Quimiorradiación preoperatoria para el cáncer rectal localmente avanzado no metastásico
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 1. Randomization method: telephone to a central office, minimization method. 2. Recruitment between 1993‐2003. | |
| Participants | 1. T3 and T4 resectable rectal cancer, diagnosed using proctology and CT scan. 2. 252 Participants RT only 3. 253 Participants CRT only 4. 253 Participants RT and post operative chemotherapy 5. 253 Participants CRT and post operative chemotherapy 6. Age under 80 years | |
| Interventions | 1. Radiotherapy 45 Gy over 5 weeks 2. Preoperative chemotherapy 2x5 day course of 5‐FU 350 mg/m2/day and leucovorin 3. Postoperative chemotherapy 4x5 day course of 5‐FU 350 mg/m2/day and leucovorin every 3 weeks, 3‐10 weeks post surgery 4. Surgery (TME) 3‐10 weeks after beginning radiotherapy | |
| Outcomes | 1. Acute toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information stated |
| Allocation concealment (selection bias) | Unclear risk | No information stated |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Did not include people aged over 80 years old. The two groups receiving post operative chemotherapy may confounded the results, although this was considered and discounted by the study authors. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
| Methods | 1. Randomization method: telephone to a central office, minimization method. 2. Recruitment between 1993‐2003. | |
| Participants | 1. T3 and T4 resectable rectal cancer. Diagnosed using proctology and CT scan. 2. 252 Participants RT only 3. 253 Participants CRT only 4. 253 Participants RT and post operative chemotherapy 5. 253 Participants CRT and post operative chemotherapy 6. Age under 80 years | |
| Interventions | 1. Radiotherapy 45 Gy over 5 weeks 2. Preoperative chemotherapy 2x5 day course of 5‐FU 350 mg/m2/day and leucovorin 3. Postoperative chemotherapy 4x5 day course of 5‐FU 350 mg/m2/day and leucovorin every 3 weeks, 3‐10 weeks post surgery 4. Surgery (TME) 3‐10 weeks after beginning radiotherapy | |
| Outcomes | 1. Overall survival (5 years) 2. Local recurrence 3. 30 Day Mortality 4. Sphincter preservation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information stated |
| Allocation concealment (selection bias) | Unclear risk | No information stated. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | Did not include people aged over 80 years old. The two groups receiving post operative chemotherapy may confounded the results, although this was considered and discounted by the study authors. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
| Methods | 1. Randomisation by telephone, telex or telegram. 9 centres in four European countries. 2. Recruitment between 1972‐1976. | |
| Participants | Histologically proven localised adenocarcinoma of the rectum, no known metastasis Of the 168 people randomised to radiotherapy 121 were evaluated in the analysis. There were 26 who had advanced disease discovered during surgery and 10 lost to follow up. In the chemoradiation group 126 of 171 participants were evaluated. There were 22 who had advanced disease, 10 refused surgery and 19 lost to follow up. No other information was given regarding the other missing participants. | |
| Interventions | 1. RT 34.5 Gy 2. CRT 34.5 Gy with 5‐FU 10mg/Kg for the first 4 days of radiotherapy 3. Surgery at 2 weeks (not TME) | |
| Outcomes | 1. Overal Survival (5 years) 2. Local recurrence 3. 30 Day Mortality | |
| Notes | The trial is now over 25 years old. Wide radiotherapy field and unconventional chemotherapy dose and high post operative death rates compared to modern standards. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information stated. |
| Allocation concealment (selection bias) | Unclear risk | No information stated. |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias. |
| Other bias | High risk | Surgical techniques, including the use of TME, not applicable to current surgical practice. Wide radiotherapy fields were used. The dose of chemotherapy is also not the dose currently used. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
| Methods | 1. Randomization method: telephone to a central office, minimization method. 2. Recruitment 1999‐2002. | |
| Participants | 1. Rectal cancer 2. Location: accessible by digital rectal examination 3. Resectability: resectable without sphincter infiltration 4. Endoanal ultrasound or pelvic CT 5. RT155 6. CRT157 | |
| Interventions | 1. Surgery ‐ TME for low lying cancers and subtotal TME for mid level tumours. 2. Timing of surgery: 7 days later for RT arm and 4‐6 weeks for CRT arm 3. RT: 25Gy for RT arm and 50.4Gy for CRT arm 4. Chemotherapy: CRT arm, 2 courses of 5‐fluorouracil(325mg/m2 per day for 5 days) and (leucovorin(20mg/m2 per day for 5 days)) concomitant with RT(weeks 1 and 5). 5. Optional postoperative chemotherapy was offered to all the trial participants (6 months 5FU in the RT and 4 months 5FU in the CRT group). In the RT arm, 65 people and 43 people in the RCT arm, took up this option. | |
| Outcomes | 1. Sphincter preservation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information stated. |
| Allocation concealment (selection bias) | Unclear risk | No information stated. |
| Selective reporting (reporting bias) | Low risk | Four patients were excluded as they did not meet the entry criteria and were not included in the intention to treat analysis; 2 had a fixed tumour and 2 had begun radiotherapy before randomisation. Therefore 312 participants were included in the intention to treat analysis. There were 155 participants in the RT arm and 157 in the RCT arm. Seven participants did not undergo surgery after randomisation; two refused surgery, three died before surgery and two were found to have distant metastasis. Two of these participants were in the RT arm and five in the CRT arm. |
| Other bias | Unclear risk | Not all participants underwent TME |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
| Methods | 1. Randomization method ‐ by telephone to central office, minimization method. 2. Recruitment 1999‐2002. | |
| Participants | 1. Rectal cancer 2. Location: accessible by digital rectal examination 3. Resectability: resectable without sphincter infiltration 4. Endoanal ultrasound or pelvic CT 5. Study arm;RT 155 6. Control arm:CRT 157 | |
| Interventions | Two arms: preop RT vs preop CRT 1. Surgery ‐ TME for low lying cancers and subtotal TME for mid level tumours. 2. Timing of surgery: 7 days later for RT arm and 4‐6 weeks for CRT arm 3. RT: 25Gy for RT arm and 50.4Gy for CRT arm 4. Chemotherapy: CRT arm, 2 courses of 5‐fluorouracil (325mg/m2 per day for 5 days) and (leucovorin(20mg/m2 per day for 5 days)) concomitant with RT (weeks 1 and 5). | |
| Outcomes | 1. 30 Day Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information stated. |
| Allocation concealment (selection bias) | Unclear risk | No information stated. |
| Selective reporting (reporting bias) | Unclear risk | See above |
| Other bias | Unclear risk | Not all participants underwent TME |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
| Methods | 1. Randomization method ‐ by telephone to central office, minimization method. 2. Recruitment 1999‐2002. | |
| Participants | 1. Rectal cancer 2. Location: accessible by digital rectal examination 3. Endoanal ultrasound or pelvic CT 4. Resectability: resectable without sphincter infiltration 5. Study arm;RT 155 5. Control arm:CRT 157 | |
| Interventions | 1. Surgery ‐ TME for low lying cancers and subtotal TME for mid level tumours. 2. Timing of surgery: 7 days later for RT arm and 4‐6 weeks for CRT arm 3. RT: 25Gy for RT arm and 50.4Gy for CRT arm 4. Chemotherapy: CRT arm, 2 courses of 5‐fluorouracil(325mg/m2 per day for 5 days) and (leucovorin(20mg/m2 per day for 5 days)) concomitant with RT(weeks 1 and 5). | |
| Outcomes | 1. Overall survival (4 years) 2. Local recurrence 3. Acute toxicity 4. Late toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information stated |
| Allocation concealment (selection bias) | Unclear risk | No information stated |
| Selective reporting (reporting bias) | Low risk | See above |
| Other bias | Unclear risk | Not all participants underwent TME |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
| Methods | 1. Randomisation via the minimisation method 2. Recruitment 1993‐2003 | |
| Participants | 1. Rectal cancer 2. Location: accessible by digital rectal examination. All underwent proctology with or without CT scan. 3. In total 762 patients were randomised. Of these, 20 were ineligible, 14 due to protocol violations, such as not receiving chemotherapy and 6 were immediately lost to follow up. 4. Study arm;RT 375 5. Control arm; CRT 375 6. Age under 75 years | |
| Interventions | 1. RT 45 Gy, CRT 45 Gy 2. CRT 5‐FU 350 mg/m2/d for 5 days with leucovorin 3. Surgery ‐ TME recommended, 3‐10 weeks post treatment | |
| Outcomes | 1. Overall Survival (5 years) 2. Local recurrence 3. Sphincter preservation 4. Acute toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information stated. |
| Allocation concealment (selection bias) | Unclear risk | No information stated. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | None |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
| Methods | 1. Randomisation, method not stated. 2. Recruitment ongoing. | |
| Participants | 1. Stage 2 and 3 disease, rectal cancer 2. 37 participants SCRT, 46 participants CRT 3. Diagnosed using endoanal ultrasound, CT and MRI 4. Aged up to 80 years | |
| Interventions | 1. Surgery, not information regarding TME 2. Timing of surgery: All surgery took place at 6 weeks 3. RT: 25Gy for SCRT arm and 50Gy for CRT arm 4. Chemotherapy: CRT arm, 2 courses of 5‐fluorouracil(400mg/m2 per day for 5 days) and (leucovorin(20mg/m2 per day for 5 days)) concomitant with RT(weeks 1 and 5). | |
| Outcomes | 1. Sphincter preservation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | High risk | Imbalance of participants within each arm |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
| Methods | 1. Randomised controlled trial, full methods not yet published | |
| Participants | 1. Rectal Cancer 2. MRI‐staged 3 within 12 cm of the anal verge 3. RT 163 participants 4. CRT 163 participants | |
| Interventions | 1. RT, 25 Gy followed by surgery at one week (plus 6 post operative chemotherapy sessions of 5‐FU and leucovorin) 2. CRT, 50.4 Gy and 5‐FU 225 mg/m2/day followed by surgery at 4‐6 weeks (plus 4 post operative chemotherapy sessions of 5‐FU and leucovorin) | |
| Outcomes | 1. Acute toxicity | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not yet reported |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not yet reported |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Limited data is available due to the abstract format. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
| Methods | 1. Randomised controlled trial, full methods not yet published | |
| Participants | 1. Rectal Cancer 2. MRI‐staged 3 within 12 cm of the anal verge 3. RT 163 participants 4. CRT 163 participants | |
| Interventions | 1. RT, 25 Gy followed by surgery at one week (plus 6 post operative chemotherapy sessions of 5‐FU and leucovorin) 2. CRT, 50.4 Gy and 5‐FU 225 mg/m2/day followed by surgery at 4‐6 weeks (plus 4 post operative chemotherapy sessions of 5‐FU and leucovorin) | |
| Outcomes | 1. Local recurrence at 3 years 2. Late toxicity | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not yet reported |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not yet reported |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Limited data is available due to the abstract format. |
| Blinding of participants and personnel (performance bias) | High risk | The trial was blinded, however, when some people receive chemotherapeutic agents and some do not this can be a potential source of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| From January 1983 to December 2000, 157 patients with stage II and III rectal cancer. Patients were enrolled consecutively into three groups; preoperative radiotherapy of 35‐45Gy and 5‐FU followed by anterior resection, radiotherapy alone followed by anterior resection or abdomino‐perineal resection only. Outcomes included 5 year survival, disease free survival, local recurrence and distant metastases.The allocation to each group occurred consecutively and was not randomised. | |
| Using the study data from the Polish Colorectal Study Group, further outcomes were obtained. They analysed the 312 patients who were included in the Polish study, who had been followed up for a median of 48 months.Their outcomes were local recurrence, disease‐free survival and overall survival. But these outcomes were based on macro and microscopic bowel resection margin as the exposure variable. The results were not stratified by RT or RCT grouping and as such could not be included. | |
| From October 2001 until October 2006, this group included 485 patients with rectal adenocarcinoma. Patients with suspected nodal involvement underwent neoadjuvant short course radiotherapy (5 x 5Gy) followed by surgery 3 days later. Patients with T4 disease underwent neoadjuvant chemoradiation (28 x 1.8 Gy over 6 weeks and 5‐FU 300mg/m2) followed by surgery 6 weeks later. Outcomes included mortality, medical complications (pneumonia, UTI, cardiac complications), anastomotic leak, wound infection and length of hospital stay. This trial was not included as the patients were not randomised. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Preoperative short‐course radiotherapy versus combined radiochemotherapy in locally advanced rectal cancer: a multi‐centre prospectively randomised study of the Berlin Cancer Society |
| Methods | Central randomisation using the minimisation method |
| Participants | 1. Patients with histological proven rectal cancer 2. T2N+ or T3 staging 3. 760 participants planned study size |
| Interventions | 1. RT, 25 Gy in five fractions of 5 Gy plus TME‐surgery within 5 days 2. RCT, 50.4 Gy in 28 fractions of 1.8 Gy, continuous infusion 5‐fluorouracil (225 mg/m2/day) plus TME‐surgery 4–6 weeks later. 3. All patients receive adjuvant chemotherapy (12 weeks continuous infusional 5‐FU) 4. Follow up planned for 5 years. |
| Outcomes | 1. Local recurrence at 5 years 2. Overall survival 3. Disease‐free survival and quality of life (including long term bowel function) 4. Complete resection rate 5. The rate of sphincter saving resection 6. Acute toxicity 7. Late toxicity |
| Starting date | First patient enrolled in 2004 |
| Contact information | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall Survival Show forest plot | 4 | 2312 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.20] |
| Analysis 1.1  Comparison 1 Primary Outcome Parameters, Outcome 1 Overall Survival. | ||||
| 2 Local Recurrence Show forest plot | 5 | 2138 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.42, 0.75] |
| Analysis 1.2  Comparison 1 Primary Outcome Parameters, Outcome 2 Local Recurrence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 30 Day Mortality Show forest plot | 3 | 1568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.88, 3.38] |
| Analysis 2.1  Comparison 2 Secondary Outcome Parameters, Outcome 1 30 Day Mortality. | ||||
| 2 Sphincter Preservation Show forest plot | 4 | 2148 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.20] |
| Analysis 2.2  Comparison 2 Secondary Outcome Parameters, Outcome 2 Sphincter Preservation. | ||||
| 3 Acute Toxicity Show forest plot | 4 | 2178 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.96 [3.03, 5.17] |
| Analysis 2.3  Comparison 2 Secondary Outcome Parameters, Outcome 3 Acute Toxicity. | ||||
| 4 Late Toxicity Show forest plot | 2 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.50, 1.54] |
| Analysis 2.4  Comparison 2 Secondary Outcome Parameters, Outcome 4 Late Toxicity. | ||||

Forest plot of comparison: 1 Primary Outcome Parameters, outcome: 1.1 Overall Survival.

Forest plot of comparison: 1 Primary outcome: 1.2 Local Recurrence CRT versus RT.
Due to potential interaction identified by the Bosset study authors this analysis does not include participants who underwent post‐operative chemotherapy.
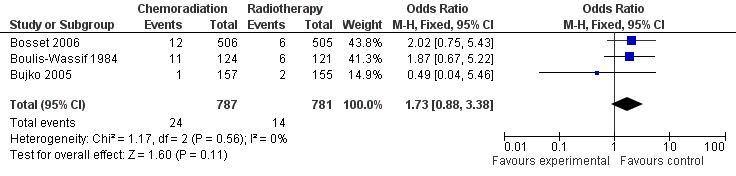
Forest plot of comparison: 2 Secondary Outcome Parameters, outcome: 2.1 30 Day Mortality CRT versus RT.

Forest plot of comparison: 2 Secondary Outcome Parameters, outcome: 2.2 Sphincter Preservation CRT versus RT.

Forest plot of comparison: 2 Secondary Outcome Parameters, outcome: 2.3 Acute Toxicity CRT versus RT.
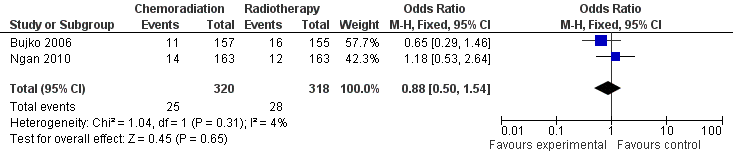
Forest plot of comparison: 2 Secondary Outcome Parameters, outcome: 2.4 Late Toxicity CRT versus RT.
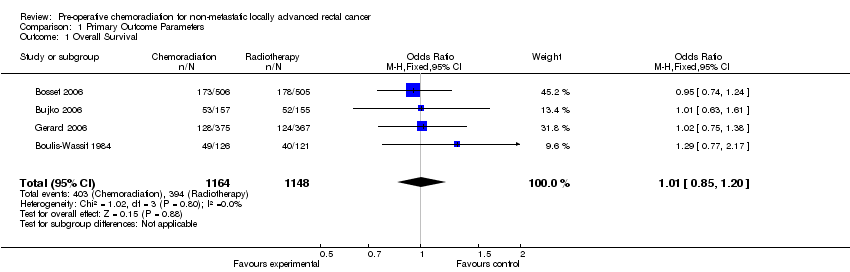
Comparison 1 Primary Outcome Parameters, Outcome 1 Overall Survival.

Comparison 1 Primary Outcome Parameters, Outcome 2 Local Recurrence.

Comparison 2 Secondary Outcome Parameters, Outcome 1 30 Day Mortality.
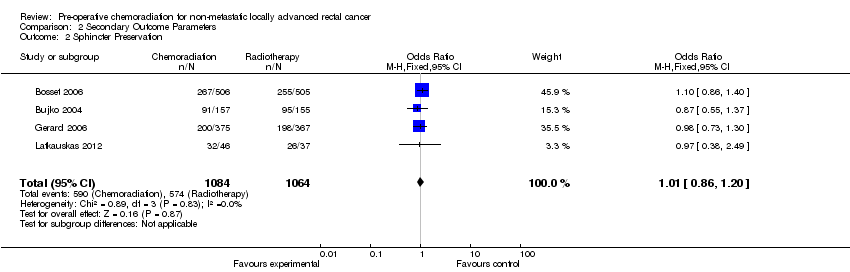
Comparison 2 Secondary Outcome Parameters, Outcome 2 Sphincter Preservation.

Comparison 2 Secondary Outcome Parameters, Outcome 3 Acute Toxicity.

Comparison 2 Secondary Outcome Parameters, Outcome 4 Late Toxicity.
| Tumour | |
| T1 | Tumour invades submucosa. |
| T2 | Tumour invades muscularis propria. |
| T3 | Tumour invades through to subserosa or into perirectal tissues. |
| T4 | Tumour invades into surrounding structures/organs. |
| Nodes | |
| N0 | no lymph nodes involved. |
| N1 | 1‐3 local lymph nodes involved. |
| N2 | >4 lymph nodes involved. |
| Metastases | |
| M0 | no distant metastases. |
| M1 | Distant metastases. |
| Stage | Dukes classification | 5 year survival (%) | |
| T1‐2N0M0 | I | A | 80 |
| T3N0M0 | IIA | B | 40‐60 |
| T4N0M0 | IIB | B | 40‐60 |
| T1‐2N1M0 | IIIA | C | 40 |
| T3‐4N1M0 | IIIB | C | 30 |
| TanyN2M0 | IIIC | C | 12 |
| TanyNanyM1 | IV | D | 15 (2 year survival) |
| Study | Outcome Measure(s) | CRT | RT |
| Toxicity was measured using the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (EORTC) scale grade III‐IV. Toxicity was classified as late toxicity if it occurred more than 30 days after surgery. | 18.2% | 3.2% | |
| One or more adverse early event. Published in abstract form so limited information currently available. | 28% | 1.9% | |
| Early grade III‐IV toxicity, assessed using the WHO scale. | 14.6% | 2.7% | |
| Patients enrolled before January 2001, n=798 | Grade II toxicity assessed using the WHO acute morbidity scale or the occurrence of acute diarrhoea. | 34.3% | 17.3% |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall Survival Show forest plot | 4 | 2312 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.20] |
| 2 Local Recurrence Show forest plot | 5 | 2138 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.42, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 30 Day Mortality Show forest plot | 3 | 1568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.88, 3.38] |
| 2 Sphincter Preservation Show forest plot | 4 | 2148 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.20] |
| 3 Acute Toxicity Show forest plot | 4 | 2178 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.96 [3.03, 5.17] |
| 4 Late Toxicity Show forest plot | 2 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.50, 1.54] |

