
Conclusiones de los autores
Summary of findings
| Mirror therapy compared to all other interventions: primary and secondary outcomes for improving motor function after stroke | |||||
| Participants: people with paresis of the upper or lower limb, or both, caused by stroke Setting: inpatient and outpatient Intervention: mirror therapy Control: no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | № of participants | Quality of the evidence | Comment | |
| Assumed risk | Corresponding risk | ||||
| Control | Mirror therapy versus all other interventions | ||||
| Motor function at the end of intervention phase: all outcome measures | The mean motor function at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor function at the end of intervention phase ‐ all studies in the intervention groups was 0.47 SDs higher (0.27 to 0.67 higher) | 1173 | ⊕⊕⊕⊝ | SMD 0.47, 95% CI 0.27 to 0.67; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Motor impairment at the end of intervention phase: all outcome measures | The mean motor impairment at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor impairment at the end of intervention phase ‐ all studies in the intervention groups was 0.49 SDs higher (0.32 to 0.66 higher) | 1292 | ⊕⊕⊕⊝ Moderatea | SMD 0.49, 95% CI 0.32 to 0.66; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Fugl‐Meyer Assessment upper extremity at the end of intervention phase | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the control groups was NA | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the intervention groups was 4.32 pointshigher (2.46 to 6.19 higher) | 898 | ⊕⊕⊝⊝ | MD 4.32, 95% CI 2.46 to 6.19; the minimum important difference is approximately 5.25 |
| Activities of daily living at the end of intervention phase: all studies | The mean activities of daily living at the end of intervention phase ‐ all studies in the control groups was NA | The mean activities of daily living at the end of intervention phase ‐ all studies in the intervention groups was 0.48 SDs higher (0.29 to 0.67 higher) | 622 | ⊕⊕⊕⊝ | SMD 0.48, 95% CI 0.30 to 0.65; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase: all studies | The mean pain at the end of intervention phase ‐ all studies in the control groups was NA | The mean pain at the end of intervention phase ‐ all studies in the intervention groups was 0.89 SDs lower (1.67 to 0.11 lower) | 248 | ⊕⊕⊝⊝ | SMD −0.89, 95% CI −1.67 to −0.11; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase after excluding studies with CRPS | The mean pain at the end of intervention phase ‐ studies without CRPS in the control groups was NA | The mean pain at the end of intervention phase ‐ studies without CRPS in the intervention groups was 0.23 SDs lower (0.53 lower to 0.08 higher) | 176 (4 RCTs) | ⊕⊕⊕⊝ | SMD −0.23, 95% CI −0.53 to 0.08; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Visuospatial neglect at the end of intervention: all studies | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the control groups was NA | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the intervention groups was 1.06SDs higher (0.10 lower to 2.23 higher) | 175 | ⊕⊕⊝⊝ | SMD 1.06, 95% CI −0.10 to 2.23; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded due to several ratings in one or more items with high or unknown risk of bias. | |||||
Antecedentes
Descripción de la afección
Las enfermedades cerebrovasculares, junto con las cardiopatías isquémicas, son las causas principales de muerte en todo el mundo. El accidente cerebrovascular es una de las principales causas de discapacidad a largo plazo, en particular en los países de ingresos altos y medianos (Murray 2013). Inmediatamente después de la aparición del accidente cerebrovascular, cerca del 80% de los supervivientes tienen una deficiencia motora de los miembros superiores o inferiores (Barker 1997; Jorgensen 1995; Nakayama 1994). Casi el 80% de los pacientes con paresia leve logran una función completa del miembro superior, pero solo el 20% de los pacientes con paresia grave del miembro superior logran este resultado (Nakayama 1994). De los pacientes con un miembro superior pléjico al inicio, solo la mitad recupera la motricidad en el miembro superior parético seis meses después (Kwakkel 2003). Dos tercios de los pacientes con deficiencia de miembros inferiores no son capaces de caminar de forma independiente poco después del accidente cerebrovascular, y después de la rehabilitación solo la mitad tiene una función de la marcha independiente (Jorgensen 1995). La gravedad inicial de la paresia de los miembros superior e inferior es una de las variables predictivas más importantes de la recuperación funcional a largo plazo después de un accidente cerebrovascular (Hendricks 2002; Jorgensen 1995; Nakayama 1994), pero la variabilidad es alta, posiblemente debido a las intervenciones terapéuticas.
Hasta el 50% de los pacientes presentan dolor del miembro superior durante los 12 primeros meses después del accidente cerebrovascular, especialmente dolor en el hombro y síndrome de dolor regional complejo tipo I (SDRC tipo I) (Jönsson 2006; Kocabas 2007; Lundström 2009; Sackley 2008). El dolor después del accidente cerebrovascular puede limitar las actividades cotidianas y reducir la calidad de vida (Jönsson 2006; Lindgren 2007).
Además, cerca del 40% de los pacientes con un accidente cerebrovascular hemisférico agudo izquierdo y cerca del 20% con uno derecho presentan inatención unilateral (Ringman 2004), especialmente inatención visuoespacial. Después de tres meses, cerca del 15% de los pacientes con un accidente cerebrovascular en el hemisferio derecho y cerca del 5% de los pacientes con uno izquierdo tuvieron inatención unilateral (Ringman 2004). Además de los déficit de atención espacial, la inatención es un factor negativo para la recuperación funcional (Farnè 2004; Katz 1999), y se encontró que se asocia con una reducción de la calidad de vida relacionada con la salud (Franceschini 2010).
Por lo tanto, se necesitan estrategias efectivas de entrenamiento para promover la recuperación motora y las actividades cotidianas, así como para aliviar el dolor o la inatención visuoespacial, o ambos, y reducir la carga del accidente cerebrovascular.
Descripción de la intervención
La evidencia indica que las intervenciones terapéuticas efectivas para la recuperación de la motricidad se deben centrar potencialmente en la práctica de tareas funcionales (Van Peppen 2004). Sin embargo, las estrategias de entrenamiento dirigidas a tareas, como la terapia de movimiento inducido por restricción (Corbetta 2015; French 2016; Liepert 1998; Miltner 1999; Taub 1993), requieren cierto grado de movimiento voluntario y, por lo tanto, no son aplicables a los pacientes con paresia grave después de un accidente cerebrovascular. La estrategias de entrenamiento nuevas para esta población de pacientes utilizan dispositivos de entrenamiento electromecánicos (Mehrholz 2015; Mehrholz 2017), estimulación muscular eléctrica (Hatem 2016; Urton 2007), o estimulación con movimientos repetitivos pasivos o de apoyo (Feys 2004; Platz 2005).
Como enfoque terapéutico alternativo, se ha propuesto la terapia del espejo como potencialmente beneficiosa (Ramachandran 1994). A diferencia de otras intervenciones que utilizan el aporte somatosensorial para ayudar a la recuperación motora (Feys 2004), la terapia del espejo se basa en la estimulación visual. En la terapia del espejo, se coloca un espejo en el plano mediosagital del paciente, de forma tal que se reflejen los movimientos del lado no parético como si fuera el lado afectado (Ramachandran 1995). Mediante esta configuración los movimientos del miembro no parético crean la ilusión de movimientos normales del miembro parético (Deconinck 2015). Una de las ventajas de la terapia del espejo es su administración relativamente fácil y la posibilidad de un tratamiento domiciliario autoadministrado, incluso para los pacientes con déficit motores graves. Los estudios clínicos han informado efectos de la terapia del espejo sobre la reducción del dolor en los brazos amputados o SDRC‐tipo I (Ramachandran 1995; Ramachandran 1996; Thieme 2016). Además, se ha señalado que la terapia del espejo alivia la hemiparesia después del accidente cerebrovascular (Ramachandran 1994), lo que se confirmó en un estudio piloto (Altschuler 1999).
Recientemente algunos autores han descrito videos "similares al espejo" o configuraciones de gráficos informáticos, en los que un video o una imagen de un gráfico informático del miembro móvil se presenta como si fuera el del lado contrario (Adamovich 2009; Eng 2007; Gaggioli 2004; Hoermann 2017; In 2012; Laver 2017; Morganti 2003).
De qué manera podría funcionar la intervención
El concepto de la terapia del espejo tiene un fundamento neurofisiológico. Desde hace mucho tiempo hay evidencia de que la observación de los movimientos y la realización de las acciones observadas comparten áreas motoras corticales similares (Grèzes 2001). La imagen en espejo del movimiento (es decir, la inversión de la retroalimentación visual) da lugar a una activación adicional del hemisferio contralateral para la lateralidad del miembro percibida (Deconinck 2015; Dohle 2004; Matthys 2009; Shinoura 2008). La ilusión del espejo puede aumentar la excitabilidad corticomuscular (Fukumura 2007; Garry 2005; Kang 2011; Kang 2012). Sin embargo, los mecanismos precisos del efecto de la terapia del espejo en los pacientes con accidente cerebrovascular aún son especulativos. Como la imagen visual del miembro parético se percibe de manera similar a la del propio miembro móvil de la persona (Dohle 2004), la ilusión del espejo podría evitar o revertir la no utilización aprendida del miembro parético (Liepert 1995). Además, mediante la modulación de la excitabilidad corticomuscular, la terapia del espejo podría estimular directamente la recuperación motora. Finalmente, la terapia del espejo se consideró una variante del entrenamiento motor con imágenes, que se basa en la imaginación repetitiva y el ensayo mental de las tareas motoras (Miltner 1998; Stevens 2003). Los estudios conductuales indican que la experiencia de autonomía (la atribución de las imágenes visuales de las partes corporales está controlada por uno mismo) depende de un acoplamiento temporal ajustado de la retroalimentación visual de los movimientos activos, pero no de los pasivos (Longo 2009). Es esta función activa la que parece diferenciar la terapia del espejo de la terapia de observación de movimientos (Wang 2013b).
Estudios de imagenología indican de manera adicional que las imágenes de espejo de los gráficos informáticos se procesan de igual manera que los movimientos reales (Adamovich 2009; Dohle 2011), siempre que la consistencia temporal y espacial con los movimientos reales no descienda por debajo de ciertos umbrales (Franck 2001). Por lo tanto, incluso las imágenes técnicamente generadas de un miembro humano móvil se pueden integrar en el esquema corporal con el mismo sentido de autonomía que durante la imagen en espejo "real".
Con respecto a los síntomas no motores, algunos estudios también encontraron efectos significativos de la terapia del espejo sobre la deficiencia somatosensorial después del accidente cerebrovascular (Acerra 2007; Dohle 2009). Los efectos corticales podrían ser diferentes de los de la rehabilitación de la motricidad (Fritzsch 2014). Además, la terapia del espejo se propuso para reducir la inatención visuoespacial unilateral después del accidente cerebrovascular (Dohle 2009). Se ha señalado que el fuerte estímulo visual de mirar los movimientos autoinducidos en el hemicampo inatendido es el responsable de este efecto. Sin embargo, lo anterior solo se podría confirmar si el espejo se coloca en el lado del cuerpo afectado, en lugar del no afectado (Ramachandran 1999).
Finalmente, se encontró que la terapia del espejo fue efectiva para aliviar el dolor en diferentes afecciones (Bowering 2013; Thieme 2016). Se ha formulado la hipótesis de que la terapia del espejo puede normalizar el procesamiento sensorial central al proporcionar una imagen fisiológica del miembro afectado (McCabe 2003).
Por qué es importante realizar esta revisión
Desde la primera publicación de esta revisión Cochrane se han publicado varios estudios clínicos nuevos acerca de la terapia del espejo después del accidente cerebrovascular. Por lo tanto, se necesita una actualización de la revisión para proporcionar una estimación actual de la evidencia disponible y abordar las limitaciones encontradas en la revisión original.
Objetivos
Resumir la efectividad de la terapia del espejo comparada con ningún tratamiento, placebo o tratamiento inactivo, u otros tratamientos para mejorar la motricidad y la deficiencia motora después del accidente cerebrovascular. También se intentó evaluar los efectos de la terapia del espejo sobre las actividades cotidianas, el dolor y la inatención visuoespacial.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se incluyeron los ensayos controlados aleatorios (ECA) y los ECA cruzados que compararon la terapia del espejo (proporcionada por un espejo o un video simultáneo o una configuración virtual) con otra modalidad de tratamiento, ningún tratamiento o tratamiento simulado. Cuando se incluyeron ECA cruzados, solo se analizó el primer período como un ensayo de grupo paralelo.
Tipos de participantes
Se incluyeron estudios que examinaron participantes con más de 18 años de edad con una paresia del miembro superior o inferior, o ambos, causada por un accidente cerebrovascular (todos los tipos, gravedad y estadios de accidente cerebrovascular). Cuando se identificaron estudios con poblaciones mixtas de pacientes con afecciones neurológicas, dichos estudios se incluyeron si estuvieron disponibles los datos separados para los pacientes con accidente cerebrovascular.
Tipos de intervenciones
La terapia del espejo se define como una intervención que utiliza un espejo para crear un reflejo del miembro superior o inferior no parético, por lo que le brinda al paciente la retroalimentación visual de un movimiento normal del miembro parético. Al utilizar esta configuración son posibles diferentes variaciones en el protocolo experimental (Bieniok 2011; Dohle 2005). Se incluyeron los estudios que utilizaron la imagen en espejo directa del movimiento de cualquier régimen y variación (es decir, incluido el video o los contextos de realidad virtual). Sin embargo, estos estudios solo se incluyeron cuando fue posible identificar el régimen y la administración de la terapia del espejo. Además, para los estudios con una combinación de terapia del espejo y otras terapias en la condición experimental, solo se incluyeron los estudios en los que se aplicó como mínimo el 50% del tiempo de la intervención experimental a la terapia del espejo.
El brazo control del estudio podría incluir un grupo de no tratamiento, práctica habitual o generalizada, u otro tratamiento control (es decir, placebo o tratamiento simulado). Se excluyeron los estudios en los que no fue posible aislar la influencia de la terapia del espejo debido a la comparación de diferentes regímenes o administraciones de terapia del espejo. Se estableció contacto con los investigadores cuando no estuvieron claros el régimen o la administración de la terapia del espejo o el control (o ambos).
Tipos de medida de resultado
Las medidas de resultado se evaluaron postintervención y en el seguimiento después de seis meses o más.
Resultados primarios
El resultado primario fue la motricidad. Debido a la amplia variedad de medidas de resultado, se seleccionaron medidas de resultado para facilitar el agrupamiento cuantitativo. Cuando se dispuso de más de una medida de resultado, las medidas se priorizaron de la siguiente manera:
-
Motricidad del miembro superior y la mano: Action Research Arm Test (Lyle 1981), Wolf Motor Function Test (Wolf 2001), Motor Assessment Scale ‐ motricidad del miembro superior y la mano o ambos (Carr 1985), Manual Function Test (Miyamoto 2009), Box and Bock Test (Mathiowetz 1985).
-
Motricidad del miembro inferior: Motor Assessment Scale ‐ Items 4 ó 5 (o ambos) (Carr 1985), Berg Balance Scale (Berg 1992).
-
Función motora global: Motor Assessment Scale (Carr 1985), Rivermead Motor Assessment Scale (Collen 1991).
Sin embargo, cuando estas escalas no estuvieron disponibles se aceptaron otras mediciones que evalúan la motricidad.
Resultados secundarios
Los resultados secundarios incluyeron medidas de deficiencia motora (deficiencia motora del miembro superior: Fugl‐Meyer Assessment ‐ motricidad del miembro superior o la mano o ambos (Fugl‐Meyer 1975); Brunnstrom Stages of the Upper Extremity (Brunnstrom 1966); Motricity Index ‐ puntuación del brazo, fuerza muscular o del puño (Demeurisse 1980)); deficiencia motora de miembros inferiores: Fugl‐Meyer Assessment ‐ motricidad del miembro inferior (Fugl‐Meyer 1975); Brunnstrom Stages of the Lower Extremity (Brunnstrom 1966), actividades cotidianas (p.ej. Functional Independence Measure: Keith 1987), Barthel Index: Mahoney 1965)); dolor (escala analógica visual o Numeric Rating Scale), e inatención visuoespacial. También se buscaron los efectos adversos informados (p.ej. tumefacción) y la tasa de abandonos.
Métodos de búsqueda para la identificación de los estudios
See the 'Specialised register' section in the Cochrane Stroke Group module. We searched for relevant trials in all languages and arranged translation of trial reports where necessary.
Búsquedas electrónicas
We searched the Cochrane Stroke Group's Trials Register (last searched on 16 August 2017); Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8) in the Cochrane Library (last searched on 16 August 2017); MEDLINE Ovid (1946 to August 2017); Embase Ovid (1974 to August 2017); Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO; 1982 to August 2017); Allied and Complementary Medicine (AMED Ovid; 1985 to August 2017); PsycINFO Ovid (1806 to August 2017); and the Physiotherapy Evidence Database (PEDro; searched August 2017).
We developed the MEDLINE search strategy for this review with the assistance of the Cochrane Stroke Group's Information Specialist and adapted it to search the other databases (Appendix 1; Appendix 2). We included all languages, and imposed no date limits. As the subject area of this review is quite specific, we did not include a trials filter to maximise the sensitivity of the search.
We also searched ongoing trials and research registers:
-
ISRCTN Registry (www.isrctn.com/, searched December 2016);
-
ClinicalTrials.gov (www.clinicaltrials.gov/, searched December 2016);
-
StrokeTrials Registry (www.strokecenter.org/trials/, searched December 2016);
-
International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/, searched December 2016);
Búsqueda de otros recursos
In an effort to identify further published, unpublished and ongoing trials not available in the major databases, we:
-
handsearched the following conference proceedings:
-
Deutsche Gesellschaft für Neurologie (2008 to 2016);
-
Deutsche Gesellschaft für Neurorehabilitation (2000, 2001, 2003, 2005, 2007, 2009, 2010, 2012, 2013, 2014, 2016);
-
Deutsche Gesellschaft für Neurotraumatologie und klinische Neurorehabilitation (2005, 2007, 2009, 2010, 2014, 2016);
-
European Stroke Conference (2001 to 2015);
-
European Congress of Neurorehabilitation (2011, 2013, 2015);
-
World Congress of Neurorehabilitation (1999, 2002, 2006, 2010, 2012, 2014, 2016);
-
World Congress of Physical Therapy (2003, 2007, 2011, 2015);
-
World Stroke Congress (2000, 2004, 2008, 2010, 2012, 2014);
-
-
screened reference lists of all relevant articles and books;
-
contacted trialists, experts, researchers and commercial companies (Reflex Pain Management Ltd) in our field of study to obtain information of unpublished studies and studies not available in the electronic databases;
-
searched System for Information on Grey Literature in Europe (OpenSIGLE ‐ www.opengrey.eu/, searched December 2016); and
-
searched the REHABDATA database (www.naric.com/research/rehab, searched December 2016).
Obtención y análisis de los datos
Selección de los estudios
Two of three review authors (HT, NM and CD) independently screened titles of the references identified from the electronic database searches and ruled out obviously irrelevant references. We obtained abstracts or full texts, or both, of the remaining studies and used our inclusion criteria (types of studies, types of participants, types of interventions and outcome measures) to assess whether they were eligible for inclusion. We resolved disagreements by discussion. If the inclusion of a study was unclear due to missing information, we tried to contact the authors of the studies for further details. Otherwise, we listed the study as 'awaiting classification'.
Extracción y manejo de los datos
Two of three review authors (HT, NM and CD) independently extracted trial and outcome data of the included trials using a checklist. Because two of the review authors (HT, CD) are principal investigators of included trials, other authors (JB, JM) did the data extraction of those study. The checklists for data extraction contained:
-
methods of randomisation;
-
methods of concealment of allocation;
-
blinding;
-
use of an intention‐to‐treat (ITT) analysis (all participants initially randomised were included in the analysis in their originally‐allocated groups);
-
adverse events;
-
dropouts for all reasons;
-
imbalance of important prognostic factors;
-
participants (country, number of participants, age, gender, type of stroke, time since stroke onset to study entry);
-
inclusion and exclusion criteria;
-
details of interventions in treatment and control groups;
-
outcomes;
-
time points of measurement.
We tried to establish all unclear characteristics of the studies by contacting the trial co‐ordinator or principal investigator. We checked the extracted data for agreement between review authors and entered the data into Review Manager 5 (RevMan 2014).
Evaluación del riesgo de sesgo de los estudios incluidos
We used the 'Risk of bias' assessment tool according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions to assess the adequacy of methods for sequence generation (selection bias), concealment of allocation (selection bias), completeness of outcome data or handling of incomplete outcome data (attrition bias), and blinding of assessors (detection bias) (Higgins 2011).
We did not integrate blinding of therapists and participants as an item in the 'Risk of bias' assessment, since this appeared not to be possible for the type of interventions in this review.
We resolved disagreements in methodological assessment by consulting a third review author (MP, JM or JB), and reached consensus through discussion. If an article did not contain information on any methodological criteria, we contacted the study authors for additional information. If no further information was available, we rated the criteria as 'unclear'.
GRADE and 'Summary of findings' table
We assessed the quality of the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), for the following main outcomes of analysis: motor function, motor impairment, Fugl‐Meyer Assessment, activities of daily living, pain, pain at the end of intervention phase after excluding studies with CRPS, and visuospatial neglect, each at the end of the intervention phase. We presented key findings of the review, including a summary of the amount of data, the magnitude of the effect size, and the overall quality of the evidence, in Summary of findings table 1.
Medidas del efecto del tratamiento
The primary and secondary outcome variables of interest were continuous outcomes. We entered data of post‐intervention assessment and follow‐up assessment at six months as means and standard deviations (SDs) and calculated the standardised mean difference (SMD) or mean difference (MD) with 95% confidence intervals (CIs) for each trial. We pooled data through calculation of the overall SMD/MD and 95% CI. For dichotomous data (adverse events, dropouts) we calculated odds ratios (ORs) between groups.
Cuestiones relativas a la unidad de análisis
We considered randomised cross‐over trials prior to cross‐over and analysed only the first intervention phase.
Manejo de los datos faltantes
We contacted study authors if appropriate data for analysis were not adequately reported. If study authors did not respond within one month after contact, we tried to contact them at least once more. If data were not sufficient to decide on inclusion or exclusion of studies, we rated the studies as 'awaiting classification'. If data were insufficient for meta‐analysis, we excluded the studies from meta‐analysis. If we were unable to get the missing data for participants who dropped out, we only analysed the participants for which we had data. However, we considered an ITT analysis as part of the 'Risk of bias' assessment and performed a sensitivity analysis in which we excluded studies with no or unreported ITT analysis. We also conducted a sensitivity analysis, excluding studies with missing methodological data (therefore rated as 'unclear' risk of bias).
Evaluación de la heterogeneidad
We evaluated clinical heterogeneity through reported clinical and methodological diversity, variability of participants, interventions, and outcomes in an additional table. We used the I2 statistic to assess heterogeneity. We used a random‐effects model, regardless of the level of heterogeneity. Thus, in the case of heterogeneity, we did not violate the preconditions of a fixed‐effect model approach.
Evaluación de los sesgos de notificación
We tried to minimise reporting bias through an extensive search of databases, handsearching of references lists and conference abstracts, and by contacting study authors, trialists, and experts in the field for other unpublished or ongoing trials. We also conducted a sensitivity analysis, excluding studies of low methodological quality.
Síntesis de los datos
Where possible, we conducted a pooled analysis of primary outcomes (motor function) and secondary outcomes (motor impairment, activities of daily living, pain, visuospatial neglect, dropout rate) as described above, using a random‐effects model.
Análisis de subgrupos e investigación de la heterogeneidad
We performed a subgroup analysis to establish the effectiveness of mirror therapy focused on upper or lower extremity. We also investigated heterogeneity regarding time since stroke. We performed a subgroup analysis separating participants in an acute/subacute stage from those in a chronic stage after stroke; the cut‐off point for separating these subgroups was six months after stroke. We also investigated heterogeneity by the type of control intervention used. We separated subgroups using no (additional) control intervention, another control intervention, and sham intervention with restricted view on the paretic extremity.
Análisis de sensibilidad
We conducted a sensitivity analysis to test the robustness of the results, removing studies that we assessed to be of lower or ambiguous methodological quality (studies with risk of bias for at least one method of sequence generation, concealment of allocation, ITT analysis, or blinded assessors). We also reanalysed the data by removing cross‐over RCTs.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, Characteristics of ongoing studies and Table 1.
| Study ID | Mean age | Sex | Side of paresis | Time since stroke | Type of stroke | |||
| Years | Women | Men | Left | Right | Mean time | Ischaemic | Haemorrhagic | |
| 68 | 22 | 18 | 16 | 24 | 5.3 days | 40 | 0 | |
| 50.9 | 9 | 15 | 15 | 9 | n/r | n/r | n/r | |
| 58.2 | 4 | 5 | 8 | 1 | 4.8 years | n/r | n/r | |
| 58.8 | 11 | 13 | 8 | 16 | 5.3 months | 24 | 0 | |
| 45.6 | 8 | 25 | 7 | 26 | 12.9 months/12.3 months. | 17 | 16 | |
| 46.4 | 6 | 30 | 16 | 20 | 15.9 months | 17 | 9 | |
| 53.9 | 7 | 13 | 13 | 7 | 4.6 months | 9 | 11 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 58.4 | 26 | 22 | 34 | 14 | 5 months | 35 | 13 | |
| 62 | 13 | 11 | 15 | 9 | 15.7 months | 19 | 5 | |
| 58.7 | 17 | 19 | n/r | n/r | 1.8 months | n/r | n/r | |
| 59.3 | 12 | 15 | 14 | 13 | 13.2 months/15.5 months | 17 | 10 | |
| 53.5 | 5 | 26 | 24 | 7 | 551 days | 23 | 8 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 56.5 | 10 | 26 | 25 | 11 | 27 days | 48 | 0 | |
| n/r | 3 | 3 | n/r | n/r | n/r | n/r | n/r | |
| 60.9 | 14 | 17 | 14 | 17 | 44.3 days | 25 | 6 | |
| 67.5 | 6 | 8 | 6 | 8 | 47 days | 9 | 5 | |
| 63.9 | 8 | 11 | 9 | 10 | 14.1 months | 10 | 9 | |
| 55.9 | 10 | 15 | 13 | 12 | 13.1 days | 16 | 9 | |
| 66.6 | 9 | 17 | 13 | 13 | 23 days | 26 | 0 | |
| 52.6 | 13 | 22 | 14 | 21 | 8.9 months | 19 | 16 | |
| 64.1 | 24 | 43 | 35 | 32 | 32.3 days | 28 | 39 | |
| 55.8 | 9 | 14 | 13 | 10 | 34.5 days | 14 | 9 | |
| 57.7 | 9 | 20 | 20 | 9 | 404.4 days | 14 | 15 | |
| 49.1 | 9 | 16 | 16 | 9 | n/r | 8 | 17 | |
| 69.1 | 3 | 10 | 5 | 8 | 78.8 days | 10 | 3 | |
| 57.3 | 8 | 22 | n/r | n/r | n/r | n/r | n/r | |
| 61.4 | 10 | 10 | 10 | 10 | n/r | n/r | n/r | |
| 57.1 | 11 | 15 | 11 | 15 | 3.6 months | n/r | n/r | |
| 54.7 | 13 | 14 | 8 | 19 | 39.6 months | 8 | 20 | |
| 64.9 | 21 | 39 | 31 | 29 | 52 days | 19 | 41 | |
| 55 | 11 | 32 | 22 | 21 | 19.6 months | 20 | 28 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 68.7 | 8 | 7 | 9 | 6 | 24.3 days | 10 | 5 | |
| 57 | 20 | 20 | 28 | 12 | 4.6 years | 28 | 12 | |
| 57.5 | 8 | 7 | 5 | 10 | 53.2 days | 15 | 0 | |
| 63 | 10 | 12 | 6 | 16 | 6.4 days | 14 | 8 | |
| 53.5 | 4 | 4 | 4 | 4 | 4.5 months | n/r | n/r | |
| 44.9 | 20 | 40 | n/r | n/r | 4.2 months | 60 | 0 | |
| 63.4 | 20 | 28 | 37 | 11 | 2 days | 26 | 22 | |
| 56.3 | 13 | 17 | 14 | 16 | 20.9 months | 16 | 14 | |
| 60 | 15 | 15 | 17 | 13 | 8.2 months | 17 | 13 | |
| 56 | 19 | 21 | 25 | 15 | 7.2 months | 27 | 13 | |
| 58 | 9 | 21 | 3 | 27 | 5 months | 20 | 10 | |
| 56.3 | n/r | n/r | n/r | n/r | 83.9 days | n/r | n/r | |
| 57.5 | 6 | 10 | 11 | 5 | 34.8 months | 16 | 0 | |
| 73.4 | 10 | 6 | 8 | 8 | 9.5 months | 16 | 0 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 51.2 | 4 | 16 | 9 | 11 | 4.1weeks | 14 | 6 | |
| 63 | 13 | 19 | 15 | 17 | 50 days | 27 | 5 | |
| 51.4 | 22 | 18 | n/r | n/r | 4.0 months | n/r | n/r | |
| 63.4 | 17 | 23 | 27 | 13 | 3.7 months | 33 | 7 | |
| 63.7 | 9 | 6 | 6 | 9 | 32.7 days | n/r | n/r | |
| 67.2 | 25 | 35 | 37 | 23 | 45 days | 45 | 15 | |
| 64 | 34 | 60 | 56 | 38 | 29 days | 76 | 18 | |
| 64.9 | 40 | 50 | 39 | 51 | 63.7 days | 57 | 33 | |
| 54.2 | 10 | 23 | 18 | 15 | 20.6 months | 20 | 13 | |
| 63.3 | 17 | 19 | 21 | 19 | 5.5 months | 29 | 7 | |
| 57.8 | 10 | 16 | 15 | 11 | 22.7 days | 16 | 10 | |
| 63.3 | 21 | 39 | 31 | 29 | 25.8 days | 46 | 14 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
n/r: not reported
Results of the search
We identified 33 new studies from the updated search of the Cochrane Stroke Group's Trials Register. We also identified 8879 references from other electronic databases and 14 references from other sources. After excluding all duplicate references we identified 3588 references from the updated search in all electronic databases (5408 with references in the first version of this review). Two review authors (HT, NM or CD) identified 519 possibly eligible studies (652 with studies in the first version of this review). We discarded 470 studies (599 with studies in the first version of this review). There was insufficient information to determine inclusion eligibility for six trials (Amimoto 2008; ISRCTN40903497; Magni 2014; May 2011; Wang 2013a; Yeldan 2015), but we failed to get in contact with the authors, so the studies are listed as 'awaiting classification' (see Characteristics of studies awaiting classification). We also identified 15 ongoing trials (see Characteristics of ongoing studies). We therefore include 49 new studies (62 with studies in the first version of this review) in this updated version of the review (see Figure 1).

Study flow diagram of updated search and selection process
Included studies
Sixty‐two trials met the inclusion criteria of our review (Acerra 2007; Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Arya 2015; Arya 2017; Bae 2012; Bahrami 2013; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009; Geller 2016; Gurbuz 2016; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Manton 2002; Marquez 2012; Michielsen 2011; Mirela 2015; Mohan 2013; Moustapha 2012; Nagapattinam 2015; Pandian 2014; Park 2015a; Park 2015b; Piravej 2012; Rajappan 2016; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Salhab 2016; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wang 2015; Wu 2013; Yavuzer 2008; Yoon 2014; Yun 2011; Zacharis 2014) (see Characteristics of included studies).
We now exclude one study which we had included in the first version of this review, as only 15% of the experimental intervention was spent in mirror therapy (Ietswaart 2011).
Because the two groups in Rothgangel 2004 received significantly different treatment sessions, we decided to split the data and analyse them separately (outpatient group: Rothgangel 2004a, and inpatient group: Rothgangel 2004b).
Design
Fifty‐seven studies were RCTs with a parallel‐group design (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Bae 2012; Bahrami 2013; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009; Geller 2016; Gurbuz 2016; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kim 2016; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Manton 2002; Marquez 2012; Michielsen 2011; Mirela 2015; Mohan 2013; Nagapattinam 2015; Pandian 2014; Park 2015a; Park 2015b; Piravej 2012; Rajappan 2016; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Thieme 2013; Tyson 2015; Wang 2015; Wu 2013; Yavuzer 2008; Yoon 2014; Yun 2011; Zacharis 2014), and five studies used a cross‐over design with random allocation to the order of treatment (Altschuler 1999; Kojima 2014; Moustapha 2012; Salhab 2016; Tezuka 2006).
Sample Size
The 62 studies included a total of 1982 participants. Individual sample sizes of identified trials ranged from six (Geller 2016) to 94 (Tyson 2015). A detailed description of individual sample sizes can be found in Characteristics of included studies.
Participants
Not all studies provided data on characteristics of participants. Detailed descriptions of participant characteristics are given in Table 1.
The mean age of participants in the included studies was 59 years, with a range from 30 years (Moustapha 2012) to 78 years (Tezuka 2006). There were more participants with a hemiparesis of the left side (53%). There were more men (60%) than women (40%). Twenty‐four studies included participants after their first‐ever stroke. Mean time post‐stroke ranged between five days (Acerra 2007), and five years (Altschuler 1999). Twenty‐nine studies included participants in the acute or subacute phase after stroke (within six months post‐stroke) and 21 trials included participants in the chronic phase (more than six months). Among those participants with known aetiology, 67% had an ischaemic and 33% a haemorrhagic stroke.
Fifty‐two studies provided information on the study setting: 39 inpatient rehabilitation settings or hospitals; three inpatient and outpatient rehabilitation settings; four home settings; two inpatient and home setting; and four outpatient settings (Table 2). The included studies were conducted in 21 different countries.
| Study ID | Extremity | Mirror therapy variation | Control intervention | Type of movements | Minutes per session | Sessions per week | Total duration (weeks) | Total amount of therapy (minutes) | Setting |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Functional motor tasks (i.e. with objects); motor co‐ordination tasks; sensory discrimination tasks; grip strength; active range of motion | 20 to 30 | 7 | 2 | 280 ‐ 420 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities without mirror | n/r | 30 | 5 | 3 | 450 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities; transparent plastic between limbs | Proximal and distal movements | 15 (2 times a day) | 12 | 4 (1st period) | 720 | n/r | |
| Upper extremity | Activities of the unaffected limb | 1. EMG‐triggered electrostimulation; | Wrist, hand flexion, extension and forearm circumduction, and supination–pronation | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy based on Brunnstrom and Bobath principles | Task‐based mirror therapy: finger dexterity, mass grasp/finger flexion, release/finger extension, wrist dorsiflexion, | 45 | 5 | 8 | 1800 | Inpatient hospital, home after discharge | |
| Lower extremity | Activities of the unaffected limb | Conventional motor therapy based on neurophysiological approaches | Activity‐based MT: ball‐rolling, rocker‐board and pedaling | 60 | n/r | 3 ‐ 4 (30 session) | 1800 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Activities of the non‐paretic arm, without mirror | Flexion/extension of the shoulder, radial/ulnar deviation and pro‐/supination of the forearm, flexion/extension of the fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper and lower extremity | Activities of the unaffected limbs | Routine programme (physiotherapy and neuromuscular stimulation) | Range of motion of the healthy limbs | 30 | 5 | 4 | 600 | n/r | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient and outpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror (control group 1); imagination of movements of the affected limb (control group 2) | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 | Daily | 4 | 840 | Inpatient and outpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb + rTMS | Activities of the unaffected limb; covered mirror + rTMS | Flexing and extending the hip, knee, and ankle at a self‐selected speed under supervision but without additional verbal feedback | 20 | 5 | 4 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb + tDCS /anode attached over primary motor cortex | Activities of the unaffected limb; covered mirror + tDCS | Pronation, supination, flexion, and extension of both wrists, flexion and extension of the fingers, and flexion and extension of the elbows (10 sets, 20 repetitions per motion and set, 2 min rest between sets) | 20 | 3 | 6 | 360 | n/r | |
| Upper extremity | Activities of the unaffected limb | Passive mobilisation of the affected limb | Flexion and extension of shoulder, pronation and supination of forearm, gross and fine motor movements of wrist, hand and fingers (also with objects) | 45 | 3 | 8 | 1080 | Outpatient rehabilitation centre | |
| Upper extremity | 10 Hz TMS applied by 8‐coil on the ipsilesional somatosensory cortex, followed by MT | TMS only | n/r | 30 | 3 | 4 | 360 | n/r | |
| Upper extremity | Bilateral activities | Bilateral activities; without mirror | Execution of arm, hand and finger postures | 30 | 5 | 6 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral and unilateral activities | Traditional occupational therapy | n/r | 30 | 5 | 6 | 900 | Home setting | |
| Upper extremity | Activities of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion and extension of wrist and finger | 20 | 5 | 4 | 400 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | No additional therapy | Supination and eversion of the forearm, flexion and extension of the wrist and finger, grasp a block | 30 | 6 or 7 | 4 | 720 ‐ 840 | Inpatient Hospital | |
| Upper extremity | Bilateral activities; virtual mirror on a screen; arm projected by a camera | Bilateral activities; without mirror (screen was off) | 1st week: wrist flexion/ extension, forearm pro‐/supination, clenching and opening the hand, 2nd week gross motor tasks, 3rd and 4th week fine motor tasks; 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Lower extremity | Uni‐ and bilateral activities; virtual mirror on the screen, leg projected by a camera | Uni‐ and bilateral activities; without mirror (screen was off) | 1st week: dorsiflexion and plantarflexion (lifting of the heel) of the unaffected ankle; adduction and abduction of forefoot and rear foot; and adduction and abduction of the hip (moving the knees inward and outward), 2nd week mimicked the movements (1st week) of the unaffected lower limb on the monitor with the affected lower limb, 3rd dorsiflexion, adduction and abduction of the unaffected ankle; plantar flexion, adduction and abduction of the ankle; and adduction and abduction of the hip; 4th week: complex movements and different tasks (remote control with up and down buttons); 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Movements of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist, pro‐ /supination of the forearm, self selected speed, no additional verbal feedback | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: MT: Movements of the unaffected limb + rTMS; Experimental 2: MT: Movements of the unaffected limb | Activities of the unaffected limb, covered mirror | Experimental 1: finger flexion and extension + 10Hz rTMS on lesioned hemisphere; | 15 | 5 | 6 | 450 | University hospital | |
| Lower extremity | Bilateral activities and activities of the unaffected limb | 4 control groups: (1) EMG triggered electrical muscle stimulation; (2) electrical muscle stimulation; (3) repetitive facilitation exercises; (4) passive and active‐assistive range of motion exercises | Dorsiflexion of the ankle joint, stepping over, and abduction/adduction of the hip joint) | 20 | 7 | 4 | 560 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities + FES | Bilateral activities + FES; covered mirror | Extension of wrist and fingers to lift of the hand from an FES switch, at the same time attempt to extend affected hand supported by electrical stimulation (20 Hz), pulse rate 300 μs, individual intensity for muscle contraction and complete extension | 30 | 5 | 4 | 600 | University hospital | |
| Upper extremity | Bilateral activities + FES | No additional therapy | 2 experimental groups: (1) EMG‐triggered FES (due to unaffected limb) of affected wrist extension + physiological and object‐related movements; (2) FES of affected wrist extension + physiological and object‐related movements | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy | Arm bicycling, peg board exercise, skateboard‐supported exercises on a tabletop, donut on base putty kneading, double curved arch, bimanual placing cone, block stacking, graded pinch exercise, plastic cone stacking, shoulder curved arch | 30 | 5 | 4 | 300 | Outpatient hospital | |
| Upper extremity | Bilateral activities + EMTS | No additional therapy | Extension of wrist and fingers to reach EMG threshold on 50 ‐ 70% of maximum wrist extension, neuromuscular stimulation 10 seconds symmetrical biphasic pulses at 50 Hz, pulse width 200 μs, followed by 20 seconds of rest to assist full range of motion; bimanual wrist and finger extension during 'on' and 'off' period, difficulty of exercises dependent upon participants’ levels of functioning with regard to wrist and finger flexion and extension or thumb opposition | 20 (2 times a day) | 5 | 4 | 800 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | No additional therapy | Flexion/ extension of the knee and ankle; self‐selected speed; under supervision | 2 times daily for 15 minutes | 5 | 2 | 300 | n/r | |
| Upper extremity | n/r | No additional therapy | Wrist extension | 4 times daily for 15 minutes | 5 | 4 | 1200 | n/r | |
| Upper extremity | Bilateral activities | No additional therapy | Lifting both arms, flexion/ extension of the elbow, pronation of the forearm, wrist extension, internal/ external rotation of the wrist, clenching and opening the fist, tapping on the table; self‐performed; supervision of a guardian | 2 times daily for 25 minutes | 5 | 4 | 1000 | Inpatient rehabilitation ward | |
| Lower extremity | Bilateral activities + NMES | Conventional therapy | Dorsiflexion movements of the ankle | n/r | 5 | 4 | n/r | Rehabilitation hospital | |
| Upper extremity | Bilateral activities | Bilateral activities, covered mirror | Task‐oriented MT: forearm pronation‐supination and wrist flexion/extension, finger flexion‐extension, counting numbers, tapping, and opposing; simple manipulating tasks (such as picking up coins and beans, flipping over cards); complicated tasks (plugging and unplugging pegboards, drawing simple figures, and colouring) | 20 | 5 | 4 | 400 | Inpatient rehabilitation ward | |
| Upper extremity | Experimental 1: MT: Bilateral activities; Experimental 2: MT and sensory electrical stimulation by a mesh‐glove | Task‐oriented training | Transitive movements (e.g. gross motor tasks, such as reaching out to put a cup on a shelf, or fine motor tasks, such as picking up marbles); intransitive movements (e.g. gross motor movements, such as pronation and supination, or fine motor movements, such as finger opposition) | 60 | 5 | 4 | 1200 | In‐ and outpatient setting | |
| Upper extremity | n/r | n/r; transparent plastic between limbs | n/r | n/r | n/r | 4 | n/r | Home | |
| Lower extremity | Bilateral activities | 1: Bilateral activities, covered mirror; | Alternate dorsiflexion and plantarflexion in both ankles as best as possible, self‐paced speed | 15 | 5 | 3 | 225 | Inpatient rehabilitation unit | |
| Upper extremity | Bilateral activities | Bilateral activities | Exercises based on the Brunnstrom phases of motor recovery; functional tasks (i.e. with objects) | 60 | 1 (under supervision) + 5 (at home) | 6 | 2160 | Home | |
| Upper extremity | Bilateral activities | No additional therapy | Flexion and extension of shoulder, elbow, wrist and finger, prone‐supination of the forearm | 30 | 5 | 6 | 900 | Inpatient | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Lying position: hip‐knee‐ankle flexion, with the hip and knee placed in flexion, moving the knee inward and outward, hip abduction with external rotation followed by hip adduction with internal rotation; sitting position: Hip‐knee‐ankle flexion, knee extension with ankle dorsiflexion, knee flexion beyond 90 °; each exercise 2 sets of 10 repetitions | 60 | 6 | 2 | 720 | Inpatient rehabilitation | |
| Upper extremity | Bilateral activities | Landscape images were shown to participants, they should try to describe the images, without movements | Finger and hand movements | 30 | 5 | 1 | 150 | n/r | |
| Upper extremity | Bilateral activities | functional electrical stimulation, covered mirror | Experimental 1: wrist and finger extension, grasping and releasing a bottle; Experimental 2: combined MT and functional electrical stimulation | 30 | 6 | 2 | 360 | Hospital | |
| Upper extremity | Bilateral activities, therapist supported if patients were not able to move paretic limb | Bilateral activities, covered mirror | Flexion and extension movements of wrist and fingers | 60 | 5 | 4 | 1200 | inpatient rehabilitation and home training after discharge | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Pronation and supination of the forearm and the flexion and extension movements of the wrist and fingers; 5 sets each motion, 30 repetitions per set | 30 | 5 | 4 | 600 | Inpatient | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Task‐oriented activities consisted with reaching, grasping, lifting and releasing objects | n/r | 5 | 6 | n/r | Rehabilitation unit | |
| Upper extremity | Not stated | Same tasks; covered mirror | Task‐oriented activities consisting of grasping and releasing objects | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | bilateral activities | Same tasks; covered mirror | Finger and wrist movements, grasping different objects | 30 | 5 | 4 | 600 | Nursing homes | |
| Upper extremity | Bilateral activities | Motor relearning programme | Hand‐opening, wrist flexion/ extension, forearm pronation/ supination, hand sliding on surface | n/r | 6 | 4 | n/r | Outpatient | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Task‐orientend activities consisted with manipulating objects | 60 | 3 | 4 | 720 | Home | |
| Upper extremity | Bilateral activities (hypotone muscles); unilateral activities (hypertone muscles) | Bilateral activities; without mirror | Gross motor arm and hand movements; functional activities (i.e. with objects); fine motor activities (i.e. with objects) | 30 | Total number of sessions: 17 | 5 | 510 | Outpatient centre | |
| See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | 30 | Total number of sessions: 37 | 5 | 1110 | Inpatient rehabilitation centre | |
| Lower extremity | MT + Electrical stimulation | Conventional therapy | n/r | 50 | 4 | 2 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb | No additional therapy | Wrist flexion, extension, radial and ulnar deviation, circumduction, fisting, releasing, abduction, and adduction of all fingers; activities such as squeezing a ball, stacking rings, flipping cards, placing pegs on a board | 2 times for 30 | 5 | 3 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Electromyographic‐triggered muscular electrical stimulation | Grasping movements in combination with electromyographic‐triggered muscular electrical stimulation | 30 | 5 | 3 | 450 | 3 inpatient rehabilitation centres | |
| Upper extremity | Activities of the unaffected limb | No therapy | 5 movements of wrist and fingers, each 6 minutes | 30 | 5 | 4 | 500 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Dorsiflexion movements of the ankle | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb; affected limb passively moved by therapist | Activities of the unaffected limb; affected limb passively moved by therapist; without mirror | 13 kinds of movements, i.e. flexion/extension of wrist, pinching fingers, gripping ball | 10 to 15 | Daily | 4 (1st period) | 280 to 420 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | 1st week: isolated movements of fingers, wrist, lower arm, elbow and shoulder in all degrees of freedom, up to 50 repetitions per series, up to 4 series; | 30 | 3 ‐ 5 | 4 ‐ 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Not stated; self‐performed, daily checking by therapist | Lower limb activities; without a mirror | n/r | 30 | 5 | 4 | 600 | 12 inpatient stroke services | |
| Upper extremity | n/r | 1: no additional therapy; | n/r | n/r | n/r | n/r | n/r | n/r | |
| Upper extremity | Bilateral activities | Usual occupational therapy | Transitive movements: fine motor tasks of squeezing sponges, placing pegs in holes, flipping a card, gross motor tasks (reaching out for touch); intransitive movements (repetitive wrist flexion/extension, finger opposition, forearm pro‐/supination) | 60 | 5 | 4 | 1200 | 4 hospitals | |
| Upper extremity | Bilateral activities | Bilateral activities; nonreflecting side of the mirror | Flexion/extension of wrist and fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | 1: constraint induced movement therapy (6 hours/day) + palliative rehabilitation programme + self‐exercise; | Flexion/extension of the shoulder, elbow, wrist, finger, and pronation/supination of the forearm | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: activities of the unaffected limb Experimental 2: activities of the unaffected limb and additionally neuromuscular electrical stimulation of the affected arm | Neuromuscular electrical stimulation of finger and wrist extensors of the affected arm | Flexion/extension of wrist and fingers | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| n/r | n/r | n/r | n/r | 30 | Total: 20 ‐ 24 | 8 | 600 ‐ 720 | n/r |
EMG: electromyography
ETMS: electromyography‐triggered neuromuscular stimulation
FES: functional electrical stimulation
Hz: hertz
MT: mirror therapy
NMES: neuromuscular electrical stimulation
n/r: not reported
rTMS: repetitive transcranial magnetic stimulation
tDCS: transcranial direct current stimulation
TMS: transcranial magnetic stimulation
μs: microsiemens
Inclusion and exclusion criteria of studies are listed in Characteristics of included studies.
Interventions
Characteristics of interventions are summarised in Table 2. All except two included studies (In 2012; In 2016), provided mirror therapy using a mirror or a mirror box in the midsagittal plane between the upper or lower limbs. Thus the mirror reflected movements of the non‐affected side as if these movements were executed with the affected side. In 2012 and In 2016 used a virtual reflection setting where the affected extremity was placed under a screen while the non‐affected extremity was placed under a camera. The screen displayed the mirrored picture of the unaffected limb.
Ten studies examined the effects of mirror therapy for the lower extremity (Arya 2017; Cha 2015; In 2016; Kawakami 2015; Kumar 2013; Lee 2016; Marquez 2012; Mohan 2013; Salhab 2016; Sütbeyaz 2007); all other studies examined the effects of mirror therapy for the upper extremity.
Eleven studies used a combination of mirror therapy and other interventions. Kim 2014, Kim 2015a, Lee 2016, and Yun 2011 integrated a combination of mirror therapy with functional or neuromuscular electrical stimulation, Kojima 2014 and Schick 2017 with electromyographic‐triggered electrical muscle stimulation, and Lin 2014a combined mirror therapy with electrical sensory stimulation using a mesh‐glove. Mirror therapy was further combined with transcranial direct current stimulation (Cho 2015), or transcranial magnetic stimulation (Cha 2015; Dalla Libera 2015; Ji 2014a). If studies used two experimental groups, we combined both intervention groups for analysis.
Mirror therapy was provided for between three and seven days a week, and for between two and eight weeks. Each session lasted between 15 and 60 minutes. The total time for experimental intervention was between 225 and 2160 minutes.
Rothgangel 2004 included 16 participants and randomised them to mirror therapy or bilateral arm training. However, six of the participants were treated in an outpatient rehabilitation centre, and 10 in an inpatient care facility, which led to a significant difference in treatment time: the outpatient group received 17 treatment sessions of 30 minutes each; the inpatient group received 37 treatment sessions of 30 minutes each. Because these two groups are considerably different in total treatment time, we decided to analyse them separately (outpatient group: Rothgangel 2004a, and inpatient group: Rothgangel 2004b).
In 29 studies participants performed bilateral movements, moving the affected limb behind the mirror as best they could. In 22 studies participants only moved the unaffected side while looking in the mirror. In two studies participants performed both uni‐ and bilateral movements (In 2016; Kawakami 2015). In Rothgangel 2004 participants with muscle hypotonia had to move the affected arm as best they could; participants with muscle hypertonia should only move the unaffected arm while looking into the mirror. In two studies, a therapist passively moved the affected arm behind the mirror according to the movements of the unaffected one (Pandian 2014; Tezuka 2006).
In 11 studies the control group received no additional intervention other than standard rehabilitation. Twenty‐two studies used a form of sham therapy where the reflecting side of the mirror was covered, or the non‐reflecting side of the mirror was placed in the direction of the unaffected arm while practising. Eleven studies provided interventions with an unrestricted view of the affected side using the same training as in the experimental groups but without a mirror or with a plexiglas between limbs. Eighteen studies used other interventions in the control groups: electromyographic‐triggered muscle stimulation (Amasyali 2016; Kawakami 2015; Schick 2017; Wang 2015); (functional) electrical muscle stimulation (Kawakami 2015; Nagapattinam 2015; Yun 2011); conventional therapy (Arya 2015; Arya 2017; Geller 2016; Kim 2016; Salhab 2016; Wu 2013); motor imagery (Cacchio 2009b); passive mobilisation of the affected limb (Colomer 2016; Kawakami 2015); transcranial magnetic stimulation (Dalla Libera 2015); task‐oriented training (Lin 2014a); motor relearning programme (Rehani 2015); lower limb activities (Tyson 2015); or constraint‐induced movement therapy (Yoon 2014). In one study a therapist passively moved the affected arm according to the movements of the unaffected one, but without a mirror between limbs (Tezuka 2006). If studies integrated two control groups we combined both groups for analysis (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9). However, for testing the influence of different control treatments, we analysed single control groups in a subgroup analysis. Based on the difference of using a covered mirror, another intervention without mirror (also transparent plexiglas), or no additional therapy, we performed a subgroup analysis differentiating the effects of types of control intervention (covered mirror versus another intervention with unrestricted view versus no additional therapy) (Analysis 3.1).
Outcome
The included studies used a number of different outcomes. A description of the outcome measures used can be found in Characteristics of included studies.
Primary outcome: motor function
For analysis of our primary outcome of motor function we used the Motor Assessment Scale Item 7 (Acerra 2007; Marquez 2012; Piravej 2012), the Box and Block Test (Alibakhshi 2016; Amasyali 2016; Cho 2015; Kim 2015a; Lin 2014a; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a), the Action Research Arm Test (Dohle 2009; Geller 2016; Invernizzi 2013; Kim 2016; Michielsen 2011; Nagapattinam 2015; Thieme 2013; Tyson 2015), the Wolf Motor Function Test (functional ability) (Cacchio 2009a; Cacchio 2009b; Colomer 2016; Hiragami 2012; Kojima 2014; Yoon 2014), the Manual Function Test (Bae 2012; In 2012; Kim 2014; Lee 2012; Park 2015b; Seok 2010), the Berg Balance Scale (Cha 2015; In 2016; Lee 2016), the Brunnel Balance Assessment (Mohan 2013), the CAHAI (Rehani 2015), and the TEMPA (Rodrigues 2016).
Secondary outcomes: motor impairment, activities of daily living, pain and visuospatial neglect
For analysing motor impairment we used the Fugl‐Meyer score (Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009;Geller 2016; Gurbuz 2016; Hiragami 2012; In 2012; Kim 2014; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lim 2016; Lin 2014a; Michielsen 2011; Mirela 2015; Mohan 2013; Park 2015a; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Tezuka 2006; Thieme 2013; Wang 2015; Wu 2013; Yoon 2014; Yun 2011), the Brunnstrom stages of motor recovery (Piravej 2012; Sütbeyaz 2007; Yavuzer 2008), muscle or grip strength (Acerra 2007; Lee 2016; Marquez 2012), the Motricity Index (Invernizzi 2013; Tyson 2015), and the Manual Muscle Test (Seok 2010).
In our pooled analysis of the secondary outcome activities of daily living we used the Functional Independence Measure (Dohle 2009; Geller 2016; Hiragami 2012; Invernizzi 2013; Kim 2015a; Kim 2016; Pandian 2014; Park 2015a; Park 2015b; Sütbeyaz 2007; Yavuzer 2008), the Barthel Index (Kuzgun 2012; Lim 2016; Piravej 2012; Schick 2017; Thieme 2013 ; Yoon 2014), and the Motor Activity Log (amount of use) (Kojima 2014; Lin 2014a; Wu 2013).
For the analysis of the secondary outcome of pain we included the measurement of pain at rest (Acerra 2007; Cacchio 2009b; Michielsen 2011), and during movement (Cacchio 2009a; Dohle 2009). The investigators used Numerical Rating Scales between 0 and 10 (Acerra 2007), Visual Analogue Scales between 0 and 10 (Cacchio 2009a), or between 0 mm and 100 mm (Cacchio 2009b; Michielsen 2011), or the pain section of the Fugl‐Meyer Assessment, normalised on the average score for each item (0 to 2; 2 indicating no pain) (Dohle 2009, Thieme 2013).
Visuospatial neglect as an outcome was analysed using the Star Cancellation Test (Moustapha 2012; Pandian 2014; Thieme 2013; Tyson 2015), and a self‐developed score (Dohle 2009).
Follow‐up assessment
For analysis of sustained treatment effects for our primary outcome of motor function, we used only the data of follow‐up assessments after six months (Cacchio 2009a; Michielsen 2011), as well as for motor impairment (Michielsen 2011; Sütbeyaz 2007; Yavuzer 2008).
Adverse effects
Twenty‐one studies explicitly reported the assessment of adverse effects (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Colomer 2016; Hiragami 2012; Invernizzi 2013; Kojima 2014; Kuzgun 2012; Lin 2014a; Marquez 2012; Mohan 2013; Rodrigues 2016; Nagapattinam 2015; Schick 2017; Sütbeyaz 2007; Tyson 2015; Wu 2013; Yavuzer 2008; Zacharis 2014). No adverse events were reported.
Excluded studies
We discarded 470 studies following consideration of abstracts, full texts or both (see: Characteristics of excluded studies). In the Excluded studies section, we mention only those studies that might in a superficial view appear to meet the eligibility criteria and those studies that we classified as well‐known and likely to be considered relevant by some readers (Characteristics of excluded studies).
Risk of bias in included studies
All details about the methodological quality of the included studies using the 'Risk of bias' assessment tool (Higgins 2011) are provided in Characteristics of included studies and Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We emailed all trialists of the included studies to clarify some methodological or design issues, or both. Most trialists provided at least some of the requested information. Two review authors (from HT, NM, CD, JB or JM) independently evaluated the methodological quality of the studies. The assessing authors discussed all disagreements and resolved them by contacting another author or by obtaining additional information through contact with the principal investigator of the study.
Allocation
Fifty‐two studies used adequate randomisation procedures, and were therefore at low risk of bias (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Bae 2012; Bahrami 2013; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009; Gurbuz 2016; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kim 2016; Kojima 2014; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Mohan 2013; Moustapha 2012; Nagapattinam 2015; Pandian 2014; Park 2015a; Piravej 2012; Rajappan 2016; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008; Yoon 2014; Yun 2011). We were not able to rate risk of bias for 10 trials due to missing information about the sequence generation process (Altschuler 1999; Cacchio 2009a; Geller 2016; Kumar 2013; Manton 2002; Mirela 2015; Park 2015b; Salhab 2016; Wang 2015; Zacharis 2014). Five studies used a cross‐over design with random allocation to the order of treatment (Altschuler 1999; Kojima 2014; Moustapha 2012; Salhab 2016; Tezuka 2006). We only analysed the first treatment period as a parallel‐group design in these five studies. Eight studies used block randomisation methods (Cacchio 2009b; Hiragami 2012; Kojima 2014; Lin 2014a; Mohan 2013; Piravej 2012; Sütbeyaz 2007; Yavuzer 2008). One study randomly allocated ability‐matched pairs to treatment groups (Manton 2002).
Twenty‐five studies used an adequate concealment of allocation, and we therefore considered them to be at low risk of bias (Acerra 2007; Arya 2015; Arya 2017; Cacchio 2009b; Cha 2015; Colomer 2016; Dohle 2009; Hiragami 2012; Invernizzi 2013; Kim 2016; Marquez 2012; Michielsen 2011; Moustapha 2012; Pandian 2014; Piravej 2012; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Nagapattinam 2015; Schick 2017; Sütbeyaz 2007; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008). There was no description of the allocation concealment process, so we rated 35 trials at unclear risk of bias (Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Bae 2012; Bahrami 2013; Cacchio 2009a; Cho 2015; Dalla Libera 2015; Geller 2016; Gurbuz 2016; In 2012; In 2016; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Manton 2002; Mirela 2015; Mohan 2013; Park 2015a; Park 2015b; Rajappan 2016; Salhab 2016; Samuelkamaleshkumar 2014; Seok 2010; Wang 2015; Yoon 2014; Zacharis 2014). Two studies were at high risk of bias because the authors of the trials confirmed that no concealment of allocation process had occurred (Tezuka 2006; Yun 2011). The methods used for concealment of allocation are presented in Characteristics of included studies.
Blinding
We rated 37 studies at low risk of bias, since at least the primary outcome measures were assessed by people blinded to group allocation (Acerra 2007; Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Arya 2015; Arya 2017; Cacchio 2009a; Cacchio 2009b; Cha 2015; Colomer 2016; Dohle 2009; Gurbuz 2016; Hiragami 2012; In 2016; Invernizzi 2013; Ji 2014a; Kim 2014; Kim 2016; Kuzgun 2012; Lee 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Moustapha 2012; Pandian 2014; Piravej 2012; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008). In 22 studies the process of blinding was not described (Bae 2012; Bahrami 2013; Cho 2015; Dalla Libera 2015; Geller 2016; In 2012; Kawakami 2015; Kim 2015a; Kumar 2013; Lee 2012; Lim 2016; Manton 2002; Mirela 2015; Mohan 2013; Park 2015a; Park 2015b; Rajappan 2016; Rehani 2015; Salhab 2016; Wang 2015; Yoon 2014; Zacharis 2014). In three trials the study authors stated that the assessors of the primary outcome measure were not blinded, so we considered them to be at high risk of bias (Kojima 2014; Nagapattinam 2015; Yun 2011)
Incomplete outcome data
Seventeen studies conducted an ITT analysis that included incomplete outcome data (Acerra 2007; Amasyali 2016; Arya 2015; Arya 2017; Cacchio 2009a; Cacchio 2009b; Hiragami 2012; Invernizzi 2013; Marquez 2012; Michielsen 2011; Mohan 2013; Nagapattinam 2015; Pandian 2014; Rodrigues 2016; Rothgangel 2004; Schick 2017; Thieme 2013). No description of handling incomplete outcome data was available in 28 studies, and we considered them to be at unclear risk of bias for this domain (Alibakhshi 2016; Altschuler 1999; Bae 2012; Bahrami 2013; Cha 2015; Cho 2015; Dalla Libera 2015; Geller 2016; Gurbuz 2016; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lim 2016; Manton 2002; Mirela 2015; Park 2015a; Park 2015b; Salhab 2016; Samuelkamaleshkumar 2014; Seok 2010; Wang 2015; Wu 2013; Yoon 2014; Zacharis 2014). Seventeen studies reported that no ITT analysis was performed, and we rated them at high risk of bias (Colomer 2016; Dohle 2009; In 2012; In 2016; Kim 2015a; Lee 2012; Lee 2016; Lin 2014a; Moustapha 2012; Piravej 2012; Rajappan 2016; Rehani 2015; Sütbeyaz 2007; Tezuka 2006; Tyson 2015; Yavuzer 2008; Yun 2011)
Selective reporting
We did not evaluate studies for selective reporting.
Other potential sources of bias
Twenty studies did not report whether or not participants dropped out during the intervention. In the remaining 42 studies, 109 participants dropped out, which is a rate of 5.5%. Seventeen studies reported no dropouts during the intervention period, 17 trialists reported dropout rates of 15% or less, and in eight studies the dropout rate was above 15%. Fifty‐nine participants dropped out of the experimental groups and 51 participants dropped out of the control groups, giving balanced dropout rates between groups. A detailed description of study characteristics can be found in Characteristics of included studies.
Effects of interventions
Comparison 1: Mirror therapy versus all other interventions
Outcome 1.1: Motor function at the end of the intervention phase
We included 36 studies in a pooled analysis of motor function after study end, with a total of 615 participants in the intervention and 558 in the control groups in the post‐assessment data analysis (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Bae 2012; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dohle 2009; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Kim 2014; Kim 2015a; Kim 2016;Kojima 2014; Lee 2012; Lee 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Mohan 2013; Park 2015b; Piravej 2012; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Nagapattinam 2015; Seok 2010; Thieme 2013; Tyson 2015; Wang 2015; Yoon 2014). Mirror therapy had a statistically significant effect on motor function in participants after stroke compared with all other types of interventions (SMD 0.47, 95% CI 0.27 to 0.67; 1173 participants; 36 studies; I2 = 62%; Analysis 1.1).
Based on our sensitivity analysis for the influence of trial methodology, we found robust effects on motor function except for concealment of allocation. By analysing only those studies with adequate methods of concealment, the effect on motor function was not significant (Analysis 5.1). We therefore downgraded the quality of evidence to moderate, due to several ratings of unclear risk of bias.
Outcome 1.2: Motor impairment at the end of intervention phase
We included 39 studies in a pooled analysis of motor impairment after study end, with a total of 672 participants in the intervention and 620 in the control groups in the post‐assessment data analysis (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Cho 2015; Colomer 2016; Dohle 2009;Gurbuz 2016; In 2012; Invernizzi 2013; Kim 2014; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lin 2014a; Lim 2016; Marquez 2012; Michielsen 2011; Mirela 2015; Mohan 2013; Piravej 2012; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wang 2015; Wu 2013; Yavuzer 2008; Yun 2011; Yoon 2014). Mirror therapy has a statistically significant effect on motor impairment in participants after stroke compared with all other types of interventions (SMD 0.49, 95% CI 0.32 to 0.66; 1292 participants; 39 studies; I2 = 53%; Analysis 1.2). The quality of evidence for motor impairment was moderate.
The effect was robust even after excluding studies with no or inadequate methods of allocation concealment (Analysis 5.2)
Outcome 1.3: Fugl‐Meyer Assessment for the upper extremity at the end of intervention phase
Since 29 studies used the Fugl‐Meyer Asssessment for analysing treatment effects on motor impairment, we analysed the effect on motor impairment for this outcome measure, using mean differences. We included 28 studies in a pooled analysis on Fugl‐Meyer Assessment for the upper extremity after study end, with a total of 463 participants in the intervention and 435 in the control groups in the post‐assessment data analysis (Alibakhshi 2016; Amasyali 2016; Arya 2015; Cho 2015; Colomer 2016; Dohle 2009; Gurbuz 2016; In 2012; Kim 2014; Kim 2015a; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lin 2014a; Lim 2016; Michielsen 2011; Mirela 2015; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Tezuka 2006; Thieme 2013; Wang 2015; Wu 2013; Yun 2011; Yoon 2014). Mirror therapy had a statistically significant effect on Fugl‐Meyer‐Assessment in participants after stroke compared with all other types of interventions (MD 4.32, 95% CI 2.46 to 6.19; 898 participants; 28 studies; I2 = 77%; Analysis 1.3). We rated the evidence for this outcome as of low quality.
Outcome 1.4: Activities of daily living at the end of the intervention phase
We included 19 studies in the analysis of the outcome of activities of daily living (Dohle 2009; Gurbuz 2016; Hiragami 2012; Invernizzi 2013; Kim 2014; Kim 2015a; Kojima 2014; Kuzgun 2012; Lim 2016; Lin 2014a; Pandian 2014; Park 2015a; Piravej 2012; Schick 2017; Sütbeyaz 2007; Thieme 2013; Wu 2013; Yavuzer 2008; Yoon 2014). These studies included 333 participants in the intervention and 289 in the control groups. Mirror therapy had a statistically significant effect on activities of daily living for participants with stroke, compared with all other interventions (SMD 0.48, 95% CI 0.30 to 0.65; 622 participants; 19 studies; I2 = 15%; Analysis 1.4). We rated the evidence for this secondary outcome as of moderate quality.
Outcome 1.5: Pain at the end of the intervention phase
For analysing the effects of mirror therapy on pain at the end of the intervention, we included six studies presenting data on pain at rest or during movement (Acerra 2007; Cacchio 2009a; Cacchio 2009b; Dohle 2009; Michielsen 2011; Thieme 2013). These studies included 129 participants in the intervention and 119 in the control groups. Mirror therapy had a statistically significant effect on pain reduction for participants after stroke, compared with all other interventions (SMD −0.89, 95% CI −1.67 to −0.11; 248 participants; 6 studies; I2 = 87%; Analysis 1.5). We rated the quality of the evidence for this secondary outcome pain as low.
However, two studies only included participants after stroke with a diagnosis of CRPS‐type I, which might have influenced the effects of the intervention (Cacchio 2009a; Cacchio 2009b). We therefore performed a post hoc sensitivity analysis and removed the studies that only included participants with CRPS after stroke. After removing those two studies, we were left with four studies with 97 participants in the intervention and 79 in the control groups (Acerra 2007; Dohle 2009; Michielsen 2011; Thieme 2013). We found no statistically significant effect on pain for mirror therapy compared with all other interventions in this subgroup (SMD −0.23, 95% CI −0.53 to 0.08; 176 participants; 4 studies; I2 = 0%; Analysis 6.1).
Outcome 1.6: Visuospatial neglect at the end of the intervention
Five studies reported outcome on visuospatial neglect (Dohle 2009; Moustapha 2012; Pandian 2014; Thieme 2013; Tyson 2015). These studies included 109 participants in the intervention and 66 in the control groups. Based on these data, we found a statistically non‐significant effect of mirror therapy versus all other interventions on visuospatial neglect after stroke (SMD 1.06, 95% CI −0.10 to 2.23; 175 participants; 5 studies; I2 = 89%; Analysis 1.6). We rated the quality of evidence for this secondary outcome as low.
Outcome 1.7: Motor function at follow‐up after six months
Two studies provided data on motor function at a follow‐up period of six months (Cacchio 2009a; Michielsen 2011). These studies included 44 participants each in the experimental and control groups. At follow‐up after six months from the end of intervention, mirror therapy had a statistically non‐significant effect on motor impairment in people after stroke, compared with all other interventions (SMD 1.20, 95% CI −0.78 to 3.18; 88 participants; 2 studies; I2 = 94%; Analysis 1.7).
Outcome 1.8: Motor impairment at follow‐up after six months
Three studies provided data on motor impairment at a follow‐up period of six months (Michielsen 2011; Sütbeyaz 2007; Yavuzer 2008). These studies included 54 participants in the experimental and 55 in the control groups. At follow‐up after six months from the end of intervention, mirror therapy had a statistically significant effect on motor function in people after stroke, compared with all other interventions (SMD 0.69, 95% CI 0.26 to 1.12; 109 participants; 3 studies; I2 = 17%; Analysis 1.8).
Outcome 1.9: Dropouts at the end of intervention phase
We included 42 studies that provided data for the dropout rate at the end of the intervention phase in this analysis (Acerra 2007; Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Arya 2015; Arya 2017; Cacchio 2009a; Cacchio 2009b; Colomer 2016; Dohle 2009; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Kawakami 2015; Kim 2014; Kim 2015a; Kojima 2014; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Mohan 2013; Moustapha 2012; Pandian 2014; Piravej 2012; Rajappan 2016; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Nagapattinam 2015; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008; Yun 2011). We found a statistically non‐significant effect for dropping out in the mirror‐therapy groups compared with the control groups (OR 1.14, 95% CI 0.74 to 1.76; 1438 participants; 42 studies; I2 = 0%; Analysis 1.9).
Comparison 2: Subgroup analysis ‐ upper versus lower extremity
Outcome 2.1: Motor function at the end of the intervention phase
We performed a subgroup analysis for those studies examining mirror therapy for the upper extremity (subgroup 2.1.1) and lower extremity (subgroup 2.1.2) (Analysis 2.1). We included 31 studies in the analysis of motor function after mirror therapy for the upper extremity. Studies included 553 participants in the experimental and 495 in the control groups (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Bae 2012; Cacchio 2009a; Cacchio 2009b; Cho 2015; Colomer 2016; Dohle 2009; Hiragami 2012; In 2012; Invernizzi 2013; Kim 2014; Kim 2015a; Kim 2016; Kojima 2014; Lee 2012; Lin 2014a; Michielsen 2011; Park 2015b; Piravej 2012; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Seok 2010; Nagapattinam 2015; Thieme 2013; Tyson 2015; Wang 2015; Yoon 2014). We found a statistically significant effect of mirror therapy on motor function of the upper extremity for people after stroke compared to all other interventions (SMD 0.46, 95% CI 0.23 to 0.69; 1048 participants; 31 studies; I2 = 66%; Analysis 2.1.1).
Five studies with 62 participants in the experimental and 63 in the control groups were included in the analysis for the lower extremity (Cha 2015; In 2016; Lee 2016; Marquez 2012; Mohan 2013). The positive effect of mirror therapy on motor function of the lower extremity for people after stroke compared with all other interventions was statistically significant (SMD 0.56, 95% CI 0.19 to 0.92; 125 participants; 5 studies; I2 = 0%; Analysis 2.1.2). There was a statistically non‐significant difference between subgroups.
Comparison 3: Subgroup analysis ‐ sham intervention (covered mirror) versus other intervention (unrestricted view) versus no intervention
We found two different groups of control interventions. In all studies, participants in the control group performed the same movements as participants in the experimental groups. However, in one type of control intervention the view of the affected side was obscured with a covered mirror, or with the non‐reflective side of the mirror (sham intervention). In the other type of control intervention participants had an unrestricted view of both; the unaffected and the affected limb (other intervention). Because we believed that this may have influenced the effect of therapy, we performed a subgroup analysis, differentiating between these two types of studies.
Outcome 3.1: Motor function at the end of the intervention phase
Sixteen studies with the outcome of motor function used a covered mirror in the control group (Acerra 2007; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; In 2016; Invernizzi 2013; Ji 2014a; Kim 2014; Marquez 2012; Mohan 2013; Nagapattinam 2015; Park 2015b; Piravej 2012; Rodrigues 2016; Thieme 2013). These studies included 281 participants in the intervention and 225 in the control groups. For this subgroup we found a statistically significant effect of mirror therapy on motor function after stroke (SMD 0.67, 95% CI 0.36 to 0.99; 506 participants; 16 studies; I2 = 63%).
Fourteen studies with an intervention using an unrestricted view in the control groups, thus providing a view of both limbs, were analysed in this subgroup (Alibakhshi 2016; Amasyali 2016; Bae 2012; Colomer 2016; Dohle 2009; In 2012; Kim 2016; Lee 2016; Lin 2014a; Michielsen 2011; Schick 2017; Tyson 2015; Wang 2015; Yoon 2014). These studies included 259 participants in the experimental and 215 in the control groups. The effect of mirror therapy on motor function after stroke in these studies was not statistically significant (SMD 0.27, 95% CI −0.05 to 0.59; 474 participants; 14 studies; I2 = 62%).
We included eight studies with no additional control therapy in this analysis (Amasyali 2016; Hiragami 2012; Kim 2015a; Kojima 2014; Lee 2012; Samuelkamaleshkumar 2014; Seok 2010; Wang 2015). Studies included 114 participants in the experimental and 105 in the control groups. This subgroup showed no statistically significant effect in favour of mirror therapy (SMD 0.57, 95% CI −0.02 to 1.15; 219 participants; 8 studies; I2 = 75%; Analysis 3.1).
However, subgroup differences did not demonstrate statistical significance.
Comparison 4: Subgroup analysis: subacute versus chronic stage after stroke
In this subgroup analysis we differentiated between studies that included participants within six months (subacute stage) and those at more than six months after stroke (chronic stage). Eighteen studies with participants in the subacute stage after stroke were included in this analysis. We found a statistically significant effect of mirror therapy compared to all other interventions for this subgroup (SMD 0.45, 95% CI 0.18 to 0.73; 596 participants; 18 studies; I2 = 59%). Fourteen studies in this analysis included participants in the chronic phase after stroke. The effect on motor function was also significant for this subgroup (SMD 0.43, 95% CI 0.06 to 0.81; 398 participants; 14 studies; I2 = 68%; Analysis 4.1). Subgroup difference did not demonstrate statistical significance.
Comparison 5: Sensitivity analysis by trial methodology
We tested the robustness of the results by analysing only RCTs and excluding randomised cross‐over trials, and by using specific methodological variables that could influence the observed treatment effects (randomisation procedure, concealment of allocation, blinding of assessors and ITT analysis; Analysis 5.1).
Outcome 5.1: Motor function at the end of the intervention phase
All studies without randomised cross‐over trials
We included 35 studies in a subgroup analysis of all studies without randomised cross‐over trials. The studies included 609 participants in the experimental and 551 in the control groups. Based on this analysis, mirror therapy had a statistically significant effect on motor function in people after stroke, compared to all other treatments (SMD 0.47, 95% CI 0.27 to 0.68; 1160 participants; 35 studies; I2 = 63%; Analysis 5.1.1).
All studies with adequate sequence generation
We analysed 33 studies with 546 participants in the intervention and 459 in the control groups in this subgroup analysis of studies that we rated as having adequate sequence generation. We found a statistically significant effect of mirror therapy compared with all other therapies for people after stroke (SMD 0.36, 95% CI 0.19 to 0.54; participants = 1005; studies = 33; I2 = 45%; Analysis 5.1.2)
All studies with adequate concealed allocation
We analysed 16 studies as having used an adequate method of allocation concealment. These studies included 313 participants in the experimental and 259 in the control groups. Based on this analysis, we found a non‐significant effect of mirror therapy compared with all other therapies for people after stroke (SMD 0.21, 95% CI −0.04 to 0.47; 572 participants; 16 studies; I2 = 51%; Analysis 5.1.3).
All studies with adequate intention‐to‐treat (ITT) analysis
We included 12 studies in our analysis of studies with an adequate ITT analysis. Based on our analysis of 204 participants in the experimental and 184 in the control groups with post‐intervention data, mirror therapy had a significant effect on motor function compared with all other interventions (SMD 0.55, 95% CI 0.14 to 0.95; 388 participants; 12 studies; I2 = 70%; Analysis 5.1.4).
All studies with blinded assessors
In this analysis we included 25 studies with 437 participants in the experimental and 383 in the control groups. Mirror therapy had a statistically significant positive effect on motor function compared with all other interventions (SMD 0.44, 95% CI 0.17 to 0.70; 820 participants; 25 studies; I2 = 69%; Analysis 5.1.5).
Outcome 5.2: Motor impairment at the end of the intervention phase
All studies with adequate sequence generation
We analysed 36 studies with 620 participants in the intervention and 537 in the control groups in this subgroup analysis of studies that we rated as having adequate sequence generation. We found a statistically significant effect of mirror therapy compared with all other therapies for people after stroke (SMD 0.46, 95% CI 0.29 to 0.63; 1157 participants; 36 studies; I2 = 47%; Analysis 5.2).
Discusión
Resumen de los resultados principales
El objetivo principal de esta revisión fue evaluar el efecto de la terapia del espejo para mejorar la motricidad, la deficiencia motora y las actividades cotidianas, así como reducir el dolor y la inatención visuoespacial en los pacientes después del accidente cerebrovascular. Se incluyeron 62 estudios (57 ECA y cinco estudios aleatorios cruzados), con un total de 1982 participantes incluidos, que compararon la terapia del espejo con otras intervenciones. Se encontró evidencia de calidad moderada de que la terapia del espejo mejora la motricidad y la deficiencia motora, así como las actividades cotidianas. Además, con evidencia de baja calidad se encontró alivio del dolor después de un accidente cerebrovascular y de mejoría de la deficiencia motora seis meses después del final de la intervención. Sin embargo, después de excluir los estudios que incluyeron participantes con sólo un síndrome de dolor regional complejo, no se encontró un efecto estadísticamente significativo sobre el dolor, según evidencia de calidad moderada. Los resultados para la motricidad después de seis meses y para la inatención visuoespacial no fueron estadísticamente significativos y la evidencia fue de baja calidad. La aceptabilidad de la intervención fue alta, no hubo significativamente más abandonos en los grupos de intervención en comparación con los grupos control, ni se informaron eventos adversos durante o después de la terapia del espejo.
Cincuenta y dos de los estudios incluidos evaluaron el efecto de la terapia del espejo sobre la motricidad del miembro superior, y diez estudios evaluaron el efecto de la terapia del espejo sobre el miembro inferior. La terapia del espejo fue efectiva para mejorar la motricidad del miembro superior y del inferior.
Según un análisis de subgrupos, se encontraron efectos estadísticamente significativos sobre la motricidad en los estudios que compararon la terapia del espejo con una intervención simulada mediante un espejo cubierto (por lo que se evitó cualquier observación del miembro afectado), pero no en los estudios que no utilizaron restricciones en la observación (ningún espejo o un acrílico transparente) o ninguna intervención adicional en los grupos control. Sin embargo, no hubo diferencias estadísticamente significativas entre los subgrupos con diferentes intervenciones control.
En un análisis de subgrupos adicional se compararon los estudios que incluyeron participantes en la fase aguda/subaguda después del accidente cerebrovascular (en los seis meses posteriores al accidente cerebrovascular) y participantes en la fase crónica (más de seis meses después del accidente cerebrovascular). La terapia del espejo fue efectiva para ambos subgrupos de participantes.
Compleción y aplicabilidad general de las pruebas
Según la evidencia disponible e incluida, fue posible responder la pregunta de investigación, especialmente para los resultados de la motricidad y la deficiencia motora para el miembro superior e inferior, así como para las actividades cotidianas y el dolor. Sin embargo, para la inatención visuoespacial el número de estudios y participantes fue escaso, por lo que no fue posible establecer conclusiones definitivas. Además, se encontraron algunas indicaciones de un efecto selectivo de la terapia del espejo sobre el dolor en los participantes con SDRC. Sin embargo, estos hallazgos se basan en solamente dos estudios, por lo que no fue posible establecer conclusiones definitivas. Los resultados positivos para la deficiencia motora fueron consistentes con la evaluación de seguimiento después de seis meses, pero no para la motricidad. Los resultados son limitados porque el análisis de subgrupos indica evidencia de un mayor efecto de la terapia del espejo sobre la motricidad cuando se compara con una intervención simulada (en la que se utiliza un espejo cubierto), que cuando se compara con otra intervención (en la que no hay restricciones para la observación) o con ninguna. Por lo tanto, los efectos positivos en esta revisión indican al menos que la terapia del espejo como complemento al tratamiento habitual puede mejorar la motricidad de los pacientes después de un accidente cerebrovascular. Además, el efecto sobre la motricidad fue estadísticamente significativo para los participantes agudos/subagudos y para los participantes crónicos.
Una de las posibles ventajas de la terapia del espejo comparada con otras intervenciones se puede deber a la posibilidad de proporcionar entrenamiento mediante el movimiento el brazo no afectado, o ambos brazos, mientras se mira al espejo. Por lo tanto, incluso los pacientes con paresia grave podrían entrenar solos, sin un terapeuta. Además, la terapia del espejo se podría aplicar en el domicilio, al menos después del entrenamiento durante la hospitalización, como se ha evaluado en cinco estudios (Arya 2015; Manton 2002; Michielsen 2011; Pandian 2014; Rodrigues 2016).
Calidad de la evidencia
Se utilizaron varios dominios metodológicos (generación adecuada de la secuencia, ocultación adecuada de la asignación, tratamiento adecuado de los datos de resultado faltantes y cegamiento de los evaluadores) para evaluar los riesgos de sesgo de los estudios incluidos. En nueve estudios la generación de la secuencia se consideró incierta. Se encontraron 33 estudios que no utilizaron o fue incierto el uso de la ocultación de la asignación de los participantes a los grupos de estudio, 40 estudios en lo que no se utilizó o fue incierto si se utilizó un tratamiento adecuado de los datos de resultado faltantes y 24 estudios sin cegamiento o con un cegamiento incierto de los evaluadores. Por lo tanto, los resultados deben interpretarse con precaución debido a los riesgos de sesgo. Debido a lo anterior se disminuyó la calidad de la evidencia.
Algunos de los análisis demostraron heterogeneidad significativa. Sin embargo, en el caso de la motricidad y la deficiencia motora la heterogeneidad desapareció cuando se excluyeron del análisis los estudios con métodos de generación de la secuencia inciertos.
Para probar los sesgos potenciales en los temas metodológicos, se realizó un análisis de sensibilidad que excluyó los estudios aleatorios cruzados, los estudios con adecuación incierta de la generación de la secuencia, los estudios con ocultación inadecuada de la asignación, los estudios con un tratamiento inadecuado de los datos de resultado faltantes y los estudios en los que los evaluadores no estaban cegados a la intervención. Según estos análisis de sensibilidad, los efectos de la terapia del espejo sobre la motricidad para los pacientes después del accidente cerebrovascular fueron consistentes, excepto para los estudios con métodos adecuados de ocultación de la asignación. Para dichos estudios, los efectos sobre la motricidad, pero no sobre la deficiencia motora, no mostraron significación estadística.
Además, las limitaciones generales de los estudios incluidos fueron los tamaños pequeños de la muestra en la mayoría de los estudios y las diferencias de los participantes de los estudios (p.ej. gravedad de la deficiencia motora), así como la administración de las terapias entre los estudios (es decir, cantidad y frecuencia del período de tratamiento).
Sesgos potenciales en el proceso de revisión
Mediante un proceso de búsqueda extenso existe certeza de que se han identificado todos los estudios relevantes en el campo. Sin embargo, se mantiene el riesgo de sesgo de publicación hacia una selección de los resultados positivos. Además, hay una posibilidad pequeña de que no se hayan identificado estudios adicionales (publicados o no publicados). Como se señaló anteriormente, hubo heterogeneidad entre los estudios en cuanto al diseño de los ensayos (es decir, ensayos de grupos paralelos y cruzados, duración del seguimiento y criterios de selección de los participantes), las características de los participantes (es decir, gravedad de la deficiencia motora y tiempo desde el inicio del accidente cerebrovascular) y las características de las intervenciones (es decir, cantidad total de tiempo de tratamiento, porcentaje de la intervención dedicada sólo a la terapia del espejo y tratamiento para el miembro superior o inferior). También se identificaron las limitaciones metodológicas de los estudios. Sin embargo, como se señaló anteriormente, un análisis de sensibilidad de las limitaciones metodológicas y las características de los participantes mostró solidez en los resultados entre los posibles factores de confusión declarados, excepto para la ocultación de la asignación. El cegamiento de los terapeutas y los participantes sería un elemento adicional en la evaluación del "riesgo de sesgo", pero se decidió no integrar este elemento porque el cegamiento de los terapeutas o los participantes parecen no ser no practicables para el tipo de intervención en esta revisión.
Acuerdos y desacuerdos con otros estudios o revisiones
Los resultados de esta revisión coinciden con los de otras revisiones (Ezendam 2009; Rothgangel 2011). Estas revisiones fueron sistemáticas en términos de sus métodos. Sin embargo, tuvieron estrategias de búsqueda más limitadas, solo incluyeron estudios que se publicaron antes de 2009 y no utilizaron el análisis agrupado de los estudios identificados. Una revisión narrativa también describe los efectos positivos de la terapia del espejo después del accidente cerebrovascular (Ramachandran 2009).
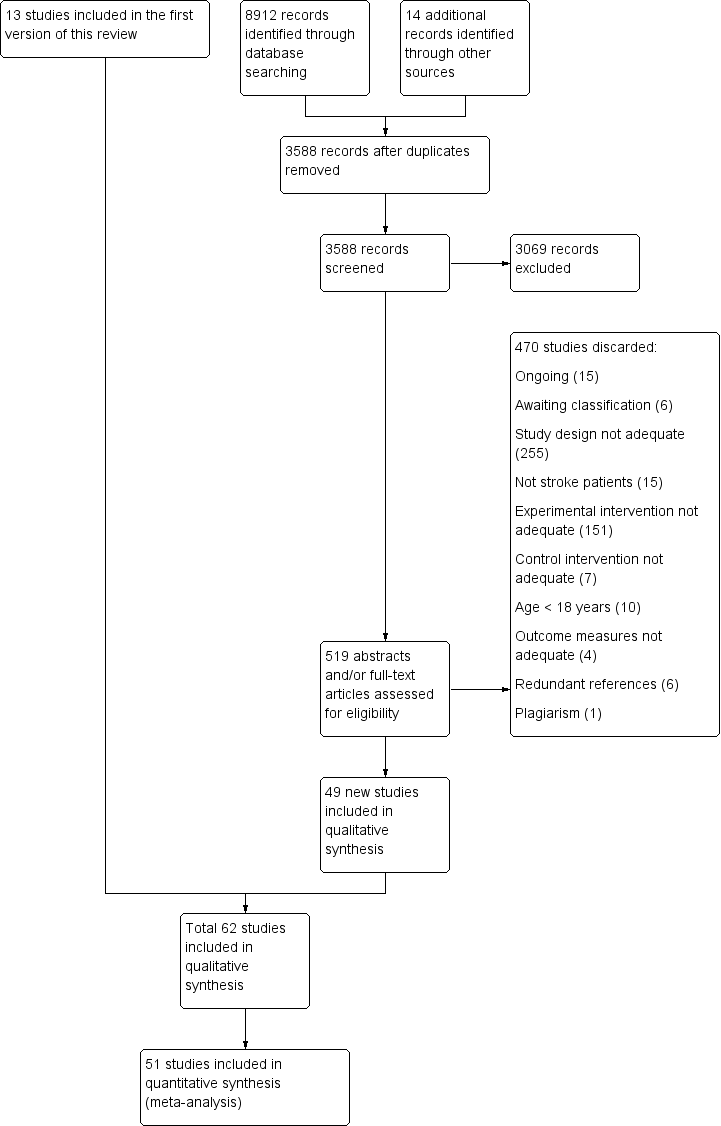
Study flow diagram of updated search and selection process

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
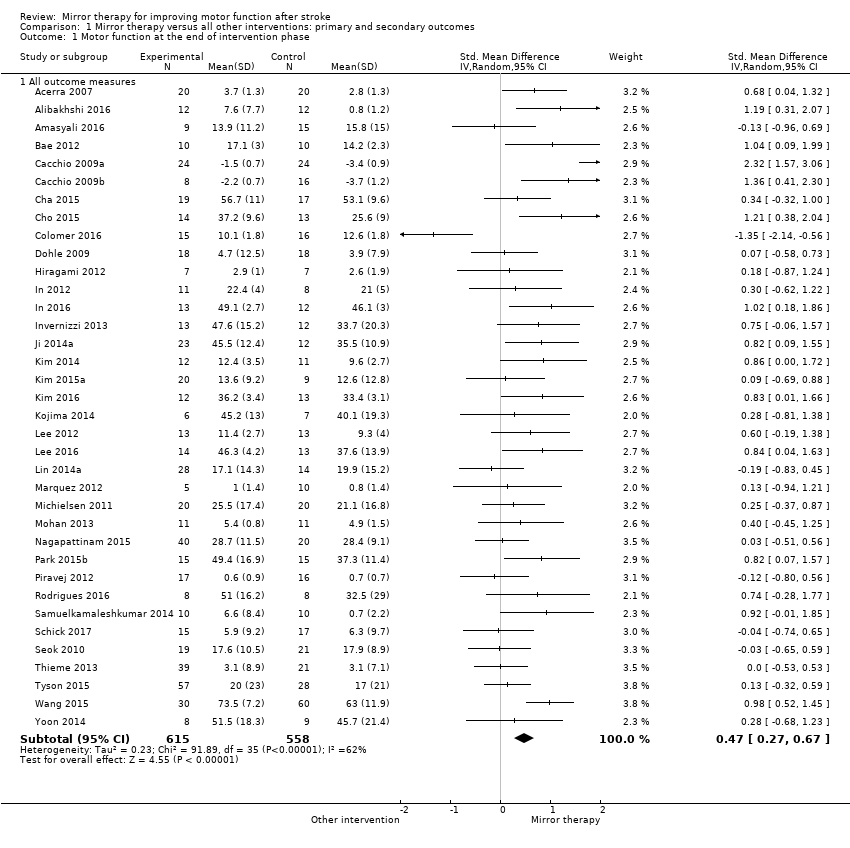
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 1 Motor function at the end of intervention phase.
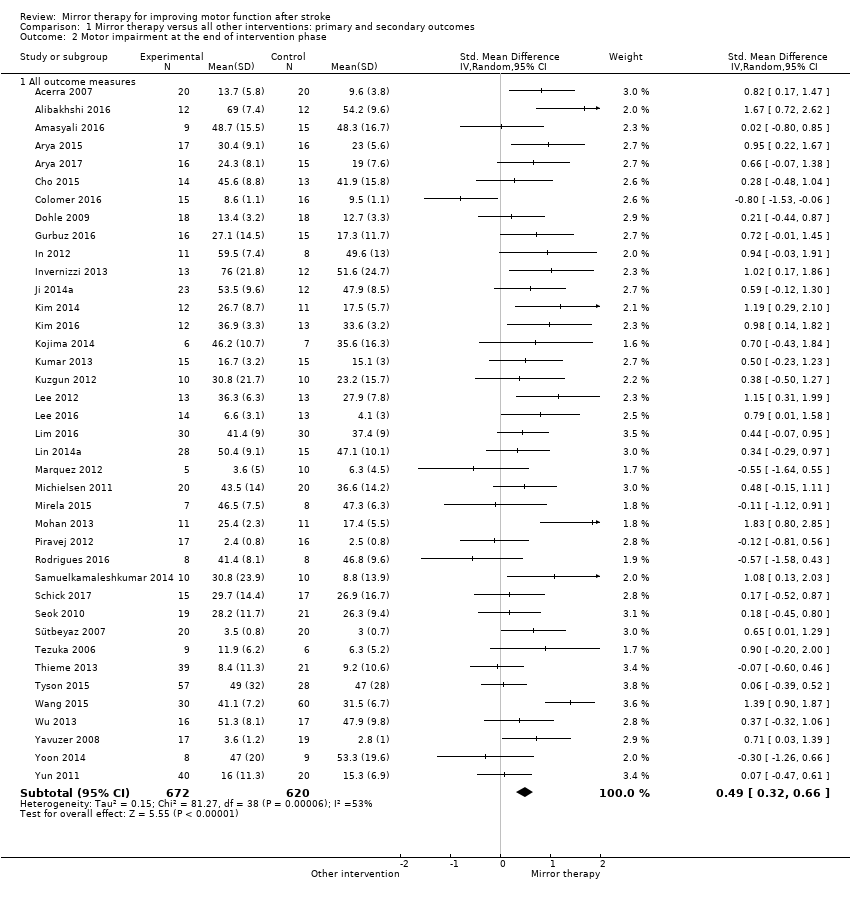
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 2 Motor impairment at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 4 Activities of daily living at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 5 Pain at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 6 Visuospatial neglect at the end of intervention.
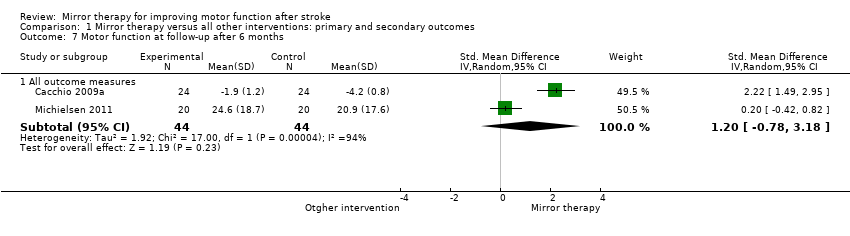
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 7 Motor function at follow‐up after 6 months.
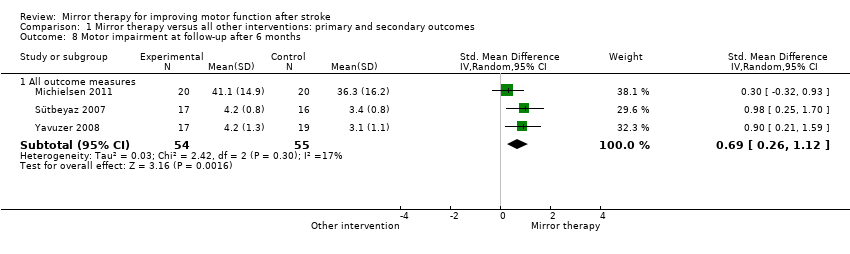
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 8 Motor impairment at follow‐up after 6 months.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 9 Dropouts at the end of intervention phase.

Comparison 2 Subgroup analysis: upper versus lower extremity, Outcome 1 Motor function at the end of intervention.

Comparison 3 Subgroup analysis: sham intervention (covered mirror) versus other intervention (unrestricted view), Outcome 1 Motor function at the end of intervention phase.

Comparison 4 Subgroup analysis: subacute versus chronic stage after stroke, Outcome 1 Motor function at the end of intervention phase.
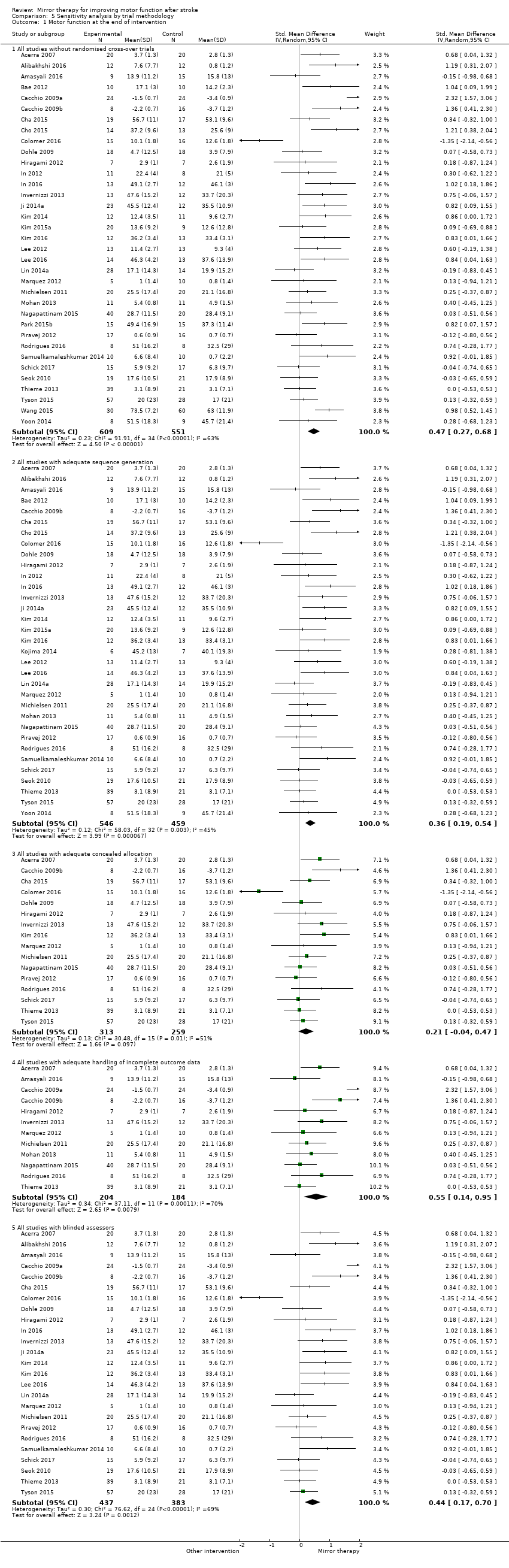
Comparison 5 Sensitivity analysis by trial methodology, Outcome 1 Motor function at the end of intervention.

Comparison 5 Sensitivity analysis by trial methodology, Outcome 2 Motor impairment at the end of intervention.

Comparison 6 Post hoc sensitivity analysis removing studies that only included participants with CRPS after stroke. Subgroup analysis: pain without complex regional pain syndrome (CRPS), Outcome 1 Pain at the end of intervention.
| Mirror therapy compared to all other interventions: primary and secondary outcomes for improving motor function after stroke | |||||
| Participants: people with paresis of the upper or lower limb, or both, caused by stroke Setting: inpatient and outpatient Intervention: mirror therapy Control: no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | № of participants | Quality of the evidence | Comment | |
| Assumed risk | Corresponding risk | ||||
| Control | Mirror therapy versus all other interventions | ||||
| Motor function at the end of intervention phase: all outcome measures | The mean motor function at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor function at the end of intervention phase ‐ all studies in the intervention groups was 0.47 SDs higher (0.27 to 0.67 higher) | 1173 | ⊕⊕⊕⊝ | SMD 0.47, 95% CI 0.27 to 0.67; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Motor impairment at the end of intervention phase: all outcome measures | The mean motor impairment at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor impairment at the end of intervention phase ‐ all studies in the intervention groups was 0.49 SDs higher (0.32 to 0.66 higher) | 1292 | ⊕⊕⊕⊝ Moderatea | SMD 0.49, 95% CI 0.32 to 0.66; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Fugl‐Meyer Assessment upper extremity at the end of intervention phase | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the control groups was NA | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the intervention groups was 4.32 pointshigher (2.46 to 6.19 higher) | 898 | ⊕⊕⊝⊝ | MD 4.32, 95% CI 2.46 to 6.19; the minimum important difference is approximately 5.25 |
| Activities of daily living at the end of intervention phase: all studies | The mean activities of daily living at the end of intervention phase ‐ all studies in the control groups was NA | The mean activities of daily living at the end of intervention phase ‐ all studies in the intervention groups was 0.48 SDs higher (0.29 to 0.67 higher) | 622 | ⊕⊕⊕⊝ | SMD 0.48, 95% CI 0.30 to 0.65; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase: all studies | The mean pain at the end of intervention phase ‐ all studies in the control groups was NA | The mean pain at the end of intervention phase ‐ all studies in the intervention groups was 0.89 SDs lower (1.67 to 0.11 lower) | 248 | ⊕⊕⊝⊝ | SMD −0.89, 95% CI −1.67 to −0.11; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase after excluding studies with CRPS | The mean pain at the end of intervention phase ‐ studies without CRPS in the control groups was NA | The mean pain at the end of intervention phase ‐ studies without CRPS in the intervention groups was 0.23 SDs lower (0.53 lower to 0.08 higher) | 176 (4 RCTs) | ⊕⊕⊕⊝ | SMD −0.23, 95% CI −0.53 to 0.08; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Visuospatial neglect at the end of intervention: all studies | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the control groups was NA | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the intervention groups was 1.06SDs higher (0.10 lower to 2.23 higher) | 175 | ⊕⊕⊝⊝ | SMD 1.06, 95% CI −0.10 to 2.23; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded due to several ratings in one or more items with high or unknown risk of bias. | |||||
| Study ID | Mean age | Sex | Side of paresis | Time since stroke | Type of stroke | |||
| Years | Women | Men | Left | Right | Mean time | Ischaemic | Haemorrhagic | |
| 68 | 22 | 18 | 16 | 24 | 5.3 days | 40 | 0 | |
| 50.9 | 9 | 15 | 15 | 9 | n/r | n/r | n/r | |
| 58.2 | 4 | 5 | 8 | 1 | 4.8 years | n/r | n/r | |
| 58.8 | 11 | 13 | 8 | 16 | 5.3 months | 24 | 0 | |
| 45.6 | 8 | 25 | 7 | 26 | 12.9 months/12.3 months. | 17 | 16 | |
| 46.4 | 6 | 30 | 16 | 20 | 15.9 months | 17 | 9 | |
| 53.9 | 7 | 13 | 13 | 7 | 4.6 months | 9 | 11 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 58.4 | 26 | 22 | 34 | 14 | 5 months | 35 | 13 | |
| 62 | 13 | 11 | 15 | 9 | 15.7 months | 19 | 5 | |
| 58.7 | 17 | 19 | n/r | n/r | 1.8 months | n/r | n/r | |
| 59.3 | 12 | 15 | 14 | 13 | 13.2 months/15.5 months | 17 | 10 | |
| 53.5 | 5 | 26 | 24 | 7 | 551 days | 23 | 8 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 56.5 | 10 | 26 | 25 | 11 | 27 days | 48 | 0 | |
| n/r | 3 | 3 | n/r | n/r | n/r | n/r | n/r | |
| 60.9 | 14 | 17 | 14 | 17 | 44.3 days | 25 | 6 | |
| 67.5 | 6 | 8 | 6 | 8 | 47 days | 9 | 5 | |
| 63.9 | 8 | 11 | 9 | 10 | 14.1 months | 10 | 9 | |
| 55.9 | 10 | 15 | 13 | 12 | 13.1 days | 16 | 9 | |
| 66.6 | 9 | 17 | 13 | 13 | 23 days | 26 | 0 | |
| 52.6 | 13 | 22 | 14 | 21 | 8.9 months | 19 | 16 | |
| 64.1 | 24 | 43 | 35 | 32 | 32.3 days | 28 | 39 | |
| 55.8 | 9 | 14 | 13 | 10 | 34.5 days | 14 | 9 | |
| 57.7 | 9 | 20 | 20 | 9 | 404.4 days | 14 | 15 | |
| 49.1 | 9 | 16 | 16 | 9 | n/r | 8 | 17 | |
| 69.1 | 3 | 10 | 5 | 8 | 78.8 days | 10 | 3 | |
| 57.3 | 8 | 22 | n/r | n/r | n/r | n/r | n/r | |
| 61.4 | 10 | 10 | 10 | 10 | n/r | n/r | n/r | |
| 57.1 | 11 | 15 | 11 | 15 | 3.6 months | n/r | n/r | |
| 54.7 | 13 | 14 | 8 | 19 | 39.6 months | 8 | 20 | |
| 64.9 | 21 | 39 | 31 | 29 | 52 days | 19 | 41 | |
| 55 | 11 | 32 | 22 | 21 | 19.6 months | 20 | 28 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 68.7 | 8 | 7 | 9 | 6 | 24.3 days | 10 | 5 | |
| 57 | 20 | 20 | 28 | 12 | 4.6 years | 28 | 12 | |
| 57.5 | 8 | 7 | 5 | 10 | 53.2 days | 15 | 0 | |
| 63 | 10 | 12 | 6 | 16 | 6.4 days | 14 | 8 | |
| 53.5 | 4 | 4 | 4 | 4 | 4.5 months | n/r | n/r | |
| 44.9 | 20 | 40 | n/r | n/r | 4.2 months | 60 | 0 | |
| 63.4 | 20 | 28 | 37 | 11 | 2 days | 26 | 22 | |
| 56.3 | 13 | 17 | 14 | 16 | 20.9 months | 16 | 14 | |
| 60 | 15 | 15 | 17 | 13 | 8.2 months | 17 | 13 | |
| 56 | 19 | 21 | 25 | 15 | 7.2 months | 27 | 13 | |
| 58 | 9 | 21 | 3 | 27 | 5 months | 20 | 10 | |
| 56.3 | n/r | n/r | n/r | n/r | 83.9 days | n/r | n/r | |
| 57.5 | 6 | 10 | 11 | 5 | 34.8 months | 16 | 0 | |
| 73.4 | 10 | 6 | 8 | 8 | 9.5 months | 16 | 0 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 51.2 | 4 | 16 | 9 | 11 | 4.1weeks | 14 | 6 | |
| 63 | 13 | 19 | 15 | 17 | 50 days | 27 | 5 | |
| 51.4 | 22 | 18 | n/r | n/r | 4.0 months | n/r | n/r | |
| 63.4 | 17 | 23 | 27 | 13 | 3.7 months | 33 | 7 | |
| 63.7 | 9 | 6 | 6 | 9 | 32.7 days | n/r | n/r | |
| 67.2 | 25 | 35 | 37 | 23 | 45 days | 45 | 15 | |
| 64 | 34 | 60 | 56 | 38 | 29 days | 76 | 18 | |
| 64.9 | 40 | 50 | 39 | 51 | 63.7 days | 57 | 33 | |
| 54.2 | 10 | 23 | 18 | 15 | 20.6 months | 20 | 13 | |
| 63.3 | 17 | 19 | 21 | 19 | 5.5 months | 29 | 7 | |
| 57.8 | 10 | 16 | 15 | 11 | 22.7 days | 16 | 10 | |
| 63.3 | 21 | 39 | 31 | 29 | 25.8 days | 46 | 14 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| n/r: not reported | ||||||||
| Study ID | Extremity | Mirror therapy variation | Control intervention | Type of movements | Minutes per session | Sessions per week | Total duration (weeks) | Total amount of therapy (minutes) | Setting |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Functional motor tasks (i.e. with objects); motor co‐ordination tasks; sensory discrimination tasks; grip strength; active range of motion | 20 to 30 | 7 | 2 | 280 ‐ 420 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities without mirror | n/r | 30 | 5 | 3 | 450 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities; transparent plastic between limbs | Proximal and distal movements | 15 (2 times a day) | 12 | 4 (1st period) | 720 | n/r | |
| Upper extremity | Activities of the unaffected limb | 1. EMG‐triggered electrostimulation; | Wrist, hand flexion, extension and forearm circumduction, and supination–pronation | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy based on Brunnstrom and Bobath principles | Task‐based mirror therapy: finger dexterity, mass grasp/finger flexion, release/finger extension, wrist dorsiflexion, | 45 | 5 | 8 | 1800 | Inpatient hospital, home after discharge | |
| Lower extremity | Activities of the unaffected limb | Conventional motor therapy based on neurophysiological approaches | Activity‐based MT: ball‐rolling, rocker‐board and pedaling | 60 | n/r | 3 ‐ 4 (30 session) | 1800 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Activities of the non‐paretic arm, without mirror | Flexion/extension of the shoulder, radial/ulnar deviation and pro‐/supination of the forearm, flexion/extension of the fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper and lower extremity | Activities of the unaffected limbs | Routine programme (physiotherapy and neuromuscular stimulation) | Range of motion of the healthy limbs | 30 | 5 | 4 | 600 | n/r | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient and outpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror (control group 1); imagination of movements of the affected limb (control group 2) | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 | Daily | 4 | 840 | Inpatient and outpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb + rTMS | Activities of the unaffected limb; covered mirror + rTMS | Flexing and extending the hip, knee, and ankle at a self‐selected speed under supervision but without additional verbal feedback | 20 | 5 | 4 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb + tDCS /anode attached over primary motor cortex | Activities of the unaffected limb; covered mirror + tDCS | Pronation, supination, flexion, and extension of both wrists, flexion and extension of the fingers, and flexion and extension of the elbows (10 sets, 20 repetitions per motion and set, 2 min rest between sets) | 20 | 3 | 6 | 360 | n/r | |
| Upper extremity | Activities of the unaffected limb | Passive mobilisation of the affected limb | Flexion and extension of shoulder, pronation and supination of forearm, gross and fine motor movements of wrist, hand and fingers (also with objects) | 45 | 3 | 8 | 1080 | Outpatient rehabilitation centre | |
| Upper extremity | 10 Hz TMS applied by 8‐coil on the ipsilesional somatosensory cortex, followed by MT | TMS only | n/r | 30 | 3 | 4 | 360 | n/r | |
| Upper extremity | Bilateral activities | Bilateral activities; without mirror | Execution of arm, hand and finger postures | 30 | 5 | 6 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral and unilateral activities | Traditional occupational therapy | n/r | 30 | 5 | 6 | 900 | Home setting | |
| Upper extremity | Activities of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion and extension of wrist and finger | 20 | 5 | 4 | 400 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | No additional therapy | Supination and eversion of the forearm, flexion and extension of the wrist and finger, grasp a block | 30 | 6 or 7 | 4 | 720 ‐ 840 | Inpatient Hospital | |
| Upper extremity | Bilateral activities; virtual mirror on a screen; arm projected by a camera | Bilateral activities; without mirror (screen was off) | 1st week: wrist flexion/ extension, forearm pro‐/supination, clenching and opening the hand, 2nd week gross motor tasks, 3rd and 4th week fine motor tasks; 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Lower extremity | Uni‐ and bilateral activities; virtual mirror on the screen, leg projected by a camera | Uni‐ and bilateral activities; without mirror (screen was off) | 1st week: dorsiflexion and plantarflexion (lifting of the heel) of the unaffected ankle; adduction and abduction of forefoot and rear foot; and adduction and abduction of the hip (moving the knees inward and outward), 2nd week mimicked the movements (1st week) of the unaffected lower limb on the monitor with the affected lower limb, 3rd dorsiflexion, adduction and abduction of the unaffected ankle; plantar flexion, adduction and abduction of the ankle; and adduction and abduction of the hip; 4th week: complex movements and different tasks (remote control with up and down buttons); 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Movements of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist, pro‐ /supination of the forearm, self selected speed, no additional verbal feedback | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: MT: Movements of the unaffected limb + rTMS; Experimental 2: MT: Movements of the unaffected limb | Activities of the unaffected limb, covered mirror | Experimental 1: finger flexion and extension + 10Hz rTMS on lesioned hemisphere; | 15 | 5 | 6 | 450 | University hospital | |
| Lower extremity | Bilateral activities and activities of the unaffected limb | 4 control groups: (1) EMG triggered electrical muscle stimulation; (2) electrical muscle stimulation; (3) repetitive facilitation exercises; (4) passive and active‐assistive range of motion exercises | Dorsiflexion of the ankle joint, stepping over, and abduction/adduction of the hip joint) | 20 | 7 | 4 | 560 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities + FES | Bilateral activities + FES; covered mirror | Extension of wrist and fingers to lift of the hand from an FES switch, at the same time attempt to extend affected hand supported by electrical stimulation (20 Hz), pulse rate 300 μs, individual intensity for muscle contraction and complete extension | 30 | 5 | 4 | 600 | University hospital | |
| Upper extremity | Bilateral activities + FES | No additional therapy | 2 experimental groups: (1) EMG‐triggered FES (due to unaffected limb) of affected wrist extension + physiological and object‐related movements; (2) FES of affected wrist extension + physiological and object‐related movements | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy | Arm bicycling, peg board exercise, skateboard‐supported exercises on a tabletop, donut on base putty kneading, double curved arch, bimanual placing cone, block stacking, graded pinch exercise, plastic cone stacking, shoulder curved arch | 30 | 5 | 4 | 300 | Outpatient hospital | |
| Upper extremity | Bilateral activities + EMTS | No additional therapy | Extension of wrist and fingers to reach EMG threshold on 50 ‐ 70% of maximum wrist extension, neuromuscular stimulation 10 seconds symmetrical biphasic pulses at 50 Hz, pulse width 200 μs, followed by 20 seconds of rest to assist full range of motion; bimanual wrist and finger extension during 'on' and 'off' period, difficulty of exercises dependent upon participants’ levels of functioning with regard to wrist and finger flexion and extension or thumb opposition | 20 (2 times a day) | 5 | 4 | 800 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | No additional therapy | Flexion/ extension of the knee and ankle; self‐selected speed; under supervision | 2 times daily for 15 minutes | 5 | 2 | 300 | n/r | |
| Upper extremity | n/r | No additional therapy | Wrist extension | 4 times daily for 15 minutes | 5 | 4 | 1200 | n/r | |
| Upper extremity | Bilateral activities | No additional therapy | Lifting both arms, flexion/ extension of the elbow, pronation of the forearm, wrist extension, internal/ external rotation of the wrist, clenching and opening the fist, tapping on the table; self‐performed; supervision of a guardian | 2 times daily for 25 minutes | 5 | 4 | 1000 | Inpatient rehabilitation ward | |
| Lower extremity | Bilateral activities + NMES | Conventional therapy | Dorsiflexion movements of the ankle | n/r | 5 | 4 | n/r | Rehabilitation hospital | |
| Upper extremity | Bilateral activities | Bilateral activities, covered mirror | Task‐oriented MT: forearm pronation‐supination and wrist flexion/extension, finger flexion‐extension, counting numbers, tapping, and opposing; simple manipulating tasks (such as picking up coins and beans, flipping over cards); complicated tasks (plugging and unplugging pegboards, drawing simple figures, and colouring) | 20 | 5 | 4 | 400 | Inpatient rehabilitation ward | |
| Upper extremity | Experimental 1: MT: Bilateral activities; Experimental 2: MT and sensory electrical stimulation by a mesh‐glove | Task‐oriented training | Transitive movements (e.g. gross motor tasks, such as reaching out to put a cup on a shelf, or fine motor tasks, such as picking up marbles); intransitive movements (e.g. gross motor movements, such as pronation and supination, or fine motor movements, such as finger opposition) | 60 | 5 | 4 | 1200 | In‐ and outpatient setting | |
| Upper extremity | n/r | n/r; transparent plastic between limbs | n/r | n/r | n/r | 4 | n/r | Home | |
| Lower extremity | Bilateral activities | 1: Bilateral activities, covered mirror; | Alternate dorsiflexion and plantarflexion in both ankles as best as possible, self‐paced speed | 15 | 5 | 3 | 225 | Inpatient rehabilitation unit | |
| Upper extremity | Bilateral activities | Bilateral activities | Exercises based on the Brunnstrom phases of motor recovery; functional tasks (i.e. with objects) | 60 | 1 (under supervision) + 5 (at home) | 6 | 2160 | Home | |
| Upper extremity | Bilateral activities | No additional therapy | Flexion and extension of shoulder, elbow, wrist and finger, prone‐supination of the forearm | 30 | 5 | 6 | 900 | Inpatient | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Lying position: hip‐knee‐ankle flexion, with the hip and knee placed in flexion, moving the knee inward and outward, hip abduction with external rotation followed by hip adduction with internal rotation; sitting position: Hip‐knee‐ankle flexion, knee extension with ankle dorsiflexion, knee flexion beyond 90 °; each exercise 2 sets of 10 repetitions | 60 | 6 | 2 | 720 | Inpatient rehabilitation | |
| Upper extremity | Bilateral activities | Landscape images were shown to participants, they should try to describe the images, without movements | Finger and hand movements | 30 | 5 | 1 | 150 | n/r | |
| Upper extremity | Bilateral activities | functional electrical stimulation, covered mirror | Experimental 1: wrist and finger extension, grasping and releasing a bottle; Experimental 2: combined MT and functional electrical stimulation | 30 | 6 | 2 | 360 | Hospital | |
| Upper extremity | Bilateral activities, therapist supported if patients were not able to move paretic limb | Bilateral activities, covered mirror | Flexion and extension movements of wrist and fingers | 60 | 5 | 4 | 1200 | inpatient rehabilitation and home training after discharge | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Pronation and supination of the forearm and the flexion and extension movements of the wrist and fingers; 5 sets each motion, 30 repetitions per set | 30 | 5 | 4 | 600 | Inpatient | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Task‐oriented activities consisted with reaching, grasping, lifting and releasing objects | n/r | 5 | 6 | n/r | Rehabilitation unit | |
| Upper extremity | Not stated | Same tasks; covered mirror | Task‐oriented activities consisting of grasping and releasing objects | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | bilateral activities | Same tasks; covered mirror | Finger and wrist movements, grasping different objects | 30 | 5 | 4 | 600 | Nursing homes | |
| Upper extremity | Bilateral activities | Motor relearning programme | Hand‐opening, wrist flexion/ extension, forearm pronation/ supination, hand sliding on surface | n/r | 6 | 4 | n/r | Outpatient | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Task‐orientend activities consisted with manipulating objects | 60 | 3 | 4 | 720 | Home | |
| Upper extremity | Bilateral activities (hypotone muscles); unilateral activities (hypertone muscles) | Bilateral activities; without mirror | Gross motor arm and hand movements; functional activities (i.e. with objects); fine motor activities (i.e. with objects) | 30 | Total number of sessions: 17 | 5 | 510 | Outpatient centre | |
| See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | 30 | Total number of sessions: 37 | 5 | 1110 | Inpatient rehabilitation centre | |
| Lower extremity | MT + Electrical stimulation | Conventional therapy | n/r | 50 | 4 | 2 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb | No additional therapy | Wrist flexion, extension, radial and ulnar deviation, circumduction, fisting, releasing, abduction, and adduction of all fingers; activities such as squeezing a ball, stacking rings, flipping cards, placing pegs on a board | 2 times for 30 | 5 | 3 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Electromyographic‐triggered muscular electrical stimulation | Grasping movements in combination with electromyographic‐triggered muscular electrical stimulation | 30 | 5 | 3 | 450 | 3 inpatient rehabilitation centres | |
| Upper extremity | Activities of the unaffected limb | No therapy | 5 movements of wrist and fingers, each 6 minutes | 30 | 5 | 4 | 500 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Dorsiflexion movements of the ankle | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb; affected limb passively moved by therapist | Activities of the unaffected limb; affected limb passively moved by therapist; without mirror | 13 kinds of movements, i.e. flexion/extension of wrist, pinching fingers, gripping ball | 10 to 15 | Daily | 4 (1st period) | 280 to 420 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | 1st week: isolated movements of fingers, wrist, lower arm, elbow and shoulder in all degrees of freedom, up to 50 repetitions per series, up to 4 series; | 30 | 3 ‐ 5 | 4 ‐ 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Not stated; self‐performed, daily checking by therapist | Lower limb activities; without a mirror | n/r | 30 | 5 | 4 | 600 | 12 inpatient stroke services | |
| Upper extremity | n/r | 1: no additional therapy; | n/r | n/r | n/r | n/r | n/r | n/r | |
| Upper extremity | Bilateral activities | Usual occupational therapy | Transitive movements: fine motor tasks of squeezing sponges, placing pegs in holes, flipping a card, gross motor tasks (reaching out for touch); intransitive movements (repetitive wrist flexion/extension, finger opposition, forearm pro‐/supination) | 60 | 5 | 4 | 1200 | 4 hospitals | |
| Upper extremity | Bilateral activities | Bilateral activities; nonreflecting side of the mirror | Flexion/extension of wrist and fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | 1: constraint induced movement therapy (6 hours/day) + palliative rehabilitation programme + self‐exercise; | Flexion/extension of the shoulder, elbow, wrist, finger, and pronation/supination of the forearm | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: activities of the unaffected limb Experimental 2: activities of the unaffected limb and additionally neuromuscular electrical stimulation of the affected arm | Neuromuscular electrical stimulation of finger and wrist extensors of the affected arm | Flexion/extension of wrist and fingers | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| n/r | n/r | n/r | n/r | 30 | Total: 20 ‐ 24 | 8 | 600 ‐ 720 | n/r | |
| EMG: electromyography | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All outcome measures | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 2 Motor impairment at the end of intervention phase Show forest plot | 39 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All outcome measures | 39 | 1292 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.32, 0.66] |
| 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase Show forest plot | 28 | 898 | Mean Difference (IV, Random, 95% CI) | 4.32 [2.46, 6.19] |
| 4 Activities of daily living at the end of intervention phase Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 All outcome measures | 19 | 622 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.30, 0.65] |
| 5 Pain at the end of intervention phase Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 All outcome measures | 6 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.67, ‐0.11] |
| 6 Visuospatial neglect at the end of intervention Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 All outcome measures | 5 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 1.06 [‐0.10, 2.23] |
| 7 Motor function at follow‐up after 6 months Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 All outcome measures | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.78, 3.18] |
| 8 Motor impairment at follow‐up after 6 months Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 All outcome measures | 3 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.26, 1.12] |
| 9 Dropouts at the end of intervention phase Show forest plot | 42 | 1438 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.74, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention Show forest plot | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 1.1 Mirror therapy for the upper extremity | 31 | 1048 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.23, 0.69] |
| 1.2 Mirror therapy for the lower extremity | 5 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.19, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 36 | 1199 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [0.29, 0.72] |
| 1.1 Studies that used a covered mirror in the control group | 16 | 506 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.36, 0.99] |
| 1.2 Studies that used unrestricted view in the control group | 14 | 474 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.05, 0.59] |
| 1.3 Studies that used no additional control intervention | 8 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.02, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 32 | 994 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.22, 0.66] |
| 1.1 All studies including participants within 6 months after stroke | 18 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.18, 0.73] |
| 1.2 All studies including participants with more than 6 months after stroke | 14 | 398 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.06, 0.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies without randomised cross‐over trials | 35 | 1160 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.68] |
| 1.2 All studies with adequate sequence generation | 33 | 1005 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.19, 0.54] |
| 1.3 All studies with adequate concealed allocation | 16 | 572 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.47] |
| 1.4 All studies with adequate handling of incomplete outcome data | 12 | 388 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.14, 0.95] |
| 1.5 All studies with blinded assessors | 25 | 820 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.17, 0.70] |
| 2 Motor impairment at the end of intervention Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies with adequate sequence generation | 36 | 1157 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.29, 0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at the end of intervention Show forest plot | 4 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.08] |

