Administración de heparina subcutánea lenta versus rápida para la prevención de la equimosis y la intensidad del dolor en el sitio de inyección
Resumen
Antecedentes
La heparina es un anticoagulante que habitualmente se inyecta de forma subcutánea. La administración subcutánea de heparina puede dar lugar a complicaciones como equimosis, hematomas y dolor en el sitio de la inyección. Uno de los factores que pueden afectar el dolor, los hematomas y la equimosis es la velocidad de inyección. Para los pacientes y los profesionales de atención sanitaria, se consideran importantes las estrategias que pueden aliviar el dolor y la equimosis. La reducción del malestar y la preocupación del paciente, siempre que sea posible, es un objetivo importante de la atención de enfermería. Se han realizado varios estudios para observar si la velocidad de la inyección afecta la intensidad del dolor y el grado de equimosis en el sitio en que se administra la inyección, aunque hay diferencias entre los resultados de estos estudios y los autores no han establecido una conclusión final clara. Ésta es la primera actualización de la revisión publicada por primera vez en 2014.
Objetivos
Evaluar los efectos de la duración (velocidad) de la inyección de heparina subcutánea sobre el dolor, los hematomas y la equimosis en el sitio de inyección en pacientes que ingresan al hospital o a consultorios y que requieren tratamiento con heparina no fraccionada (HNF) o heparina de bajo peso molecular (HBPM).
Métodos de búsqueda
Para esta actualización el especialista en información del Grupo Cochrane Vascular (Cochrane Vascular Information Specialist, CIS) buscó en el registro especializado (última búsqueda marzo 2017) y en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2017, Número 2). El CIS también buscó en registros de ensayos para obtener detalles de los estudios en curso o no publicados. Los autores de la revisión efectuaron búsquedas en dos bases de datos persas ‐ Iranmedex y Scientific Information Database (SID) ‐ así como Google Scholar.
Criterios de selección
Se realizaron búsquedas de ensayos controlados aleatorizados (ECA) que compararon los efectos de diferentes duraciones de las inyecciones subcutáneas de heparina sobre el dolor, la equimosis y los hematomas en el sitio de inyección.
Obtención y análisis de los datos
Dos autores de la revisión (MM, LJ), de manera independiente, extrajeron los datos en un formulario estructurado y evaluaron la calidad de los estudios. Se utilizaron los criterios recomendados por Cochrane para evaluar el riesgo de sesgo de los estudios incluidos. Para los resultados, se calculó la diferencia de medias (DM) o la DM estandarizada (DME) con los intervalos de confianza (IC) del 95% correspondientes. Se agruparon los datos mediante los modelos de efectos fijos y de efectos aleatorios. Se utilizó GRADE para evaluar la calidad general de la evidencia que apoyaba los resultados evaluados en esta revisión.
Resultados principales
Para esta actualización, se identificaron tres estudios nuevos y por lo tanto se incluyeron en la revisión cuatro estudios con un total de 459 participantes que recibieron inyecciones subcutáneas de HBPM en el abdomen. Sólo un ensayo informó el volumen del fármaco inyectado (0,4 ml). Debido a la naturaleza de la intervención, no fue posible cegar a los participantes ni a los cuidadores (personal) en ningún estudio incluido. Dos estudios describieron el cegamiento de los evaluadores de resultados; por lo tanto, en general la calidad metodológica de los estudios incluidos fue moderada. La duración de la inyección rápida fue de 10 segundos y la duración de la inyección lenta fue de 30 segundos en todos los estudios incluidos.
Tres estudios informaron la intensidad del dolor en el sitio de inyección después de cada inyección en diferentes puntos temporales. Dos estudios evaluaron la intensidad del dolor en el sitio de inyección inmediatamente después de cada inyección, y el metanálisis de 140 participantes no mostró diferencias claras en la intensidad del dolor en el sitio de inyección inmediatamente después de la inyección lenta en comparación con la inyección rápida (evidencia de baja calidad; p = 0,15). Por el contrario, el metanálisis de dos estudios con 59 participantes mostró que, 48 horas después de la inyección de heparina, la inyección lenta se asoció con menos intensidad del dolor en comparación con la inyección rápida (evidencia de baja calidad; p = 0,007). Un estudio (40 participantes) informó la intensidad del dolor 60 y 72 horas después de la inyección. Este estudio no describió diferencias claras en la intensidad del dolor en el sitio de inyección 60 y 72 horas luego de la inyección lenta en comparación con la inyección rápida.
Los cuatro estudios incluidos evaluaron el tamaño de la equimosis 48 horas después de cada inyección. El metanálisis de 459 participantes no mostró diferencias en el tamaño de la equimosis después de la inyección lenta en comparación con la inyección rápida (evidencia de baja calidad; p = 0,07). Ninguno de los estudios incluidos midió la incidencia del hematoma como resultado.
Conclusiones de los autores
Se encontraron cuatro ECA que evaluaron el efecto de la duración de la inyección de heparina subcutánea sobre la intensidad del dolor y el tamaño de la equimosis. Debido a los números pequeños de participantes, no se encontró evidencia suficiente para determinar cualquier efecto sobre la intensidad del dolor inmediatamente después de la inyección o 60 y 72 horas después de la inyección. Sin embargo, la inyección lenta puede reducir la intensidad del dolor en el sitio de inyección 48 horas después de la inyección (evidencia de baja calidad). No se observaron diferencias claras en el tamaño de la equimosis después de la inyección lenta en comparación con la inyección rápida (evidencia de baja calidad). Esta evidencia se consideró de baja calidad debido a la imprecisión y la inconsistencia.
PICOs
Resumen en términos sencillos
¿La velocidad de la inyección implica diferencias en la intensidad del dolor y en la equimosis en los pacientes que reciben inyecciones de heparina?
Antecedentes
La heparina es un fármaco utilizado para ayudar a evitar la coagulación de la sangre. Se presenta en dos formas: heparina no fraccionada (HNF) y heparina de bajo peso molecular (HBPM). Generalmente se administran mediante una inyección debajo de la piel. La heparina inyectada ingresa en la capa de grasa debajo de la piel y se libera lentamente en el organismo. Este tipo de inyección a veces puede causar equimosis y dolor en el sitio en el que se introduce la aguja. A veces también puede causar un edema que contiene sangre, denominado hematoma. Para los pacientes y los profesionales de atención sanitaria, se consideran importantes las estrategias que pueden aliviar el dolor y la equimosis. La reducción del malestar y la preocupación del paciente, siempre que sea posible, es un objetivo importante de la atención de enfermería. Se han realizado varios estudios para observar si la velocidad de la inyección afecta la intensidad del dolor y el grado de equimosis en el sitio en que se administra la inyección, aunque hay diferencias en los resultados y los autores no han establecido una conclusión final clara.
Características de los estudios y resultados clave
Se realizaron búsquedas de estudios que investigaron los efectos de la velocidad de la inyección sobre la intensidad del dolor y el grado de equimosis en el sitio donde se administra la inyección (actuales hasta marzo de 2017), y se encontraron cuatro estudios que cumplieron con los criterios de la revisión. Estos estudios se realizaron en Turquía, Italia y China. Incorporaron un total de 459 pacientes (287 mujeres y 172 hombres). Todos los pacientes recibieron HBPM, y ninguno de los estudios utilizó HNF. Los participantes fueron tratados en el hospital, en unidades de neurología, ortopédicas y de cardiología.
Los investigadores inyectaron heparina de forma lenta o rápida en el abdomen (estómago) de los participantes. Los pacientes podían mirar cuando se administraba la inyección y sabían si la misma era rápida (10 segundos) o lenta (30 segundos).
Los participantes que recibieron las inyecciones informaron que el dolor después de 48 horas fue menor con la inyección lenta. Debido a los números pequeños de participantes, no se encontró evidencia suficiente para determinar cualquier efecto sobre la intensidad del dolor inmediatamente después de la inyección o 60 horas y 72 horas después de la inyección. La equimosis no fue más pequeña con la inyección lenta. Ninguno de los estudios incluidos informó si los participantes presentaron un edema con sangre en el interior (hematoma).
Calidad de la evidencia
Se calificó la calidad de la evidencia como baja porque solo se encontró un pequeño número de estudios publicados que informaron sobre esta cuestión. Estos estudios fueron pequeños y presentaron resultados contradictorios. El hecho de que los participantes supieran si recibieron una inyección rápida o lenta puede haber afectado a los resultados.
Authors' conclusions
Summary of findings
| Slow vs fast subcutaneous heparin injection for prevention of bruising and site pain intensity | |||||
| Patient or population: patients treated with subcutaneous heparin injections Settings: hospital outpatient and inpatient units Intervention: slow injection (injection speed of 20 or more seconds) Comparison: fast injection (injection speed of less than 20 seconds) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk with fast injection | Corresponding risk with slow injection | ||||
| Intensity of injection pain immediately after injection (VAS 0 to 10 cm) 0 (no pain) to 10 (worst possible pain) | Mean pain intensity reported by the 2 studies ranged across fast injection groups from 2 to 5. | Mean pain intensity in the slow injection group was 1.52 points less than in the fast group (3.56 lower to 0.53 higher; P = 0.15). | 140 | ⊕⊕⊝⊝a | |
| Intensity of injection pain 48 hours after injection (VAS 0 to 10 cm) 0 (no pain) to 10 (worst possible pain) | Mean pain intensity ranged across fast injection groups from 2.1 to 2.8. | Mean pain intensity in the slow injection group was 1.68 points less than in the fast group (2.91 lower to 0.45 lower; P = 0.007). | 59 | ⊕⊕⊝⊝b | |
| Bruise size 48 hours after injection (mm/mm2) | See comment. | Mean bruising size in the slow injection group was 0.6 SD lower than in the fast injection group (1.24 lower to 0.04 higher; P = 0.07). | 459 | ⊕⊕⊝⊝c | Bruise size was measured on different scales; therefore we used the SMD to pool data. |
| Haematoma at injection site | See comment. | No studies measured this outcome. | |||
| *The basis for the assumed risk (e.g. median control group risk across studies) for pain intensity was the range of mean pain score reported following fast injection by the 2 studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the mean difference of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aWe downgraded the quality of evidence by two steps owing to study limitations, as we identified few studies with small numbers of participants (imprecision) and high heterogeneity (I2 = 73%) (inconsistency). | |||||
Background
Description of the condition
Heparin is an anticoagulant medication that is used to prevent further development of an existing thrombus or new clot formation. Heparin is prescribed for treatment or prevention of thromboembolic disorders. Different forms of heparin are available. Unfractionated heparin (UFH) may be administered by subcutaneous (SC) or intravenous (IV) injection, but low molecular weight heparin (LMWH) is administered only subcutaneously (Hodgson 2007).
Description of the intervention
Subcutaneous administration of heparin is frequently carried out as a nursing intervention (Wooldridge 1988). Subcutaneous injection is normally chosen when slow and continuous absorption of a drug (e.g. insulin, heparin) is needed. The drug is injected into fat and connective tissue underlying the dermis, where less blood flow results in a slower absorption rate. Suitable sites for subcutaneous injections are often the umbilical region of the abdomen and the lateral sides of the arms and thighs (Hunter 2008). Administration and injection techniques that are used for subcutaneous heparin injection may cause adverse outcomes such as bruising, haematoma, and pain at the injection site (Chan 2001; Kuzu 2001). The incidence of bruising at the injection site when 3‐mL and 1‐mL syringes are used has been reported as 69% and 79%, respectively (Hadley 1996).
How the intervention might work
Adverse drug reactions are a relatively common problem that might cause harm to patients. Nurses should apply techniques that minimise the side effects of subcutaneous injections, including site pain, haematoma, and bruising. It can be argued that slow versus fast injection of heparin might significantly reduce pain, haematoma, and bruising at the injection site. However, a systematic review has not been conducted to explore this theory. Slow administration of heparin allows time for subcutaneous tissue to accommodate the injected volume, resulting in reduced pressure, capillary bleeding, and site pain, and minimising the likelihood of other damage (Chan 2001).
Why it is important to do this review
Some patients receive subcutaneous heparin for a long time from the time of hospital admission until after hospital discharge (Delate 2012). With daily heparin injections for several weeks, the risk of extensive bruising in addition to pain at the injection site is high. Therefore, for patients and healthcare providers, strategies that can reduce pain and bruising are considered important. Reducing patients' discomfort and concerns whenever and wherever possible is an important aim of nursing. Several studies have explored the effects of factors such as temperature, syringe size, needle gauge, injection volume, and air bubble in the syringe on the incidence of bruising, haematoma, and pain at the injection site (Chan 2001; Kuzu 2001; Rahmani 2016; Sendir 2015). Other studies have explored the effect of duration of the injection on the incidence of bruising and pain (Dadaeen 2015; Dehghani 2012; Zaybak 2008). Some investigators have recommended that subcutaneous heparin injections must be given slowly to reduce bruising and pain, but others have reported no significant differences between the two techniques in terms of bruising and pain (Balci Akpinar 2008; Chenicek 2004; Pourghaznein 2014; Rahmani 1999). Several studies have compared slow versus fast subcutaneous injection of heparin, but researchers have reported variable results and study authors were not able to reach a clear final conclusion about the exact speed of heparin injection (Chenicek 2004; Sendir 2015; Zaybak 2008). This controversy highlights the importance of conducting a systematic review to explore the effect of different durations of heparin injection on complications at the injection site.
Objectives
To assess the effects of duration (speed) of subcutaneous heparin injection on pain, haematoma, and bruising at the injection site in people admitted to hospitals or clinics who require treatment with unfractionated heparin (UFH) or low molecular weight heparin (LMWH).
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials (RCTs) that we could include in this review. We excluded clinical controlled trials (CCTs), quasi‐randomised controlled trials (QRCTs), and quasi‐experimental studies.
Types of participants
We included studies in which participants were males and females 18 years of age or older who were admitted to hospitals or clinics and treated with subcutaneous injections of heparin including LMWH and UFH. We excluded trials involving people treated with other anticoagulant drugs.
Types of interventions
We included studies in which the intervention consisted of slow subcutaneous administration of heparin (LMWH or UFH) compared to fast subcutaneous administration of heparin. We considered an injection speed of 20 or more seconds as a slow injection, and an injection speed of less than 20 seconds as a fast injection.
Types of outcome measures
Primary outcomes
-
Pain intensity (measured at injection site by any scale, including visual analogue scale (VAS), numerical rating, McGill scales, and descriptive or other pain scales)
-
Size of bruise at the injection site
Secondary outcomes
-
Incidence of haematoma at the injection site
Search methods for identification of studies
We did not restrict language of publication.
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
-
Cochrane Vascular Specialised Register (20 March 2017).
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Allied and Complementary Medicine Database (AMED), and through handsearching of relevant journals. The full list of databases, journals, and conference proceedings that have been searched, as well as the search strategies used, is presented in the Specialised Register section of the Cochrane Vascular Module in the Cochrane Library (Specialised Register; www.cochranelibrary.com).
The CIS also searched the following trials registries for details of ongoing and unpublished studies.
-
ClinicalTrials.gov (www.clinicaltrials.gov).
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
-
International Standard Registered Clinical/Social Study Number (ISRCTN) Register (www.isrctn.com/).
See Appendix 2 for details of trial registries searches.
In addition, review authors searched Google Scholar (November 2016) and updated the search of two Persian databases ‐ Iranmedex (November 2016) and the Scientific Information Database (SID) (November 2016) ‐ using the search strategies provided in Appendix 3 and Appendix 4 (http://health.barakatkns.com/irmedex/index.asp; http://www.sid.ir/En/index.asp).
Searching other resources
For this update, we reviewed the reference lists of included studies to identify other studies for inclusion. We also tried to contact relevant trial authors to identify additional studies.
Data collection and analysis
Selection of studies
Two review authors (MM, LJ) independently assessed titles and abstracts of the studies retrieved by searching to determine whether each study met the inclusion criteria. If it was not possible to include or exclude a study on the basis of the title or abstract, review authors obtained the full version of the article. The same two review authors (MM, LJ) then assessed the full papers independently to explore if they met the inclusion criteria. We resolved disagreements by discussion and, if necessary, by consultation with the third review author (AAS).
Data extraction and management
Two review authors (MM, LJ) independently extracted data from the included studies using a structured data extraction form. We resolved disagreements by discussion and, if necessary, by consultation with the third review author (AAS). We collected the following data from the included studies: study design; method of randomisation; method of concealment of allocation; blinding, details of participants, details of interventions, and duration of interventions; main inclusion and exclusion criteria; outcomes; methods of measuring pain, bruising, and haematoma; and methods of performing statistical analysis and reporting results.
Assessment of risk of bias in included studies
Two review authors (MM, LJ) independently assessed the risk of bias of included studies. We resolved disagreements by discussion and, if necessary, by consultation with the third review author (AAS). We used the Cochrane 'Risk of bias' tool as described by Higgins when assessing risk of bias (Higgins 2011).
Measures of treatment effect
Bruise size and pain intensity were continuous outcome measures. Therefore, we calculated the mean difference (MD) and 95% confidence intervals (95% CIs) for these outcomes. As investigators measured bruise size using different scales, we calculated the standardised mean difference (SMD) and 95% CIs to pool data.
Unit of analysis issues
Cross‐over trials were eligible for inclusion in this review because heparin has a temporary effect and the half‐life of heparin is 4.5 to 7 hours after administration. Therefore, the first administration of heparin has no effect on formation of bruises at the second injection. We did not expect to find cluster‐randomised trials, and we identified none for inclusion in this review. The individual participant was the unit of analysis.
Dealing with missing data
We contacted trialists to ask them to provide missing information when needed.
Assessment of heterogeneity
We visually inspected forest plots and determined the I2 and Chi2 statistics to evaluate heterogeneity among the included studies.
Assessment of reporting biases
It is recommended that the test for funnel plot asymmetry to detect publication bias should be used when at least 10 studies are included in the review (Higgins 2011). We included only four studies and determined that it was not appropriate to prepare a funnel plot.
Data synthesis
We summarised study outcomes using narrative and quantitative methods. We applied a fixed‐effect model for meta‐analyses unless heterogeneity was high (I2 > 40%), in which case we used a random‐effects model for data synthesis. We used MD or SMD to pool continuous outcomes.
Subgroup analysis and investigation of heterogeneity
For this update, we subgrouped outcomes according to different measures used by trialists to recording bruise size (mm or mm2) and different time points of assessment.
If sufficient studies are included, and if appropriate in future updates, we will perform subgroup analyses according to participants' sex, age, etc.
Sensitivity analysis
We planned to perform a sensitivity analysis to assess possible influences of the following factors on effect size, if sufficient studies met the inclusion criteria of this review.
-
Exclusion of unpublished studies.
-
Consideration of the quality of studies.
-
Exclusion of studies conducted in different countries.
'Summary of findings'
We created a 'Summary of findings' table using GRADEpro software to summarise the evidence derived when investigators compared fast subcutaneous heparin injections versus slow injections in patients requiring heparin (GRADEproGDT 2015) (summary of findings Table for the main comparison). We included outcomes of pain intensity, bruise size, and extent of haematoma, as described under Types of outcome measures. We calculated assumed control intervention risks by using mean measurements in the control groups of selected studies for each outcome. We used the GRADE system to grade the quality of evidence as high, moderate, low, and very low, upon assessing within‐study risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Atkins 2004).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
See Figure 1.

Study flow diagram.
Included studies
For this update, we identified three new studies (Palese 2013; Sendir 2015; Zhao 2016). This review includes four studies with a total of 459 participants that were conducted in Italy (Palese), Turkey (Sendir and Zaybak), and China (Zhao) (Palese 2013; Sendir 2015; Zaybak 2008; Zhao 2016). Three studies were published in English (Palese, Sendir, and Zaybak) and one in Chinese (Zhao) (Palese 2013; Sendir 2015; Zaybak 2008; Zhao 2016). Three studies were small, including from 19 to 100 participants. The largest study involved 300 participants (Palese 2013). Two trials included more females (Palese and Sendir), one included more males (Zhao), and one included equal numbers of males and females (Zaybak) (Palese 2013; Sendir 2015; Zaybak 2008; Zhao 2016).
All studies included participants who received subcutaneous injections of LMWH, and no studies included participants given UFH. All studies performed subcutaneous injections into the abdomen. Only one trial reported the injected drug volume (0.4 mL) (Palese 2013).
The included studies enrolled participants who were hospitalised in orthopaedics units (Palese and Sendir); cardiology units (Zhao); and neurology, orthopaedics, and cardiology units (Zaybak) (Palese 2013; Sendir 2015; Zaybak 2008; Zhao 2016).
Sendir randomised participants to three groups: group A (injection duration 10 seconds), group B (injection duration 30 seconds and 5‐minute dry cold local application), and group C (injection duration 30 seconds) (Sendir 2015). We did not consider results from the group with injection duration of 30 seconds that applied 5‐minute dry cold locally, as this was not an objective of the review (Sendir 2015).
Zhao divided participants into six groups. Four groups investigated the effects of different injection durations and different pressing time on the injection site (Zhao 2016). We did not consider results from these four groups, as these were not the objectives of our review. We included as the control group the group that injected heparin for 10 seconds without pressing, and as the intervention group the group that injected heparin for 30 seconds without pressing (Zhao 2016).
Two studies used an applied self‐controlled design whereby every participant received the slow injection into one side of the abdomen (as intervention) and the fast injection into the other side of the abdomen (as control) (Palese 2013; Zaybak 2008).
Two other studies applied parallel design whereby investigators randomly allocated participants to different groups (Sendir 2015; Zhao 2016).
Only one trial reported the source of funding (Zaybak 2008).
Details of all included studies are given in the Characteristics of included studies table.
Excluded studies
For this update, we excluded seven additional studies (Avsar 2013; Dadaeen 2015; Dehghani 2012; Deng 2009; Fathi 2014; Rahmani 2013; Uzun 2016). In total, we excluded 21 studies. We excluded eight studies because they used a quasi‐randomised design (Babaie Asl 2008; Balci Akpinar 2008; Chan 2001; Dadaeen 2015; Dehghani 2012; Gholam Nezhad 2004; Sanagoo 2011; Tehrani Neshat 2005). We excluded three studies because they used a non‐randomised design (Deng 2009; Nair 2008; Uzun 2016). We excluded two studies because investigators used other anticoagulant drugs during the study (Chenicek 2004; Rahmani 1999). We excluded one study because researchers compared 10‐second heparin injection versus 30‐second heparin injection plus air lock (Fathi 2014). We excluded another study because study authors compared 10‐second heparin injection versus 10‐second injection and waiting for 10 seconds before withdrawing the needle (Rahmani 2013). We excluded one study because heparin injection duration was less than 20 seconds in all comparison groups (Pourghaznein 2014).
The remaining excluded studies compared various techniques of heparin injection but did not explore the effects of injection duration on study outcomes. Two of these studies compared two techniques (McGowan 1990; Wooldridge 1988). One study compared three different techniques (Vanbree 1984). Two studies compared four techniques of heparin injection (Avsar 2013; Jesús Gómez 2005).
Details of the excluded studies are given in the Characteristics of excluded studies table.
Risk of bias in included studies
For a summary of 'Risk of bias', see the Characteristics of included studies table and Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies adequately described methods of random sequence generation (Sendir 2015; Zhao 2016). One of these studies used a computerised randomisation programme to generate random sequence (Sendir 2015). The other study used a table of random numbers (Zhao 2016). However, methods of allocation concealment were unclear in these studies (Sendir 2015; Zhao 2016).
Two studies gave every participant a slow injection into one side of the abdomen and a fast injection into the other side of the abdomen (Palese 2013; Zaybak 2008). Therefore, it was not possible to randomise participants into two separate groups. However, participants could instead be randomised into intervention or control groups according to treatment order. Each participant was given one of the two injection techniques (injection duration of 10 or 30 seconds) for the first injection and the second technique 12 hours later. For each individual, the technique to be used first (injection duration of 10 or 30 seconds) was identified randomly using a randomised sequence. Two studies did not describe the methods of sequence generation and allocation concealment used (Palese 2013; Zaybak 2008). The CIS contacted the authors of the Palese study to request information about the randomisation method used, and they stated that the random sequence was generated by computer (Palese 2013).
Blinding
Owing to the nature of the intervention, it was not possible to blind participants and care givers in all included studies. Two studies described blinding of outcome assessors (Palese 2013; Zhao 2016). The Zaybak study was at unclear risk of detection bias because, although study authors mentioned assessor blinding, they provided no description of assessor blinding for bruising (Zaybak 2008). Sendir investigators reported that they used unblinded methods, leading to high risk of performance bias (Sendir 2015).
Incomplete outcome data
All included studies provided an adequate description of individual study withdrawals and dropouts. Sendir and Zhao reported 6.25% and 10% dropouts in one group, respectively, with no described intention‐to‐treat analysis, so we assigned high risk of bias (Sendir 2015; Zhao 2016).
Selective reporting
Although we did not have access to individual study protocols, study reports suggest that investigators reported all expected outcomes.
Other potential sources of bias
Three studies did not provide clear descriptions about some aspects of heparin injection (e.g. heparin temperature, syringe size, injection volume, air bubble in the syringe) (Sendir 2015; Zaybak 2008; Zhao 2016). These factors may have affected study outcomes, and so we judged these studies as being at unclear risk of other bias.
Effects of interventions
Pain intensity
Three studies evaluated site pain intensity at different time points after each injection. One study reported pain intensity immediately after injection and at 48, 60, and 72 hours after each injection (Sendir 2015). Another reported pain intensity immediately after injection (Zaybak 2008). The third reported pain intensity at 48 hours after each injection (Zhao 2016). The fourth study did not report injection pain intensity (Palese 2013).
Heterogeneity was high for this outcome, so we pooled results of different studies using a random‐effects method. In addition, we pooled data according to different time points reported in included studies, so numbers of studies and of participants at each time point were small.
Meta‐analysis of data from two trials showed no clear differences in site pain intensity immediately after slow injection compared to fast injection (mean difference (MD) ‐1.52, 95% confidence interval (CI) ‐3.56 to 0.53; participants = 140; I2 = 73%; P = 0.15; low‐quality evidence) (Sendir 2015; Zaybak 2008). See Analysis 1.1.
Meta‐analysis of data from two RCTs showed reduced site pain intensity 48 hours after slow injection compared to fast injection (MD ‐1.68, 95% CI ‐2.91 to ‐0.45; participants = 59; I2 = 72%; P = 0.007; low‐quality evidence) (Sendir 2015; Zhao 2016). See Analysis 1.1.
Only one study reported pain intensity at 60 and 72 hours after injection (Sendir 2015). This study described no clear differences in site pain intensity at 60 hours post slow injection compared to fast injection (MD ‐1.00, 95% CI ‐2.15 to 0.15; participants = 40; I2 = 0%; P = 0.09); or at 72 hours post injection (MD ‐0.80, 95% CI ‐1.70 to 0.10; participants = 40; I2 = 0%; P = 0.08; low‐quality evidence). See Analysis 1.1.
Size of bruise
Two studies used millimetric measuring papers to measure the area of the bruise and recorded it as square millimetres (mm2) (Sendir 2015; Zaybak 2008). Two studies measured the largest diameter of the bruise and recorded it as millimetres (mm) (Palese 2013; Zhao 2016).
We used the standardised mean difference (SMD) to pool data from these different outcome measures.
All four studies reported bruise size 48 hours after injection. Heterogeneity was high for this outcome (I2= 85%), so we pooled results of different studies using the random‐effects method. We pooled data according to different time points reported in included studies, so the numbers of studies and of participants at each time point were small.
Meta‐analysis revealed no difference in bruise size after slow injection compared to fast injection after 48 hours (SMD ‐0.60, 95% CI ‐1.24 to 0.04; participants = 459; studies = 4; P = 0.07) and detected no subgroup differences (P = 0.43). See Analysis 1.2.
Only one study assessed bruise size after 60 hours, showing no clear differences between intervention and control groups when these data were pooled (MD ‐3.85, 95% CI ‐8.99 to 1.29; participants = 40; P = 0.14; low‐quality evidence) (Sendir 2015). See Analysis 1.3.
Two studies assessed bruise size after 72 hours (Sendir 2015; Zaybak 2008). Heterogeneity was low for this outcome (I2 = 38%), therefore we pooled results of these studies using a fixed‐effect method and found no clear difference between slow and fast injections of heparin (MD ‐2.29, 95% CI ‐6.57 to 1.99; participants = 140; P = 0.29). See Analysis 1.3.
Incidence of haematoma
None of the included studies measured the incidence of haematoma as an outcome.
Discussion
Summary of main results
This systematic review incorporated data from four trials enrolling 459 participants to assess the effects of duration of subcutaneous heparin injection on pain and bruise size at the injection site. Results of this review show that slow injection may reduce pain intensity after 48 hours compared to fast injection, but that this reduction may not be detected immediately, nor at 60 or 72 hours after injection (low‐quality evidence). Review results also show that the slow injection technique had no clear effect on bruise size compared to the fast injection technique (low‐quality evidence). None of the included studies measured haematoma incidence after injection.
Overall completeness and applicability of evidence
All studies included in this update injected heparin subcutaneously into the abdomen, but two excluded studies injected heparin into the arm (Babaie Asl 2008; Balci Akpinar 2008). Therefore, it should be considered that pain intensity and bruise size at the injection site could be different depending on whether heparin is injected into the arm, thigh, or abdomen.
Similarily, all included studies used low molecular weight heparin (LMWH), and it is possible that outcomes could have been different had they used unfractionated heparin (UFH). To minimise any confounding effect, we excluded studies in which investigators also treated participants with antiplatelet or anticoagulant drugs because of the possible effects of these agents on bruising and haematoma size. Included studies provided no clear description of how analgesic medications were used. In addition, studies used different injection protocols such as needle gauge, syringe size, heparin volume injected, injection technique used, experience of the injector, etc, and did not clearly describe these. Therefore, it should be considered that these factors, along with injection speed, may also affect pain intensity and bruise size at the injection site.
Quality of the evidence
See summary of findings Table for the main comparison.
All studies included in this review were randomised controlled trials (RCTs), and we assessed the overall methodological quality of these studies as moderate, as the nature of the intervention made a double‐blind study design impossible. Two trials blinded outcome assessors (Palese 2013; Zhao 2016).
We assessed the overall quality of evidence presented using the GRADE approach. We judged the body of evidence related to pain intensity and size of the bruise to be of low quality. We downgraded the quality of the evidence owing to study limitations, as we identified only a few studies with small numbers of participants. This may have affected the precision of results.
Pooling of data from all included studies in the meta‐analyses was impossible, as some studies reported outcomes at different time points or used different measurements, thus reducing the numbers of available studies and participants for each outcome. One study addressed only bruise size (Palese 2013). The other studies addressed all primary outcomes. We detected heterogeneity across studies.
Potential biases in the review process
None of the review authors was involved in any of the included or excluded trials. Furthermore, none of the review authors had any conflicts of interest. To minimise the possibility of bias, we tried to locate eligible RCTs by conducting a broad and comprehensive search of several national and international databases. However, it is still possible that we did not recognise all eligible studies. In addition, two trained review authors independently performed study selection, data collection, and quality assessment of included studies. We followed Cochrane processes as described by Higgins when assessing risk of bias (Higgins 2011).
Agreements and disagreements with other studies or reviews
A recent published systematic review evaluated the effects of heparin injection duration on injection pain intensity and bruising (Yi 2016). Some of the findings of the previous review were not in line with the findings of our review. Yi reported that slow heparin injection reduced pain intensity and bruise size at 48 and 60 hours after each injection (Yi 2016). The Yi review included three RCTs and five quasi‐experimental studies (Yi 2016). Our review did not include quasi‐experimental studies because of potential risk of bias due to inadequate allocation concealment, as this could lead to overestimation of treatment efficacy by 30% to 40% compared to trials with adequate allocation concealment (Schulz 1995). Inclusion of quasi‐experimental studies could account for the difference between results of the two reviews.
In addition, Yi and colleagues limited their search strategies to studies published only in Chinese and English, and our search strategies did not restrict the language of publication (Yi 2016). Although we identified only papers published in Chinese and English in the current version of our review, this may not be the case in future searches, and we believe our search strategy is more comprehensive.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
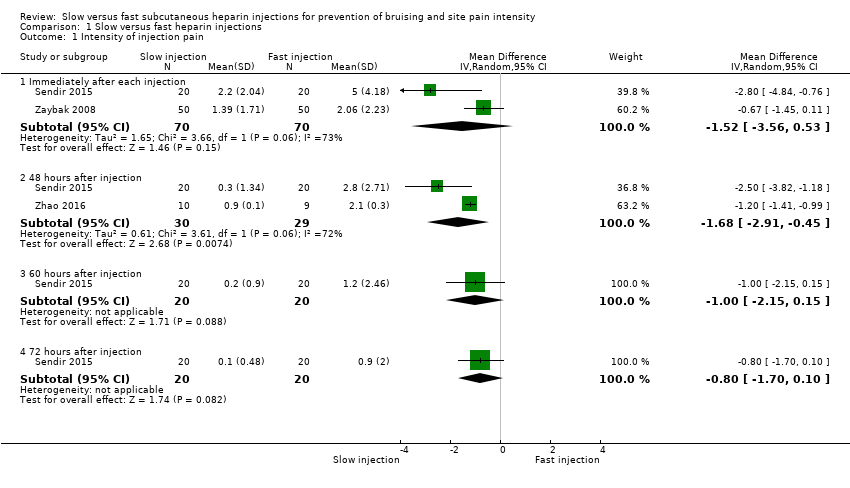
Comparison 1 Slow versus fast heparin injections, Outcome 1 Intensity of injection pain.
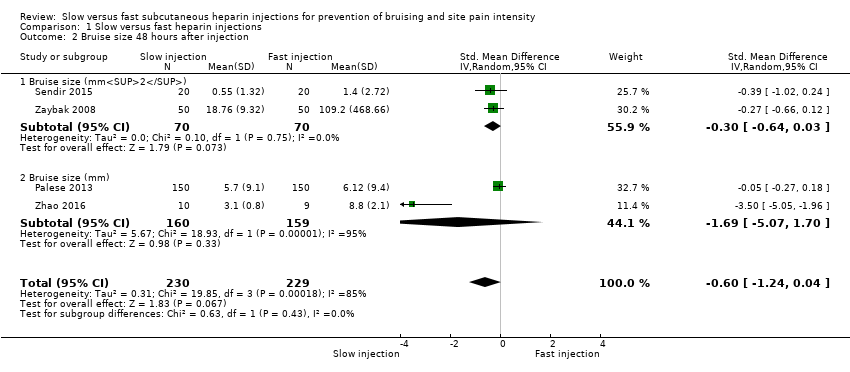
Comparison 1 Slow versus fast heparin injections, Outcome 2 Bruise size 48 hours after injection.

Comparison 1 Slow versus fast heparin injections, Outcome 3 Bruise size.
| Slow vs fast subcutaneous heparin injection for prevention of bruising and site pain intensity | |||||
| Patient or population: patients treated with subcutaneous heparin injections Settings: hospital outpatient and inpatient units Intervention: slow injection (injection speed of 20 or more seconds) Comparison: fast injection (injection speed of less than 20 seconds) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk with fast injection | Corresponding risk with slow injection | ||||
| Intensity of injection pain immediately after injection (VAS 0 to 10 cm) 0 (no pain) to 10 (worst possible pain) | Mean pain intensity reported by the 2 studies ranged across fast injection groups from 2 to 5. | Mean pain intensity in the slow injection group was 1.52 points less than in the fast group (3.56 lower to 0.53 higher; P = 0.15). | 140 | ⊕⊕⊝⊝a | |
| Intensity of injection pain 48 hours after injection (VAS 0 to 10 cm) 0 (no pain) to 10 (worst possible pain) | Mean pain intensity ranged across fast injection groups from 2.1 to 2.8. | Mean pain intensity in the slow injection group was 1.68 points less than in the fast group (2.91 lower to 0.45 lower; P = 0.007). | 59 | ⊕⊕⊝⊝b | |
| Bruise size 48 hours after injection (mm/mm2) | See comment. | Mean bruising size in the slow injection group was 0.6 SD lower than in the fast injection group (1.24 lower to 0.04 higher; P = 0.07). | 459 | ⊕⊕⊝⊝c | Bruise size was measured on different scales; therefore we used the SMD to pool data. |
| Haematoma at injection site | See comment. | No studies measured this outcome. | |||
| *The basis for the assumed risk (e.g. median control group risk across studies) for pain intensity was the range of mean pain score reported following fast injection by the 2 studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the mean difference of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aWe downgraded the quality of evidence by two steps owing to study limitations, as we identified few studies with small numbers of participants (imprecision) and high heterogeneity (I2 = 73%) (inconsistency). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intensity of injection pain Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Immediately after each injection | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐3.56, 0.53] |
| 1.2 48 hours after injection | 2 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.91, ‐0.45] |
| 1.3 60 hours after injection | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.15, 0.15] |
| 1.4 72 hours after injection | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.8 [‐1.70, 0.10] |
| 2 Bruise size 48 hours after injection Show forest plot | 4 | 459 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.24, 0.04] |
| 2.1 Bruise size (mm2) | 2 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.64, 0.03] |
| 2.2 Bruise size (mm) | 2 | 319 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐5.07, 1.70] |
| 3 Bruise size Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 60 hours after injection (mm2) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐3.85 [‐8.99, 1.29] |
| 3.2 72 hours after injection (mm2) | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐2.29 [‐6.57, 1.99] |

