Progesterona para el tratamiento del traumatismo craneoencefálico agudo
Resumen
Antecedentes
El traumatismo craneoencefálico (TCE) es una causa principal de muerte y discapacidad, y la identificación de tratamientos efectivos, de bajo coste y ampliamente practicables para la lesión cerebral es de gran importancia para la salud pública en todo el mundo. La progesterona es una hormona natural con una farmacocinética bien definida, de amplia disponibilidad, de bajo coste y tiene acciones esteroideas, neuroactivas y neurosteroideas en el sistema nervioso central. Por lo tanto, es un posible candidato para el tratamiento de los pacientes con TCE. Sin embargo, existe incertidumbre con respecto a la eficacia de este tratamiento. Esta es una actualización de la revisión anterior del mismo título, publicada en 2012.
Objetivos
Evaluar los efectos de la progesterona sobre el resultado neurológico, la mortalidad y la discapacidad en pacientes con TCE agudo. Evaluar la seguridad de la progesterona en pacientes con TCE agudo.
Métodos de búsqueda
Se actualizaron las búsquedas en las siguientes bases de datos: el Registro especializado del Grupo Cochrane de Lesiones (Cochrane Injuries Group) (30 de septiembre de 2016), el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; Número 9, 2016), MEDLINE (Ovid; 1950 hasta el 30 de septiembre de 2016), Embase (Ovid; 1980 hasta el 30 de septiembre de 2016), Web of Science Core Collection: Conference Proceedings Citation Index‐Science (CPCI‐S; 1990 a 30 de septiembre de 2016); y registros de ensayos: Clinicaltrials.gov (30 de septiembre de 2016) y en la World Health Organization (WHO) International Clinical Trials Registry Platform (30 de septiembre de 2016).
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados (ECA) de progesterona versus ninguna progesterona (o placebo) para el tratamiento de los pacientes con TCE agudo.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, examinaron los resultados de la búsqueda para identificar estudios potencialmente relevantes para inclusión. De forma independiente, dos autores de la revisión seleccionaron los ensayos que cumplieron con los criterios de inclusión de los resultados de las búsquedas revisadas, sin que hubiera desacuerdos.
Resultados principales
En la revisión se incluyeron cinco ECA con un total de 2392 participantes. Un ensayo se consideró de bajo riesgo de sesgo; dos de riesgo de sesgo incierto (en un ensayo multicéntrico la posibilidad de efectos de los centros no estuvo clara, mientras que el otro ensayo se interrumpió de manera temprana), y dos con alto riesgo de sesgo, debido a problemas de cegamiento e informe selectivo de los datos de resultado.
Todos los estudios incluidos informaron los efectos de la progesterona sobre la mortalidad y la discapacidad. Evidencia de calidad baja no mostró evidencia de una diferencia en la mortalidad general entre el grupo de progesterona y el grupo placebo (RR 0,91; IC del 95%: 0,65 a 1,28; I² = 62%; cinco estudios, 2392 participantes, 2376 agrupados para el análisis). Con el uso de los criterios GRADE, la calidad de la evidencia se consideró baja debido a inconsistencia significativa entre los estudios.
Tampoco hubo evidencia de una diferencia en la discapacidad (resultados desfavorables evaluados por la Glasgow Outcome Score) entre el grupo de progesterona y el grupo placebo (RR 0,98; IC del 95%: 0,89 a 1,06; I² = 37%; cuatro estudios; 2336 participantes, 2260 agrupados para el análisis). La calidad de esta evidencia se consideró moderada, debido a la inconsistencia entre los estudios.
No se dispuso de datos para el metanálisis de los resultados de presión intracraneal media, presión arterial, temperatura corporal ni eventos adversos. Sin embargo, los datos de tres estudios no mostraron diferencias en la presión intracraneal media entre los grupos. Los datos de otro estudio no mostraron evidencia de una diferencia en la presión arterial ni en la temperatura corporal entre los grupos de progesterona y placebo, aunque hubo evidencia de que la infusión de progesterona intravenosa aumentó la frecuencia de flebitis (882 participantes). No hubo evidencia de una diferencia en la tasa de otros eventos adversos entre el tratamiento con progesterona y placebo en los otros tres estudios que informaron los eventos adversos.
Conclusiones de los autores
Esta revisión actualizada no encontró evidencia de que la progesterona pueda reducir la mortalidad ni la discapacidad en los pacientes con TCE. Sin embargo, las inquietudes con respecto a la inconsistencia (heterogeneidad entre los participantes y la intervención utilizada) entre los estudios incluidos reducen la confianza en estos resultados.
No hay evidencia a partir de los datos disponibles de que el tratamiento con progesterona provoque más eventos adversos que placebo, aparte del único estudio con evidencia de un aumento de la flebitis (en el caso de la progesterona intravascular).
No hubo datos suficientes sobre los efectos del tratamiento con progesterona en los otros resultados de interés (presión intracraneal, presión arterial, temperatura corporal) para poder establecer conclusiones firmes.
Los ensayos futuros se beneficiarían de una clasificación más precisa del TCE y de un intento por optimizar la dosis y el régimen de administración de la progesterona.
PICO
Resumen en términos sencillos
Progesterona para el tratamiento del traumatismo craneoencefálico
Pregunta de la revisión
Determinar si el uso de la hormona progesterona para el tratamiento de las personas que han tenido un traumatismo de cráneo que causó daño cerebral (traumatismo craneoencefálico [TCE]) es útil y seguro, cuando se administra dentro de las 24 horas posteriores a la lesión.
Antecedentes
El TCE es una de las principales causas de mortalidad y discapacidad en las personas que sufren lesiones. El daño cerebral puede comenzar en el momento de la lesión, aunque también puede continuar días después de producida. La progesterona es una hormona que según algunos médicos se podría utilizar para reducir el daño cerebral si se administra poco después del TCE. Sin embargo, es importante evaluar la evidencia debido a que hay incertidumbre acerca de la efectividad de esta hormona.
Características de los estudios
Se realizaron búsquedas amplias en la bibliografía médica de ensayos controlados aleatorizados que investigaron los efectos de la progesterona en los pacientes con TCE hasta el 30 de septiembre de 2016. Los ensayos controlados aleatorizados aportan la evidencia médica más sólida.
Resultados clave
Se incluyeron cinco estudios con un total de 2392 participantes y se identificaron tres estudios en curso. Los estudios compararon un grupo de participantes que recibieron progesterona en el transcurso de las 24 horas posteriores al TCE con un grupo que recibió un fármaco sin efectos o inactivo (conocido como placebo) con la misma apariencia de la progesterona.
Los resultados de la revisión no encontró evidencia de que, en comparación con placebo, la progesterona podría reducir la muerte y la discapacidad en los pacientes con TCE. Hubo muy pocos datos disponibles de los otros resultados de interés (presión dentro del cráneo [presión intracraneal], presión arterial, temperatura corporal y eventos adversos [efectos perjudiciales]) para poder analizarlos en detalle. Sin embargo, aunque la información disponible no muestra evidencia de una diferencia en el efecto entre el grupo de progesterona y el grupo control en la presión intracraneal, la presión arterial ni la temperatura corporal, un estudio mostró un aumento en el nivel de un evento adverso llamado flebitis (inflamación de una vena) en el grupo de progesterona, posiblemente porque la progesterona se administró por vía intravenosa como infusión intravascular (“goteo”).
Calidad de la evidencia
Se consideró que la calidad de la evidencia fue baja para los datos sobre el riesgo de muerte y moderada para los datos sobre el riesgo de discapacidad. Estas valoraciones resultan de las diferencias entre los estudios, incluidas diferentes dosis de progesterona y diferentes puntos temporales para la evaluación de los participantes en los estudios incluidos. Esto significa que la confianza en las conclusiones de esta revisión es limitada.
Authors' conclusions
Summary of findings
| Progesterone compared with no progesterone or placebo for traumatic brain injury | ||||||
| Patient or population: people with acute TBI secondary to head injury Settings: hospitals, intensive care units Intervention: progesterone therapy Comparison: no progesterone or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Progesterone | |||||
| Mortality at end of scheduled follow‐up | 192 per 1000 | 175 per 1000 (125 to 246) | RR 0.91 (0.65 to 1.28) | 2376 | ⊕⊕⊝⊝ | There is no evidence of a reduction of mortality at the end of scheduled follow‐up as a result of progesterone therapy. Our confidence in this evidence is limited as we have assessed it as low quality. |
| Disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1‐3) at end of scheduled follow‐up | 533 per 1000 | 522 per 1000 (474 to 565) | RR 0.98 (0.89 to 1.06) | 2260 (4 studies) | ⊕⊕⊕⊝ | There is no evidence of a difference in disability (unfavourable outcomes) at the end of scheduled follow‐up as a result of progesterone therapy. Our confidence in this evidence is somewhat limited as we have assessed it as moderate quality. |
| Intracranial pressure (ICP) within the treatment period | ‐ | ‐ | ‐ | 3 studies | ‐ | In Xiao 2008, ICP data were presented as mean values. In Wright 2006, ICP data were presented as the mean frequency of pressures exceeding threshold values. In Skolnick 2014, ICP data were presented as the population with increased ICP. We were therefore not able to perform meta‐analysis. There was no evidence that progesterone therapy has an effect on ICP. |
| Blood pressure | ‐ | ‐ | ‐ | 1 study | ‐ | "Throughout the three‐day infusion interval, there was no difference between the progesterone and placebo groups" (Wright 2006) |
| Body temperature | ‐ | ‐ | ‐ | 1 study | ‐ | "Progesterone group experienced a lower increase in mean temperature than the control group" (Wright 2006) |
| Adverse events | ‐ | ‐ | ‐ | 4 studies | ‐ | Wright 2014 reported that phlebitis or thrombophlebitis was significantly more frequent in the progesterone group than in the placebo group (882 cases, RR 3.03; 95% CI, 1.96 to 4.66). The rates of other serious and non‐serious adverse events were similar in the 4 studies. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We judged the overall risk of bias of two studies as high, and two studies as unclear. However, most data were from studies at low or unclear risk of bias, so we did not downgrade for risk of bias. 2. Downgraded once for inconsistency (substantial heterogeneity: I² = 62%, P value 0.03) 3. Downgraded once for inconsistency (the dosage, treatment routine and vehicles of progesterone varied across studies. Different time points were involved in the analysis of mortality and unfavourable outcomes). | ||||||
Background
Description of the condition
Traumatic brain injury (TBI) is one of the main causes of death and disability in people with injuries (Ghajar 2000). Globally, at least 10 million people are killed or hospitalised every year because of TBI (Hyder 2007). The cost to society of TBI is considerable. In the USA, it is estimated that the cost of acute treatment and rehabilitation for patients with brain injury is around two billion dollars (USD) per year (McGarry 2002), not including indirect costs to families and society. The identification of effective, inexpensive and widely practicable treatments for brain injury is of great public health importance.
Although much of the neurological damage after TBI is caused at the time of the injury, secondary brain damage caused by mechanisms such as brain oedema, free radical formation or release of inflammatory mediators may exacerbate the primary injury. Severe injury sets in motion a cascade of events over several hours that can lead to secondary damage or death of brain tissue. To date, no pharmacologic agent has been shown to improve outcomes of TBI (Gultekin 2016). Methylprednisolone, once considered a mainstay of treatment, has been shown to be harmful (Alderson 2008), and there is no evidence to support the use of magnesium in patients with acute TBI (Arango 2008). It is important to search for safe and clinically effective neuroprotective drugs to prevent secondary brain damage after TBI, and progesterone has several features that make it an attractive candidate.
Description of the intervention
Progesterone, a hormone which is both widely available and inexpensive, has steroidal, neuroactive and neurosteroidal actions in the central nervous system. The pharmacokinetics of progesterone are well known, as the drug has been safely used for a long time (Allolio 1995; Goldfien 1989). Progesterone is present in the brains of men and women in small, roughly equal concentrations. Progesterone receptors are widely distributed throughout the central nervous system (Schumacher 1995). Although progesterone's non‐neurologic effects are well known, the steroid also has neuroprotective properties (Singh 2013). At the preclinical level, there is increasing evidence that progesterone could produce beneficial effects in brain and spinal cord injuries (Brotfain 2016), stroke (Yousuf 2016), brain haemorrhage (Hsieh 2016), and neurodegenerative diseases (De 2013).
How the intervention might work
A great number of preclinical studies have reported a therapeutic effect of progesterone in the central nervous system. Progesterone is thought to decrease brain oedema and help to maintain the integrity of the blood‐brain barrier (He 2014; Soltani 2016; Wang 2013), prevent apoptosis and necrosis (Li 2015; Yousuf 2016), reduce excitotoxicity by lessening the effect of neuroinflammation, reduce oxidative stress and alter glutamate receptor activity (Hong 2016; Luoma 2011; Webster 2015), and improve motor, sensory and cognitive recovery (Geddes 2016; Stein 2008; Wali 2016). However, so far, none of these encouraging preclinical results have led to evidence of any considerable improvement in clinical outcomes.
Why it is important to do this review
The limited evidence from the last version of our review published in 2012 revealed that progesterone might improve the neurologic outcome of acute TBI patients (Ma 2012). However, the previous systematic review has become outdated as the results of two recent phase III randomised controlled trials (RCTs) have now been published (Skolnick 2014; Wright 2014). An updated review was needed to provide a comprehensive assessment of the best available evidence of the safety and efficacy of progesterone treatment for acute TBI.
This updated systematic review of RCTs aims to quantify the evidence for the effects of progesterone administration on people with TBI. Because of the high incidence of TBI and its excessive cost each year, even a modest reduction in the risk of unfavourable outcomes could have major public health significance.
Objectives
To assess the effects of progesterone on neurologic outcome, mortality and disability in patients with acute TBI. To assess the safety of progesterone in patients with acute TBI.
Methods
Criteria for considering studies for this review
Types of studies
We included published randomised controlled trials (RCTs) of progesterone versus no progesterone (or placebo) for the treatment of acute TBI. In an attempt to improve the quality of our updated systematic review, we excluded non‐registered studies for which the study report was published after 2010 (Roberts 2015).
Types of participants
People, of any age, with clinically diagnosed with acute TBI secondary to head injury. All severities of head injury were included.
Types of interventions
Progesterone versus no progesterone or placebo, administered in any dose, by any route, for any duration and started within 24 hours of the head injury.
We only considered natural progesterone as the intervention. Synthetic progestin has different effects to natural progesterone in postinjury treatment. Consequently, we did not include the following compounds as interventions: medroxyprogesterone, megestrol acetate, chlormadinone, hydroxyprogesterone, norethindrone, norgestrel nor norethynodrel (Stein 2008).
Types of outcome measures
Primary outcomes
-
Mortality at end of scheduled follow‐up
-
Disability at end of scheduled follow‐up: assessed via the Glasgow Outcome Scale (GOS) and any other validated measures of neurological functioning and disability
-
Intracranial pressure within the treatment period
Secondary outcomes
-
Blood pressure
-
Body temperature
-
Complications and adverse events, including liver function abnormality, episodes of venous or arterial thromboembolism, allergic reactions, phlebitis
To determine the optimal information size we assumed a 20% control event rate (mortality) and a 25% relative risk reduction with 90% power and a 0.05 significance level. Our calculations indicated that the optimal information size needed to reliably detect a plausible treatment effect in mortality is 1212 patients in each group. For the disability outcome (GOS 1 to 3), we assumed a 50% control event and a 25% relative risk reduction with 90% power and a 0.05 significance level. The optimal information size needed to reliably detect a plausible treatment effect in disability is 329 patients in each group.
Search methods for identification of studies
The searches were not restricted by date, language or publication status.
Electronic searches
The Cochrane Injuries Group's Information Specialist updated searches of the following electronic databases and trials registries (2012 to date):
-
Cochrane Injuries Group's Specialised Register (30 September 2016);
-
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 9, 2016, via Cochrane Register of Studies (CRSO));
-
MEDLINE (Ovid) (1950 to 30 September 2016);
-
Embase (Ovid) (1980 to 30 September 2016);
-
Web of Science Core Collection: Conference Proceedings Citation Index‐Science (CPCI‐S; 1990 to 30 September 2016);
-
Clinicaltrials.gov (30 September 2016);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (30 September 2016).
The full search strategies are presented in (Appendix 1).
Strategies for earlier searches (conducted in August 2012) are presented in (Appendix 2).
Data collection and analysis
Selection of studies
Two review authors (JM and YZ) independently screened the titles and abstracts of all citations found through the searches and decided whether or not the articles were potentially eligible for inclusion in the review. We obtained the full texts of all potentially relevant articles and the two review authors (JM and YZ) independently assessed whether each met the predefined inclusion criteria. We excluded any studies that did not fulfil the inclusion criteria, and the reasons for exclusion are noted in the 'Characteristics of excluded studies' table. We resolved any disagreement by discussion or arbitration with SH. We examined all duplicate study reports to verify that they presented unique sets of data.
Data extraction and management
Two review authors (JM and YZ) independently extracted data from the included studies on sequence generation, allocation concealment, loss to follow‐up, blinding of outcome assessment, types of participants, types of interventions, types of outcomes, methods of analysis (intention‐to‐treat (ITT) analysis or per protocol analysis, or both), comparability of groups at baseline, statistical methods used and source of study funding. Where necessary, we requested unpublished information from the study authors. We extracted data to allow an ITT method. For studies with a `modified ITT' method, if we had considered the reasons for exclusion of participants to be inappropriate and the data were available to the review author, we would have conducted analyses that include participants who were excluded by the study authors. We compared the data extracted by each author and resolved any disagreement by discussion or arbitration with SH. Data extracted are noted in the 'Characteristics of included studies' table.
Assessment of risk of bias in included studies
Two review authors (JM and YZ) assessed RCTs using the 'Risk of bias' assessment tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Each review author independently evaluated risk of bias through assessing: sequence generation, allocation concealment, blinding (assessments were made for each main outcome or class of outcomes), incomplete outcome data (assessments were made for each main outcome or class of outcomes), selective outcome reporting and other sources of bias. We resolved any disagreement through discussion or arbitration from SH. We came to a judgement relating to the risk of bias for each domain and we also categorised the overall risk of bias of individual studiesas being at: low, high or unclear risk of bias as follows (Higgins 2011):
-
low risk of bias (i.e. plausible bias unlikely to alter the results seriously) if all domains were at low risk of bias; ·
-
unclear risk of bias (i.e. plausible bias that raises some doubt about the results) if one or more domains had an unclear risk of bias;
-
high risk of bias (i.e. plausible bias that seriously weakens confidence in the results) if one or more domains were at high risk of bias.
Measures of treatment effect
We calculated the risk ratio (RR) and 95% confidence interval (CI) for mortality. We split GOS data into favourable (moderate disability, good recovery; GOS scores 4 and 5) and unfavourable outcomes (death, vegetative state, severe disability; GOS scores 1 to 3). We would have split other validated functional outcome data into favourable (modified Rankin Scale score<3, Barthel Index>60, etc) and unfavourable outcomes (modified Rankin Scale score graded 3 to 6 and Barthel Index 0 to 60,etc).These were also calculated by RR and 95% CI. For continuous outcomes such as intracranial pressure, body temperature, and blood pressure, we would have used arithmetic means and standard deviations for each group.
Dealing with missing data
We contacted study authors to obtain missing information. When the missing data were unavailable, we included data on only those particpants whose results were known to generate the outcome and considered the potential impact of the missing data during the interpretation of the results.
Assessment of heterogeneity
We assessed clinical heterogeneity between comparable trials by examining the participants, interventions and outcomes of the trials. In addition, we used visual inspections of graphs to assess heterogeneity. Statistical heterogeneity was examined with the I² statistic and Chi² test. Substantial heterogeneity was considered to exist when I² exceeded 60% and the Chi² test P value was less than 0.1.
Assessment of reporting biases
We planned to assess reporting bias by funnel plots and linear regression tests, however, there were too few included studies to enable meaningful analysis. We will assess reporting biases in future updates if there are 10 or more studies included in the meta‐analysis.
Data synthesis
We calculated the RRs and 95% CIs via a fixed‐effect model and conducted a meta‐analysis for dichotomous outcomes, if we judged that the included trials were clinically and statistically homogeneous. We employed the random‐effects model to pool studies when statistical heterogeneity occurred. For continuous data, we would have calculated mean differences (MD) or standardised mean differences (SMD) with 95% CI. We assessed possible sources of heterogeneity by subgroup and sensitivity analyses. We used Review Manager 2014 software, version 5.3 for all analyses.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analysis by severity of brain injury:
severe TBI subgroup (Glasgow Coma Scale (GCS) ≤ 8) and moderate TBI subgroup (GCS 9 to 12).
Sensitivity analysis
We undertook sensitivity analyses to assess the robustness of the results, by excluding studies with an unclear or high risk of bias for allocation concealment.
Summarising findings and assessing the quality of the evidence
We developed a 'Summary of findings' table to present the results of this review. We included all outcomes: mortality, disability, intracranial pressure; blood pressure, body temperature, and complications and adverse events.
Where possible, we assessed the quality of the evidence for our effect estimates using the GRADE methods to account for the overall risk of bias of the included studies, inconsistency of the results, indirectness of the evidence, precision of the estimates and the risk of publication bias (GRADE 2004). This was done independently and in duplicate by JM and YZ. We rated the quality of the evidence as high, moderate, low or very low.
Results
Description of studies
We identified five completed studies that satisfied the inclusion criteria using the process described in Figure 1 (Skolnick 2014; Wright 2006; Wright 2014; Xiao 2007; Xiao 2008). All trials eligible for inclusion compared progesterone therapy with a control group. There were also three ongoing trials (IRCT2014042017356N1; CTRI/2009/091/000893, CTRI/2013/02/003396), which are detailed in Characteristics of ongoing studies.
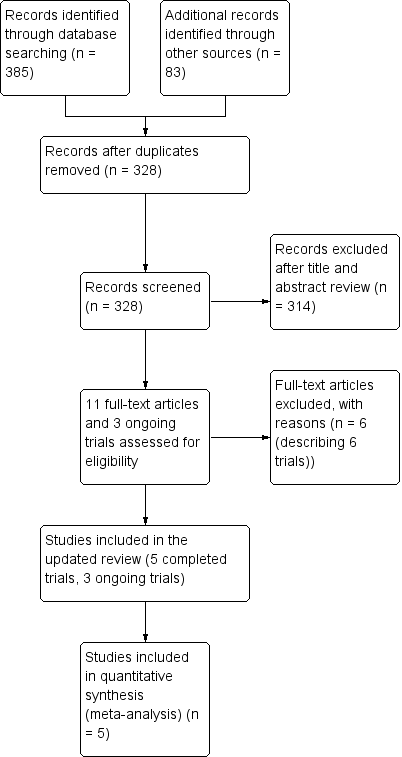
Study flow diagram.
Included studies
Full descriptions of all the included studies can be found in the Characteristics of included studies.
The five included studies had a total of 2392 participants; 1195 in Skolnick 2014;100 in Wright 2006; 882 in Wright 2014; 56 in Xiao 2007; and 159 in Xiao 2008.
Skolnick 2014: was a Mmulticenter phase III RCT that randomly assigned participants. Patients, (16 to 70 years of age, with severe TBI (GCS ≤ 8 and at least one reactive pupil)) were randomly assigned to receive progesterone or placebo. The modified ITT population excluded 16 participants (6 participants in the progesterone group and 10 in the placebo group) because they did not receive any study drug. Drug infusion (progesterone andor placebo) was started intravenously within 8eight hours afterof injury with a loading dose of 0.71 mg/kg/hour for one hour, followed by 0.50 mg/kg/hour for 119 hours.
Wright 2006 was a phase II RCT that recuited adults with acute severe TBI and a GCS score of 4 to 12 after resuscitation, and stabilisation within 11 hours of injury. Participants were assigned to eight clinical subgroups, then permuted block randomisation assigned four of every five consecutive patients to progesterone and the other to placebo. A 4:1 randomisation scheme was used to increase the number of patients receiving progesterone while maintaining blinding. The progesterone group received an infusion of 0.71 mg/kg of progesterone at 14 mL/h for the first hour and then an infusion of 0.5 mg/kg progesterone in 10 mL/h for the next 11 hours. Five additional 12‐hour maintenance infusions were delivered at the standard rate of 10 mL/hour, for a total of three days of treatment; the control group received placebo.
Wright 2014: was a Mmulticenter phase III RCT that recruited a. Adults patients with brain injury (GCS score of 4 to 12) were enrolled if the study treatment could be initiated within 4four hours afterof the injury. The study drug (progesterone or placebo) was infused continuously through a dedicated intravenous catheter at a dose of 14.3 mlL/h per hour for 1one hour and then at 10 mL/l per hour for 71 hours; the dose was then tapered by 2.5 mlL/ per hour every 8eight hours, for a total treatment duration of 96 hours.
Xiao 2007 recruited patients aged 15 to 65 years with severe TBI (GCS 5 to 8) after the time of injury, and randomised them according to a random number table. The progesterone group received 80 mg progesterone via intramuscular injection once every 12 hours for five consecutive days.
Xiao 2008 recruited adults with acute severe TBI and a GCS score ≤ 8 after resuscitation, and stabilisation within eight hours of injury. Participants were randomised according to a random number table. The progesterone group received a dose of progesterone of 1.0 mg/kg via intramuscular injection within eight hours of the time of injury, and then every 12 hours for five consecutive days. The control group received placebo.
Excluded studies
We excluded a total of six studies (Abokhabar 2012; Aminmansour 2012; Mofid 2016; Raheja 2016; Shakeri 2013; Wright 2005).
We excluded three studies because they were not prospectively registered and their reports were published after 2010. These included Aminmansour 2012 and Abokhabar 2012 for which we were unable to locate registration or protocols. Mofid 2016 claimed it was a prospective, single‐blind RCT performed from May 2013 to July 2015. However, we found the study protocol was approved by ethics committee of Kerman University of Medical Sciences in April 2014 and registered in the Iranian Registry of Clinical Trials in August 2014 (www.irct.ir, CT2014042017356N1). Because of this delay in trial registration, we judged that the study had not been prospectively registered.
We excluded two studies because they used biochemical outcome measures and did not address clinical outcomes. Wright 2005 evaluated the pharmacokinetics of progesterone given by intravenous infusion to people with TBI, while Raheja 2016 was part of a prospective blinded, randomized, placebo‐controlled phase II trial of progesterone with or without hypothermia (CTRI/2009/091/000893), which focused on the relationship between serum biomarkers and outcomes after TBI; the study report did not include the effect of progesterone on TBI.
We excluded Shakeri 2013 because medroxyprogesterone was used as the intervention rather than progesterone.
Risk of bias in included studies
Our assessments of the risk of bias in the included studies are recorded in the 'Risk of bias' tables, and are displayed in Figure 2 and Figure 3. The overall risk of bias of individual studies was judged as high for Xiao 2007 and Xiao 2008, unclear for Wright 2014 and Skolnick 2014, and low for Wright 2006.
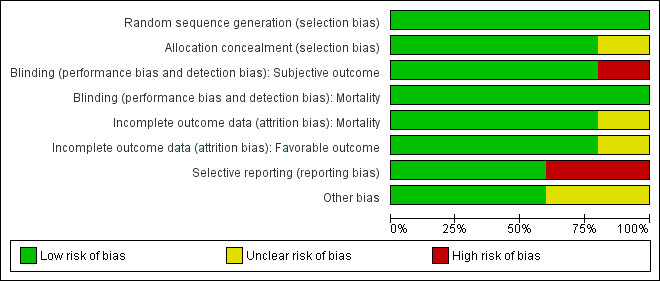
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Five studies are included in this review.
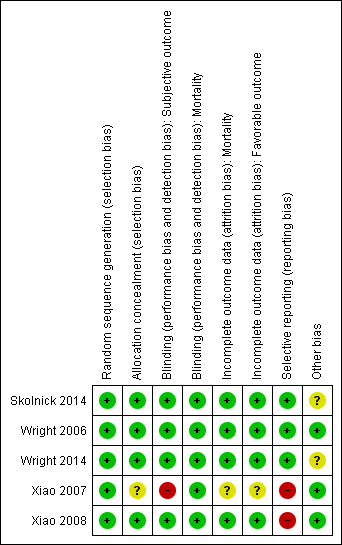
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Wright 2006 assigned four of every five consecutive participants to progesterone and the fifth to placebo via permuted block randomisation.
In Xiao 2007 and Xiao 2008, participants were allocated according to a random number table.
In Wright 2014, randomisation was performed with the use of a combination of minimisation and biased‐coin algorithms.
In Skolnick 2014, randomisation was implemented with the use of an interactive web‐based response system.
The risk of bias for this domain was judged to be low for all five studies.
Allocation concealment
In Wright 2006, Xiao 2008, Wright 2014 and Skolnick 2014, the appearance, packaging and administration of placebo and progesterone injections were the same for the two groups. Xiao 2007 did not describe the methods used for allocation concealment sufficiently for us to determine the risk of bias for this domain. We therefore assessed this study as being at unclear risk of bias.
The risk of bias for this domain was judged as low for Wright 2006, Xiao 2008, Wright 2014 and Skolnick 2014, and unclear for Xiao 2007.
Blinding
Blinding (GOS and other objective outcomes)
Wright 2006, Xiao 2008, Skolnick 2014 and Wright 2014 mentioned `double blinding', and achieved blinding by use of indistinguishable drug kits. We judged the risk of bias for this domain as low for these trials.
In the trial by Xiao 2007, there was no blinding and the outcome measurement was likely to be influenced by lack of blinding. We judged the risk of bias for this domain as high.
Blinding (mortality)
We decided that for the outcome of mortality, the outcome and its measurement were not likely to have been influenced by blinding. So we judged the risk of bias for this domain to be low for all included studies.
Incomplete outcome data
In Wright 2006, with the exception of three people who discontinued treatment (two died during infusion, one was taken into police custody), there were no withdrawals, dropouts, or losses to follow‐up by the end of the follow‐up period. In Xiao 2008, there were two withdrawals, three dropouts and 19 losses to follow‐up at the end of the follow‐up period. In Skolnick 2014, a total of 31 participants (17 in the progesterone group and 14 in the placebo group) were lost to follow‐up. Skolnick 2014 used a modified ITT population and excluded 16 participants (six in the progesterone group and 10 in the placebo group) because they did not receive any study drug. This method was described previously in its protocol, so we did not consider these exclusions were associated with industry funding or authors' conflicts of interest. In Wright 2014, 28 participants (6.3%) in progesterone group and 24 (5.5%) in placebo group were missing. Missing outcome data was balanced in numbers between progesterone group and placebo group in all of the above four studies. Attrition or exclusion of participants was not reported in Xiao 2007.
We judged the risk of bias for this domain as low for Wright 2006, Xiao 2008, Skolnick 2014 and Wright 2014, and as unclear for Xiao 2007.
Selective reporting
The protocols for Wright 2006, Wright 2014 and Skolnick 2014 were presented in Clinicaltrials.gov. It was clear that these published reports included all expected outcomes, so we judged the risk of bias for this domain as low for Wright 2006, Wright 2014 and Skolnick 2014.
Xiao 2007 and Xiao 2008 were registered in the Australian New Zealand Clinical Trials Registry (ACTRN12607000545460) and the Chinese Clinical Trial Register (ChiCTR‐TRC‐08000174), but this was done retrospectively. The dates of registration postdated the ends of the trials. We did not exclude these two trials because they were published before 2010 (Roberts 2015). We judged the risk of bias for selective reporting as high for these two trials.
Other potential sources of bias
Source of funding
Wright 2006 and Wright 2014 were supported by a grant from the National Institute for Neurological Disorders and Stroke, National Institutes of Health, USA.
Xiao 2007 and Xiao 2008 were supported by the Scientific Research Fund of Zhejiang Provincial Education Department, China.
Skolnick 2014 was funded by BHR Pharma, UK.
Stopping a trial early
The Wright 2014 trial intended to enrol 1140 participants, but the trial was abandoned after 882 people had been assessed. We did not consider reporting bias, because there was no selective revealing or suppression of the study information. After the second interim analysis, the trial was stopped because of futility (favourable outcomes were observed in 51% of participants who received progesterone after TBI, compared with 55.5% of controls. Stratification of the participants on the basis of injury severity did not reveal any effect of progesterone on recovery). We therefore assessed the risk of other bias for Wright 2014 as unclear.
Centre effects in multicenter RCTs
Skolnick 2014 was conducted in approximately 100 centres in 21 countries. The number of mortality or functional outcome events in each centre was quite low. We assessed potential bias from variation between‐centres as unclear because of factors such as different levels of expertise in treating TBI and outcome assessment.
Wright 2014 was conducted in 22 academic medical centres through the National Institute of Neurological Disorders and Stroke‐funded Neurological Emergencies Treatment Trials network in the USA. We considered the potential bias from centre effects in this trial to be low.
Effects of interventions
See: Summary of findings for the main comparison
Mortality
Overall mortality was evaluated in all five trials at the end of follow‐up (i.e. 30 days postinjury in Wright 2006, three months postinjury in Xiao 2007, and six months postinjury in Xiao 2008, Wright 2014 and Skolnick 2014).There was no evidence of a difference in mortality between the treatment and placebo groups. We assessed the quality of this evidence to be low (see summary of findings Table for the main comparison).
The ITT analysis included 2376 participants pooled for meta‐analysis. The pooled RR of death at the end of follow‐up was 0.91 (95% CI 0.65 to 1.28, P value 0.60). We used a random‐effects model as there was substantial heterogeneity (Tau² = 0.08; Chi² = 10.40, df = 4 (P = 0.03); I² = 62%; Analysis 1.1).
Disablity
All the included studies reported disability data assessed by GOS score. GOS data in Wright 2006 , Xiao 2008, Wright 2014 and Skolnick 2014 were sufficient to be dichotomised into favourable outcomes (moderate disability, good recovery; GOS 4 and 5) and unfavourable outcomes (sometimes referred to as unfavourable functional recovery: i.e. death, vegetative state, severe disability; GOS 1 to 3). Only Xiao 2007 reported the mean GOS at three months postinjury, and these data were insufficient to dichotomise into favourable and unfavourable outcomes.
We pooled unfavourable outcomes (death, vegetative state, severe disability; GOS 1 to 3) for four trials at the end of follow‐up (i.e. 30 days postinjury in Wright 2006, six months postinjury in Xiao 2008, Skolnick 2014 and Wright 2014). There was no evidence of a difference between the treatment and placebo groups and no substantial heterogeneity (RR = 0.98, 95% CI 0.89 to 1.06, P value 0.58; Chi² = 4.77, df = 3 (P = 0.19); I² = 37%; Analysis 1.2). We assessed the quality of this evidence to be moderate (see summary of findings Table for the main comparison).
In Wright 2006, disability in survivors was also assessed by the Disability Rating Score (DRS) at 30 days postinjury. Survivors with severe TBI in the placebo group were slightly less likely to be disabled than those in the progesterone group (DRS = 10.7, 95% CI 8.3 to ‐13.1 for progesterone‐treated participants versus DRS = 4.4, 95% CI 0.0 to 9.8 for placebo‐treated participants). The authors explained that a higher proportion of severely injured participants treated with progesterone survived, so survivors in the progesterone group may have been slightly more disabled. In the moderate TBI stratum, participants treated with progesterone were significantly less disabled than those who received placebo (DRS = 5.0, 95% CI 1.8 to 6.2 for progesterone‐treated participants versus DRS = 12.7, 95% CI 7.6 to 17.78 for placebo‐treated participants). The authors did not report a test for interaction between the high and moderate brain injury strata.
Xiao 2007 and Xiao 2008 presented data showing reduced disability in the progesterone therapy group relative to the control. However, these were the small studies and assessed to be at high overall risk of bias. We do not consider these data to outweigh the evidence from the much larger meta‐analysis. In Xiao 2007, disability data were assessed using GOS score, Karnofsky Performance Scale (KPS), verbal and motor function at three months postinjury. For GOS, a higher score equates to less disability, whilst for KPS, verbal and motor function scores, a lower score equates to lower disability. All of these data were presented as means and standard deviations in a table as follows:
-
the GOS score in the progesterone group was 5.0 ± 1.7 and in the control group was 4.0 ± 1.9, P < 0.05
-
the KPS score in the progesterone group was 4.9 ± 1.2 and in the control group was 4.0 ± 1.1, P < 0.05;
-
verbal function in the progesterone group was 3.1 ± 0.4 and in the control group was 2.3 ± 0.7, P < 0.05;
-
motor function in the progesterone group was 2.4 ± 0.7 and in the control group was 2.4 ± 0.4, P > 0.05.
No other details about these data were reported.
In Xiao 2008, disability was also assessed by Modified Functional Independence Measure (FIM) scores, where a higher score equates to less disability. At the three‐month follow‐up, the scores were 7.35 ± 1.89 for the placebo group and 8.02 ± 1.73 for the progesterone group (P < 0.05). At six months after injury, the scores were 8.95 ± 1.05 for the placebo group and 9.87 ± 1.17 (P < 0.01) for the progesterone group.
Intracranial pressure (ICP)
Wright 2006, Xiao 2008 and Skolnick 2014 reported intracranial pressure (ICP) data, but we were not able to perform meta‐analysis due to variations in the way the data were presented. Xiao 2008 presented ICP data as mean values, while Wright 2006 presented them as the mean frequency of pressures exceeding threshold values, and Skolnick 2014 presented them as the proportion with increased ICP.
In Wright 2006, the mean ICP level of the progesterone group remained stable, whereas that of the control group tended to increase during the first three days of treatment and for one day after treatment However, this trend was not significant. The mean ICP‐therapeutic intensity level scores presented did not differ between groups.
In Xiao 2008, there was no evidence of a difference between the two groups for mean ICP at 24 hours after trauma (progesterone group, 22.1 ± 4.3 mmHg versus placebo group, 23.2 ± 4.6 mmHg; P value 0.121). At 72 hours and at seven days after injury, there was still no evidence of a difference in the mean ICP of participants who were given progesterone and participants who received placebo (16.9 ± 3.8 mmHg and 14.8 ± 3.8 mmHg for progesterone treated participants versus 18.2 ± 5.1 mmHg and 15.9 ± 4.1 mmHg for placebo‐treated participants, respectively; P > 0.05).
In Skolnick 2014, the ICP of 130 participants in the progesterone group increased, as did the ICP of 137 participants in the placebo group. There was no evidence of a difference between the two groups.
Body temperature
No data for body temperature were available for meta‐analysis.
Wright 2006 collected detailed data on body temperature. Throughout the three‐day infusion period, the progesterone group experienced a lower increase in mean temperature than the control group; this was determined through analysis of a treatment‐by‐time interaction term for progesterone versus control participants, with had a slope of 0.0055 (95% CI ‐0.010 to ‐0.001).
Blood pressure
No data for blood pressure were available for meta‐analysis.
Wright 2006 collected detailed data on blood pressure. Throughout the three‐day infusion interval, there was no evidence of a difference between the progesterone and placebo groups.
Adverse events
A pooled analysis was not appropriate for adverse event data, so we have presented the data narratively.
Wright 2006 reported serious adverse events and adverse event rates for the progesterone‐treated group and the placebo‐treated group. There was no evidence of a difference in the rates of adverse and serious adverse events between groups. The only adverse event attributed to progesterone was a case of superficial phlebitis at the intravenous site.
Xiao 2008 did not report the specifics of any adverse events in either group, but did report that there were no additional adverse events after administration of progesterone and no further late toxicity up to six months.
Wright 2014 reported the rates of all reported adverse events and serious adverse events. Only phlebitis or thrombophlebitis was reported to be significantly more frequent in the progesterone group than in the placebo group (882 cases, RR 3.03; 95% CI, 1.96 to 4.66).
Skolnick 2014 recorded adverse events for the first 15 days, and serious adverse events were recorded throughout the duration of the study. There was no evidence of a difference in the rate of adverse events between the progesterone and placebo groups.
Subgroup analysis (severity of TBI)
The Wright 2006 and Wright 2014 trials involved 281 participants with moderate TBI and 701 participants with severe TBI. The Xiao 2007, Xiao 2008 and Skolnick 2014 studies enrolled a total of 1410 participants with severe TBI, but did not enrol people with moderate TBI. When we limited our analysis to the severe TBI subgroup or moderate TBI subgroup, there were minimal changes in the results.
In the severe TBI subgroup (GCS ≤ 8), the pooled RR for mortality at the end of follow‐up was 0.87 (95% CI 0.60 to 1.27; P value 0.48; 2111 participants, 2090 pooled for meta‐analysis). We used a random‐effects model as there was substantial heterogeneity (Tau² = 0.10; Chi² = 11.60, df = 4 (P value 0.02); I² = 66%). In the moderate TBI subgroup (GCS = 9 to 12) the pooled RR for mortality at the end of follow‐up was 1.30 (95% CI 0.70 to 2.41; P value 0.40; 281 participants, 279 pooled for meta‐analysis).There was no substantial heterogeneity (Chi² = 0.01, df = 1 (P = 0.91); I² = 0%). The test for subgroup differences showed no evidence that progesterone therapy has a differential effect on these two subgroups (P value 0.28) (Analysis 2.1).
The pooled RR in the severe TBI subgroup for death or severe disability (GOS 1 to 3) at the end of follow‐up was 0.99 (95% CI 0.87 to 1.11; P value 0.80; 2055 participants, 2002 pooled for meta‐analysis). There was no substantial heterogeneity (Chi² = 4.25, df = 3 (P value 0.24); I² = 29%). The pooled RR in the moderate group for death or severe disability (GOS 1 to 3) at the end of follow‐up was 0.68 (95% CI 0.34 to 1.37 ; P value 0.28; 281 participants, 258 pooled for meta‐analysis); we used a random‐effects model as there was substantial heterogeneity (Tau² = 0.20; Chi² = 4.41, df = 1 (P = 0.04); I² = 77%). The test for subgroup differences showed no evidence that progesterone therapy had a differential effect on these two subgroups (P value 0.31). (Analysis 2.2).
Sensitivity analysis
When we removed studies that did not report allocation concealment procedures from the analysis, there was still no evidence of a difference in mortality (RR 0.88, 95% CI 0.60 to 1.28) or disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1 to 3) (RR 0.98, 95% CI 0.89 to 1.06) between the progesterone and placebo groups (Analysis 3.1; Analysis 3.2).
Discussion
Summary of main results
We included five completed studies with 2392 participants. The main results are presented in the 'Summary of findings' table (summary of findings Table for the main comparison). The results of the meta‐analyses did not find evidence that progesterone could improve overall mortality (RR 0.91, 95% CI 0.65 to 1.28; P = 0.60, I² = 62%; 2376 participants; low quality evidence) nor disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1 to 3) (RR 0.98, 95% CI 0.89 to 1.06; P = 0.58, I² = 37%; 2260 participants; moderate quality evidence) in participants with TBI. When we performed a subgroup analysis on participants with severe TBI versus moderate TBI, and tested for a difference between subgroups, there was no evidence of a difference between the two groups. When we performed sensitivity analysis and removed the study without adequate allocation concealment procedures from analysis, there were no significant changes to the results.
Data were not available for meta‐analysis for the outcomes of mean intracranial pressure, blood pressure, body temperature and adverse events. However, data from three studies showed no evidence of a difference in mean intracranial pressure among participants in either group. Data from one study showed no evidence of a difference in blood pressure and body temperature between the progesterone and placebo groups. Intravenous progesterone infusion increased the frequency of phlebitis in one trial. There was no evidence of a difference in the rate of other adverse events between progesterone treatment and placebo in the other three studies that looked at adverse events.
Overall completeness and applicability of evidence
Completeness
We were only able to perform meta‐analysis for two of our three primary outcomes (mortality and disability), as ICP was presented in three different formats in the three trials that looked at this outcome. For our secondary outcomes, blood pressure and body temperature were only recorded in one of our five included trials (Wright 2006), which limits the completeness of our results.
We were unable to obtain some information regarding the trial described in Xiao 2007. We judged the allocation concealment as unclear due to a lack of information in the study report. In addition, in this trial the data for GOS score were presented by mean difference and 95% CI in a table. These data were insufficient to calculate the pooled RR for disability. We tried to contact the study authors of Xiao 2007 to request details, but we did not receive a response.
Applicability
In terms of the applicability of our results, TBI patients are highly diverse in terms of aetiology, pathology, function and outcome, which leads to concerns that progesterone may affect the recovery of different TBI patients differentially. This is supported by the fact that although we performed subgroup analysis according to GCS grade, there was still high heterogeneity within these subgroups. This means that the results of the meta‐analyses should be interpreted with caution. We discuss the issue of heterogeneity among TBI patients in more detail under 'Quality of the evidence ‐ Categorisation of TBI'.
Quality of the evidence
According to the GRADE approach to assessing the quality of the evidence in the included studies (summary of findings Table for the main comparison), we classified the quality of the evidence as low for mortality, and moderate for disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1 to 3)).
Although the overall risk of bias of two included studies was judged as high (Xiao 2007; Xiao 2008), and unclear for two studies (Skolnick 2014; Wright 2014), most of the data were from studies that were judged to be at low risk of bias across most domains, with no domains at high risk of bias, so we did not downgrade for risk of bias.
Although most of the trials included in this review were well conducted, several factors did influence our assessment of the quality of the overall body of evidence. Firstly, although subgroup analyses were performed according to the protocol, there was still substantial statistical heterogeneity. Secondly, there were three different time points involved in the analysis of mortality and two different time points involved in the analysis of unfavourable outcomes. Thirdly, the dosage, treatment routine and vehicles of progesterone varied across studies. Finally, we had concerns regarding the heterogeneity among TBI patients, and the fact that progesterone may affect TBI patients differentially.
Time at the end of follow‐up
For the mortality and disability outcome we pooled mortality and GOS scores at the end of the follow‐up in each trial; for Wright 2006 this was 30 days postinjury, for Xiao 2007 this was three months, and for Xiao 2008; Wright 2014; Skolnick 2014 this was six months. We should consider this as an inconsistency that has an impact on our confidence in our conclusions.
Dosage, mode of delivery and schedule of progesterone therapy
The dosage used in Wright 2006, Wright 2014 and Skolnick 2014 was six times that used in Xiao 2008 (approximately 12 mg/kg/day versus 2 mg/kg/day). The schedule and mode of delivery also varied between trials. This may explain the existence of significant heterogeneity. Examination of the pharmacokinetics of progesterone intravenous infusion in demonstrated that stable progesterone concentrations can be achieved rapidly via progesterone infusion following TBI. Unfortunately, none of the studies used allometric scaling to determine the most effective dose for humans based on the preclinical animal research (FDA 2005; Nair 2016), and there was no attempt to optimise either dose or schedule as part of any included study. It is possible that none of the doses in the included RCTs was optimal for improving motor, sensory and memory function according to preclinical dose‐response studies (Stein 2015; Wali 2014).
Categorisation of TBI
TBI is categorised according to GCS score on admission to hospital to estimate the severity of the underlying injury. As we know, TBI is regarded as a very heterogeneous and complex disease, with substantial heterogeneity in the aetiology, pathology, mechanisms, exposure time and outcome. There are obvious limitations to using the GCS for the classification of such a complicated disease (Maas 2012). The diverse mechanisms of TBI, as well as differences in age and gender, also lead to different potential for functional recovery and response to progesterone. Use of a 'one size fits all' approach for these different types of TBI is not appropriate (Meyfroidt 2016). Recently, an increasing number of articles suggest a more individualised classification and subgroup analysis of TBI based on biomarkers, age, gender, and clinical characteristics and imaging findings to identify the appropriate patients for progesterone therapy after acute TBI (Menon 2015). Unfortunately, in this review, we only performed subgroup analysis according to GCS grade due to the lack of inclusion of more detailed data in the included trials. These heterogeneities might explain the dissociation seen in the effect of progesterone between animal models and these human trials.
So far, all pharmacological interventions in TBI have failed, which led to discussion of the problematic neuroprotection end‐points in clinical trials (Gultekin 2016). It appears that the commonly used GOS and GOS‐E (Glasgow Outcome Scale‐extended), which measure global functioning after TBI, do not adequately reflect the patients' neurologic function, especially for specific deficits in behaviour, executive function, memory and emotion that may produce significant disabilities. This limitation can become even more evident when the scores are subjectively dichotomised into `unfavourable' and `favourable' outcomes (Poon 2015; Stein 2015).
Therefore, we consider that the different timescales of outcome measures, differing dosage of progesterone, and heterogeneity of participants, reduce our confidence in the conclusions of this review.
Potential biases in the review process
We have attempted to minimise bias in this review and to be as inclusive as possible. We included five studies, but we expect that there may have been other trials that have been conducted that we were unable to identify.
We excluded three studies that were not prospectively registered and published their reports after 2010 (Aminmansour 2012; Abokhabar 2012; Mofid 2016), in accordance with Roberts 2015. We consider that this will have reduced bias in the review process.
Agreements and disagreements with other studies or reviews
A series of preclinical studies and results of phase II RCTs indicated that progesterone might be an appropriate candidate for the treatment of people suffering from TBI (Stein 2008; Wright 2006; Xiao 2008). However, this updated systematic review did not find evidence that progesterone could provide a beneficial clinical outcome in people with TBI. It is possible that this negative result could be due to some of the problems with trial design that we have discussed under Quality of the evidence.
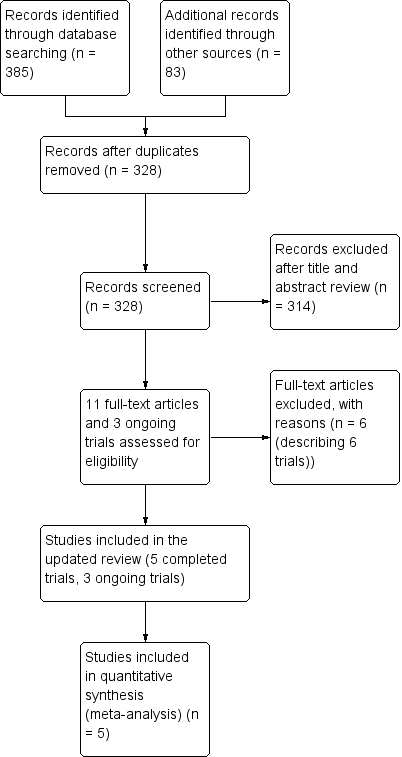
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Five studies are included in this review.
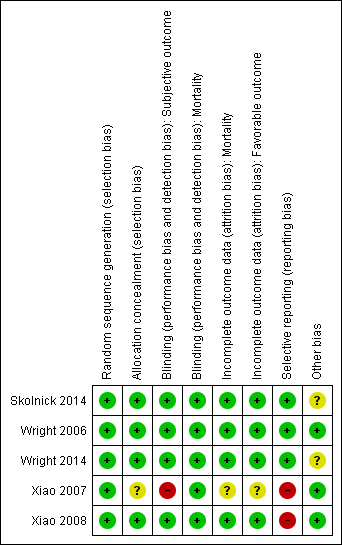
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
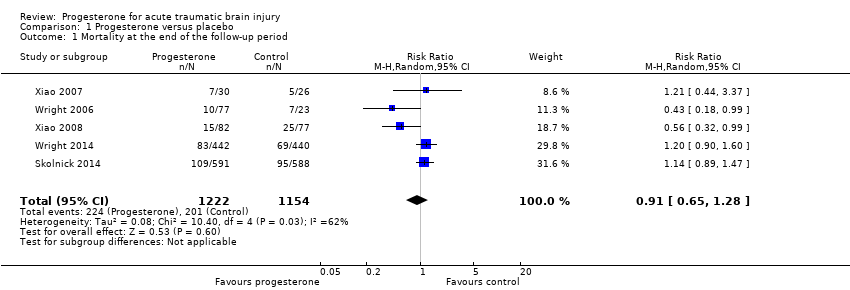
Comparison 1 Progesterone versus placebo, Outcome 1 Mortality at the end of the follow‐up period.

Comparison 1 Progesterone versus placebo, Outcome 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period.

Comparison 2 Subgroup analysis: severe TBI subgroup (GCS ≤ 8), Outcome 1 Mortality at the end of the follow‐up period.
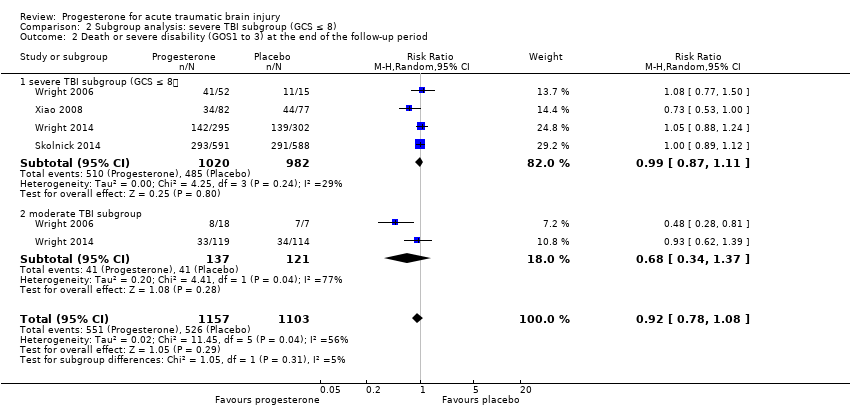
Comparison 2 Subgroup analysis: severe TBI subgroup (GCS ≤ 8), Outcome 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period.

Comparison 3 Adequate allocation concealment (sensitivity analysis), Outcome 1 Mortality at the end of the follow‐up period.

Comparison 3 Adequate allocation concealment (sensitivity analysis), Outcome 2 Death or severe disability (GOS 1 to 3) at the end of the follow‐up period.
| Progesterone compared with no progesterone or placebo for traumatic brain injury | ||||||
| Patient or population: people with acute TBI secondary to head injury Settings: hospitals, intensive care units Intervention: progesterone therapy Comparison: no progesterone or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Progesterone | |||||
| Mortality at end of scheduled follow‐up | 192 per 1000 | 175 per 1000 (125 to 246) | RR 0.91 (0.65 to 1.28) | 2376 | ⊕⊕⊝⊝ | There is no evidence of a reduction of mortality at the end of scheduled follow‐up as a result of progesterone therapy. Our confidence in this evidence is limited as we have assessed it as low quality. |
| Disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1‐3) at end of scheduled follow‐up | 533 per 1000 | 522 per 1000 (474 to 565) | RR 0.98 (0.89 to 1.06) | 2260 (4 studies) | ⊕⊕⊕⊝ | There is no evidence of a difference in disability (unfavourable outcomes) at the end of scheduled follow‐up as a result of progesterone therapy. Our confidence in this evidence is somewhat limited as we have assessed it as moderate quality. |
| Intracranial pressure (ICP) within the treatment period | ‐ | ‐ | ‐ | 3 studies | ‐ | In Xiao 2008, ICP data were presented as mean values. In Wright 2006, ICP data were presented as the mean frequency of pressures exceeding threshold values. In Skolnick 2014, ICP data were presented as the population with increased ICP. We were therefore not able to perform meta‐analysis. There was no evidence that progesterone therapy has an effect on ICP. |
| Blood pressure | ‐ | ‐ | ‐ | 1 study | ‐ | "Throughout the three‐day infusion interval, there was no difference between the progesterone and placebo groups" (Wright 2006) |
| Body temperature | ‐ | ‐ | ‐ | 1 study | ‐ | "Progesterone group experienced a lower increase in mean temperature than the control group" (Wright 2006) |
| Adverse events | ‐ | ‐ | ‐ | 4 studies | ‐ | Wright 2014 reported that phlebitis or thrombophlebitis was significantly more frequent in the progesterone group than in the placebo group (882 cases, RR 3.03; 95% CI, 1.96 to 4.66). The rates of other serious and non‐serious adverse events were similar in the 4 studies. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We judged the overall risk of bias of two studies as high, and two studies as unclear. However, most data were from studies at low or unclear risk of bias, so we did not downgrade for risk of bias. 2. Downgraded once for inconsistency (substantial heterogeneity: I² = 62%, P value 0.03) 3. Downgraded once for inconsistency (the dosage, treatment routine and vehicles of progesterone varied across studies. Different time points were involved in the analysis of mortality and unfavourable outcomes). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at the end of the follow‐up period Show forest plot | 5 | 2376 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.28] |
| 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period Show forest plot | 4 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at the end of the follow‐up period Show forest plot | 5 | 2369 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.29] |
| 1.1 severe TBI subgroup (GCS ≤ 8) | 5 | 2090 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.27] |
| 1.2 moderate TBI subgroup (GCS 9 to 12) | 2 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.70, 2.41] |
| 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period Show forest plot | 4 | 2260 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.08] |
| 2.1 severe TBI subgroup (GCS ≤ 8) | 4 | 2002 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.11] |
| 2.2 moderate TBI subgroup | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.34, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at the end of the follow‐up period Show forest plot | 4 | 2320 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.60, 1.28] |
| 2 Death or severe disability (GOS 1 to 3) at the end of the follow‐up period Show forest plot | 4 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.06] |

