Trasplante autólogo de células madre hematopoyéticas después de la quimioterapia de dosis alta para los sarcomas de tejidos blandos no rabdomiosarcomas
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008216.pub5Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 abril 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer ginecológico, neurooncología y otros cánceres
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
FP: designed and co‐ordinated the review, collected data, designed search strategies, undertook searches, screened search results, organized retrieval of papers, screened retrieved papers against eligibility criteria, appraised quality of papers, extracted data from papers, wrote to authors of papers for additional information, managed data, entered data into Review Manager 5, analyzed data, interpreted data, wrote manuscript.
HE: screened retrieved papers against eligibility criteria, reviewed manuscript.
LAS: provided methodologic advice, appraised quality of papers, reviewed manuscript.
Sources of support
Internal sources
-
University of Cologne, Germany.
Provision of full texts
External sources
-
No sources of support supplied
Declarations of interest
FP: none known.
HE: none known.
LAS: none known.
Acknowledgements
We thank the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Editorial Team for their assistance during the preparation of the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Apr 13 | Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcomas | Review | Frank Peinemann, Heike Enk, Lesley A Smith | |
| 2013 Aug 07 | Autologous hematopoietic stem cell transplantation following high dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas | Review | Frank Peinemann, Lesley A Smith, Carmen Bartel | |
| 2011 Feb 16 | Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas | Review | Frank Peinemann, Lesley A Smith, Mandy Kromp, Carmen Bartel, Nicolaus Kröger, Michael Kulig | |
| 2010 Feb 17 | Autologous stem cell transplantation following high‐dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas | Protocol | Frank Peinemann, Annegret Herrmann‐Frank, Mandy Hildebrandt, Carmen Bartel, Tatjana Burkhardt‐Hammer, Robert Grosselfinger, Michael Kulig, Nicolaus Kröger, Stefan Lange | |
| 2010 Jan 20 | Autologous stem cell transplantation following high‐dose chemotherapy for non‐rhabdomyosarcoma soft tissue sarcomas | Protocol | Frank Peinemann, Carmen Bartel, Mandy Hildebrandt, Michael Kulig, Tatjana Burkhardt‐Hammer, Nicolaus Kröger, Stefan Lange | |
Differences between protocol and review
Differences between the previous version and the current version of the review
We revised the criteria for considering studies for this review. First, we confined the types of studies to RCTs. Therefore, we removed non‐randomized studies and associated data from the review. The single RCT included in the previous version was carried over to the current version of the review. Second, we extended the previous WHO classification of soft tissue sarcomas by adding information from the recently updated version of the WHO classification of soft tissue sarcomas. We revised the search strategies to improve precision and reported the results of the update search.
We changed the items of the 'Risk of bias' tool. We removed the items that were designed to judge the risk of bias of non‐randomized studies. We extended the rest of the items to complete all items of the risk of bias for RCTs. Thus, we included the judgment of some items of risk of bias that were not part of the previous version. Subsequently, the risk of bias was different between the previous and the current version of the review and the judgment changed from low to unclear risk of bias.
We identified additional inconsistencies in the reporting of the included study and sent inquiries to two authors of that study, but we did not receive a response. We identified a published warning letter sent by the FDA to the first author. We do not know if the cause of this letter was associated with conducting the study.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Aged; Humans; Middle Aged;
PICO

Study flow diagram.
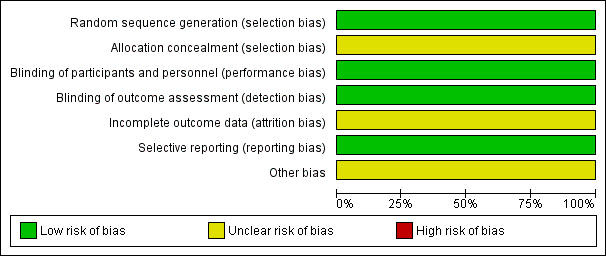
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
| Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcoma | ||||||
| Patient or population: people with non‐rhabdomyosarcoma soft tissue sarcoma Settings: specialized hospital Intervention: autologous hematopoietic stem cell transplantation following HDCT Comparison: SDCT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| SDCT | Autologous HSCT following HDCT | |||||
| Overall survival Follow‐up: median 55 months | 489 per 1000 | 571 per 1000 | HR 1.26 | 83 | ⊕⊕⊕⊕ | ‐ |
| Treatment‐related mortality Follow‐up: 24 months | See comment | See comment | Not estimable | 83 | ⊕⊕⊕⊕ | 1 event 2 years after HDCT and 0 events after SDCT |
| Disease‐free survival | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Progression‐free survival Follow‐up: median 55 months | 756 per 1000 | 849 per 1000 | HR 1.34 | 83 | ⊕⊕⊕⊕ | ‐ |
| Non‐hematologic toxicity grade 3 to 4 | See comment | See comment | Not estimable | ‐ | See comment | Not adequately reported, people from within and without the randomization were mixed in the control arm. |
| Health‐related quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Sarcoma type | Sarcoma type 'Others' | Both arms | HDCT arm | SDCT arm |
| Leiomyosarcoma | ‐ | 16 | 7 | 9 |
| Liposarcoma | ‐ | 10 | 6 | 4 |
| Synovial sarcoma | ‐ | 9 | 2 | 7 |
| Angiosarcoma | ‐ | 6 | 2 | 4 |
| Malignant peripheral nerve sheath tumor | ‐ | 2 | 1 | 1 |
| Clear cell sarcoma | ‐ | 1 | 1 | 0 |
| Desmoplastic small round cell sarcoma | ‐ | 1 | 0 | 1 |
| Rhabdomyosarcoma | ‐ | 9 | 4 | 5 |
| Malignant fibrous histiocytoma | ‐ | 16 | 8 | 8 |
| Extraskeletal osteosarcoma | ‐ | 1 | 0 | 1 |
| Melanoma* | ‐ | 1 | 1 | 0 |
| 'Others' | Leiomyosarcoma | 1 | 1 | 0 |
| Fibrosarcoma | 1 | 1 | 0 | |
| Myofibrosarcoma | 1 | 0 | 1 | |
| Undifferentiated sarcoma | 2 | 1 | 1 | |
| Desmoplastic small round cell sarcoma | 2 | 2 | 0 | |
| Gastrointestinal stromal tumor | 1 | 0 | 1 | |
| Malignant Triton tumor | 1 | 0 | 1 | |
| Unclassified sarcoma | 1 | 1 | 0 | |
| Myoepithelioma* | 2 | 2 | 0 | |
| Endometrial stromal sarcoma* | 3 | 1 | 2 | |
| Total | 87 | 41 | 46 | |
| Not listed in the WHO classification | 6 | 4 | 2 | |
| HDCT: high‐dose chemotherapy; SDCT: standard‐dose chemotherapy; WHO: World Health Organization. Bui‐Nguyen: the table lists the sarcoma types assigned to each individual of all randomized participants of the study by Bui‐Nguyen 2012. *Soft tissue sarcomas: tumor entities not listed in either versions of the WHO classification (Fletcher 2002; Fletcher 2013), or soft tissue tumors not categorized as malignant are italicized. Myoepithelioma is categorized as an intermediate soft tissue tumor. Melanoma and endometrial stromal sarcoma are not listed in the classification. | ||||

