겸상적혈구병 환자의 다리궤양 치료중재
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Desing trial: parallel (2 groups). | |
| Participants | Total: 30. Reported leg ulcers at the start of the trial: 40 (data supplied by Dr Graham Serjeant, Jamaica, February 4, 2011). Age: median (range). Antibiotic therapy group: 29 years (14 ‐ 44 years); control group: 25 years (17 ‐ 49 years). Number of ulcers: 40 (antibiotic therapy: 20; placebo: 20). Mean baseline ulcer area (cm2) ± SD. Antibiotic therapy group: 20.0 (14.9); placebo: 20.8 (19.0). Gender (male:female). Antibiotic therapy group: 4:9; control: 6:9. Inclusion criteria: Exclusion criteria: | |
| Interventions | Antimicrobial therapy (aerosol preparation, twice daily): Co‐intervention: disinfectant (Eusol). Systematic antibiotics: not allowed. | |
| Outcomes | Primary: pain. According with Dr Graham Serjeant, interviewed by one author of this review (JKM), 'ulcer area‐change' was the primary outcome (February 4, 2011). | |
| Notes | 1. This trial was described as "randomised controlled crossover trial" at Proceedings of the Commonwealth Caribean medical Research Council (31st Scientific Meeting, 1986, April 16‐19, Port Spain, Trinidad and Tobago. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were assigned, using size 4 block randomization" (page 847). Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition and exclusions to permit judgement of ‘low risk’ or ‘high risk’. |
| Selective reporting (reporting bias) | High risk | One or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded (i.e. safety). |
| Other bias | High risk | Ascertaiment bias and bias in the presentation of data (Appendix 2). |
| Methods | Desing trial: parallel (3 groups). | |
| Participants | Sample size: 32 participants. 16 (Solcoseryl®: 3); hydrocolloid dressing: 8); (Placebo: 5) Age: Mean (± SD): Solcoseryl®: 27.0 years (8.2); hydrocolloid dressing: 35.5 years (9.8); placebo: 30.7(8.2). Gender (males): Solcoseryl®: 93%; hydrocolloid dressing: 50%; placebo: 71%. Inclusion criteria: | |
| Interventions | 1. Solcoseryl® (de‐proteinized extract of calf blood) supplied in tubes as jelIy or ointment, twice daily after cleaning with Eusol, covered with a gauze dressing and supported by an Elastoweb bandage. 2. Hydrocolloid dressing (hydroactive dressing) supplied as sheets of occlusive dressing and as granules. and covered with gauze and supported by an Elastoweb bandage. The dressing was replaced at weekly intervals or more often if indicated. Control: Twice‐daily cleaning with a mild antiseptic agent (Eusol), wet dressing and a firm supportive elastic bandage. | |
| Outcomes | Healing ulcer. | |
| Notes | Centre and Country: Sickle‐cell Clinic of the University Hospital of the West Indies, Kingston, Jamaica. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Unilateral ulcers were randomized into one of three treatment schedules in blocks of three" and "In bilateral ulcers, one leg was randomized to one of the two treatment agents and the other leg to control therapy" (page 121). Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) | High risk | Dropouts: 50% (16/32) (Placebo 24% (5/21); Solcoseryl® 21.4% (3/14); hydrocolloid dressing 57.1% (8/14). Imbalance between hydrocolloid dressing group and the other groups > 10%. |
| Selective reporting (reporting bias) | High risk | 1 or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded (i.e. safety). |
| Other bias | High risk | Ascertaiment and design bias and bias in the presentation of data (Appendix 2). Comments: there is imbalance in sex and age. |
| Methods | Design trial: parallel (2 groups). | |
| Participants | Sample size: 26 participants Age: median (range): arginine butyrate plus standard local care: 36.6 (21–60); standard local care: 34.7 (20–57). Gender (female): arginine butyrate plus standard local care: 57%; standard local care: 58%. Inclusion criteria: Exclusion criteria: | |
| Interventions | Intervention*: arginine butyrate at a total daily dose of 500 mg/kg/dose given 5 d/week plus standard local care. Control arm: standard local care alone. | |
| Outcomes | Wound healing: partial healing (decrease in ulcer area by at least 25% of the baseline ulcer area) and complete healing (complete closure of the ulcer (to an area of 0 cm2). | |
| Notes | * If healing was objectively documented during the first 12‐week treatment cycle, as determined by a decrease in measured ulcer area by at least 25% of the baseline area, arginine butyrate could be continued for two additional courses of 8‐week cycles, although the responses to the extended treatment were not analysed as study endpoints. Control arm participants could cross‐over to the treatment arm if their ulcer did not heal after 12 weeks of closely monitored and supervised standard local care. Their remaining ulcers were then assessed on the treatment arm for 12 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "table of random numbers prepared by a blinded statistician." (page 517). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘low risk’ or ‘high risk’. |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | High risk | Bias in the presentation of data (Appendix 2). |
| Methods | Design trial: parallel (two groups). | |
| Participants | Enrolled: not described. 1. Reported patients: 40
4. Ulcers developed during trial: 9 (# patients: no reported). 8. Number of ulcers completing trial: 54 (isoxuprine: 32; placebo: 22). Allocated group: not mentioned. Information about haemoglobin disorder type: Inclusion criteria: information was not supplied. | |
| Interventions | Experimental group: Isoxuprine hydrochloride: 40 mg, per oral (bd). Control group: Placebo: 40 mg, per oral (bd). Co‐interventions: | |
| Outcomes | Outcomes were not describe as primary or secondary. They were reported into 'results' section: According with Dr Graham Serjeant, interviewed by one author of this review (JKM), 'ulcer area‐change' was the primary outcome (February 4, 2011). | |
| Notes | Centre and Country: Medical Research Council Laboratories, University of West India, Jamaica. Sample size estimation a priori: no. Support: Mead Johnson and Company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " patients were allotted serial numbers in a code..." (page 164). Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ Comment: Dr Searjent referred this paper as blinded (Interviewed by one author of this review (JKM), on February 4, 2011). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Incomplete outcome data (attrition bias) | High risk | The number or reasons for dropouts and withdrawals were not described. |
| Selective reporting (reporting bias) | High risk | One or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded. |
| Other bias | High risk | Quote "The code was kept by the producing company". |
| Methods | Design trial: parallel (2 groups). | |
| Participants | 1. Enrolled: not described. 6. Reported leg ulcers at the start trial: 34 (Data supplied by Dr Graham Serjeant, Jamaica, February 4, 2011). 7. Sex (male): 12 (80%). 8. Age (years): (arithmetic range): 9. Clinical characteristics of the leg ulcers: Leg ulcers of at least six months' duration and at least 3 cm diameter at the onset of treatment. 10. Inclusion criteria: 11.‐ Exclusion criteria: not described. | |
| Interventions | Baseline treatment (12‐weeks): all patients Experimental group: Control group: Co‐interventions: | |
| Outcomes | Healing rate. According with Dr Graham Serjeant, interviewed by one author of this review (JKM), 'ulcer area‐change' was the primary outcome (February 4, 2011). | |
| Notes | RCT start date: not described. Centre and Country: Conducted at Medical Research Council Laboratories, University of West India, Jamaica. A priori sample size estimation: 14 patients (7 patients by group).
Support: Sigma‐Tau Pharmaceuticals, Gaittherborg, Md (Partial grant). Founder role: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "...were randomly assigned to a treatment group, seven in the PLC group and eight in the placebo group" (pages 491‐2). Quote "randomisation within small blocks (2‐4)" This information was supplied by Dr Serjeant on February 4, 2011. Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ Comment: Dr Searjent referred this paper as blinded (Interviewed by one author of this review (JKM), on February 4, 2011). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘low risk’ or ‘high risk’. |
| Selective reporting (reporting bias) | High risk | if one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded. |
| Other bias | High risk | Design bias (Appendix 2). |
| Methods | Design trial: parallel (2 groups). | |
| Participants | Randomised: 55 (RGD peptide matrix: 32) / placebo (23). Completed study: 48 (87%). RDG peptide matrix (27/32 = 84%), placebo (21/23 = 91%) Baseline ulcer duration (months) median(± SE) and range: RGD peptide matrix: 52.0 months (12.6), range 1 ‐ 312; placebo: 45.9 months (12.4), range 1 ‐ 192). Gender (male: female): RGD peptide matrix: 21:11; placebo: 12:11. Inclusion criteria:
Exclusion criteria:
| |
| Interventions | RGD peptide matrix (Argidene gel: formerly Telio‐Derm gel, Telios Pharmaceuticals, Inc, San Diego, CA). Placebo: saline solution. Co‐intervention: debridament and cleaning at each visit. | |
| Outcomes | Primary: changes in per cent ulcer closure. | |
| Notes | Country: conducted at USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "sequentially assigned to treatment groups based on a unique randomization number list" (page 1776). Comments: imbalance exists on 'baseline ulcer area (cm2 ). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Low risk | Patient and outcome assessor were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Patient and outcome assessor were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts < 20% |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | High risk | Design bias (Appendix 2). |
bd: twice a day
SD: standard deviation
SE: standard error
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Author did not report data from the patients suffering sickle cell thalassaemia. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Case report. | |
| Not a randomised clinical trial. | |
| Case study. | |
| Case report including three types of ulcers. | |
| Not a randomised clinical trial. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in ulcer size (surface area or volume) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 L‐carnitine versus placebo, Outcome 1 Change in ulcer size (surface area or volume). | ||||
| 1.1 All randomised patients | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 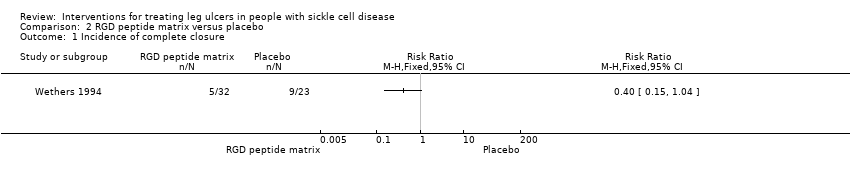 Comparison 2 RGD peptide matrix versus placebo, Outcome 1 Incidence of complete closure. | ||||
| 2 Change in size of ulcers healed Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 RGD peptide matrix versus placebo, Outcome 2 Change in size of ulcers healed. | ||||
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 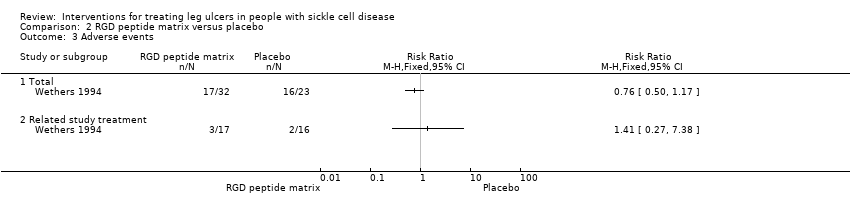 Comparison 2 RGD peptide matrix versus placebo, Outcome 3 Adverse events. | ||||
| 3.1 Total | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Related study treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Flowchart of last search of the Group's Trials Register: 25 May 2012.

Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study
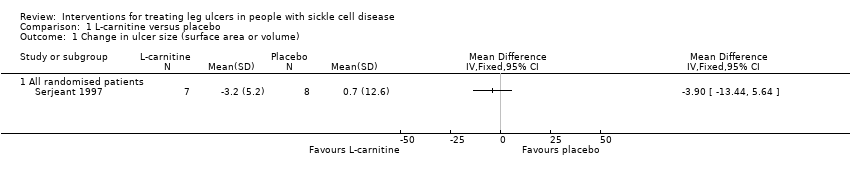
Comparison 1 L‐carnitine versus placebo, Outcome 1 Change in ulcer size (surface area or volume).

Comparison 2 RGD peptide matrix versus placebo, Outcome 1 Incidence of complete closure.

Comparison 2 RGD peptide matrix versus placebo, Outcome 2 Change in size of ulcers healed.

Comparison 2 RGD peptide matrix versus placebo, Outcome 3 Adverse events.
| Isoxuprine compared to placebo for leg ulcer in people with sickle cell disease | ||||||
| Patient or population: patients with leg ulcer in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | Isoxuprine | |||||
| Incidence of complete closure | See comment | See comment | Not estimable | 54 ulcers | ⊕⊝⊝⊝ | This trial shows inconsistency between units of randomisation (30 participants) and unit of analysis (54 ulcers). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation, allocation concealment, blinding: unclear. Incomplete outcome data and selective report. CI: confidence interval | ||||||
| arginine butyrate plus standard local care compared to standard local care for sickle cell in people with sickle cell disease | ||||||
| Patient or population: sickle cell in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| standard local care | arginine butyrate plus standard local care | |||||
| Complete healing | See comment | See comment | Not estimable | 23 participants 62 ulcers | ⊕⊝⊝⊝ | This trial shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). |
| Change in ulcer size | See comment | See comment | Not estimable | (1 study; McMahon 2010) | ⊕⊝⊝⊝ | This trial shows inconsistency between units of randomisation (23 participants) and unit of analysis (62 ulcers). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unclear allocation concealment CI: confidence interval | ||||||
| L‐carnitine compared to placebo for leg ulcer in people with sickle cell disease | ||||||
| Patient or population: leg ulcer in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | L‐carnitine | |||||
| Change in ulcer size | See comment | See comment | Not estimable | 15 | ⊕⊝⊝⊝ | Mean difference: ‐3.90 (95% CI ‐13.44 to 5.64). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation: unclear. CI: confidence interval | ||||||
| RGD peptide matrix compared to placebo for leg ulcer in people with sickle cell disease | ||||||
| Patient or population: patients with leg ulcer in people with sickle cell disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | RGD peptide matrix | |||||
| Complete closure | See comment | See comment | Not estimable | 55 | ⊕⊝⊝⊝ | Risk ratio: 0.40 (95% CI 0.15 to 1.04). |
| Change in size ulcers healed | See comment | See comment | Not estimable | 55 | ⊕⊝⊝⊝ | Mean difference: 6.60 (95% CI 5.51 to 7.69). |
| Total adverse events | See comment | See comment | Not estimable | 55 | ⊕⊝⊝⊝ | Risk ratio: 0.76 (95 CI 0.50 to 1.17). |
| Related study treatment adverse events | See comment | See comment | Not estimable | 33 | ⊕⊝⊝⊝ | Risk Ratio: 1.41 (95% CI 0.27 to 7.38). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation and allocation concealment: unclear CI: confidence interval | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in ulcer size (surface area or volume) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 All randomised patients | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of complete closure Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Change in size of ulcers healed Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Total | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Related study treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


